Interspecific Nuclear Transfer Blastocysts Reconstructed from Arabian Oryx Somatic Cells and Domestic Cow Ooplasm
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ovaries, Oocyte, and Maturation
2.3. Dissect Zona with Micropipettes
2.4. Oocyte Enucleation
2.5. Preparation of Fibroblast from Arabian Oryx
2.6. Nuclear Transfer, Fusion, and Activation
2.7. In Vitro Culture of iSCNT Embryos
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowak, R.M.; Paradiso, J.L. Mustela erminea. In Walker’s Mammals of the World; Johns Hopkins University Press: Baltimore, MD, USA, 1983; pp. 988–989. [Google Scholar]
- Spinage, C.A. Natural History of Antelopes; Croom Helm: Kent, UK, 1986. [Google Scholar]
- Wilson, D.E.; Reeder, D.M. (Eds.) Mammal Species of the World: A Taxonomic and Geographic Reference; JHU Press: Baltimore, MD, USA, 2005. [Google Scholar]
- Islam, M.Z.; Ismail, K.; Boug, A. Restoration of the endangered Arabian Oryx Oryx leucoryx, Pallas 1766 in Saudi Arabia lessons learnt from the twenty years of re-introduction in arid fenced and unfenced protected areas: (Mammalia: Artiodactyla). Zool. Middle East 2011, 54 (Suppl. S3), 125–140. [Google Scholar] [CrossRef]
- Wurster, D.H.; Benirschke, K. Chromosome studies in the superfam~ly Bovoidea. Chromosoma 1968, 2, 152–171. [Google Scholar] [CrossRef] [PubMed]
- Newnham, R.E.; Davidson, W.M. The karyotype of the South Arabian oryx, ONX ICUCOI~(PXa llas), Mamma l Chmm Ncw.sI. Oryx Leucoryx 1967, 8, 15. [Google Scholar]
- Al-Ghadi, M.Q.; Alhimaidi, A.R.; Iwamoto, D.; Al Mutary, M.G.; Ammari, A.A.; Saeki, K.O. The in vitro development of cloned sheep embryos treated with Scriptaid and Trichostatin (A). Saudi J. Biol. Sci. 2020, 27, 2280–2286. [Google Scholar] [CrossRef]
- Bondioli, K.R.; Westhusin, M.E.; Looney, C.R. Production of identical bovine offspring by nuclear transfer. Theriogenology 1990, 33, 165–174. [Google Scholar] [CrossRef]
- Collas, P.; Balise, J.J.; Robl, J.M. Influence of cell cycle stage of the donor nucleus on development of nuclear transplant rabbit embryos. Biol. Reprod. 1992, 46, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Qu, P.; Shen, C.; Du, Y.; Qin, H.; Luo, S.; Fu, S. Melatonin protects rabbit somatic cell nuclear transfer (SCNT) embryos from electrofusion damage. Sci. Rep. 2020, 10, 2186. [Google Scholar] [CrossRef] [Green Version]
- Prather, R.S.; Sims, M.M. First NL. Nuclear transplantation in early pig embryos. Biol. Reprod. 1989, 41, 414–418. [Google Scholar] [CrossRef] [Green Version]
- Kamimura, S.; Inoue, K.; Mizutani, E.; Kim, J.M.; Inoue, H.; Ogonuki, N. Improved development of mouse somatic cell nuclear transfer embryos by chlamydocin analogues, class I and IIa histone deacetylase inhibitors. Biol. Reprod. 2021, 105, 543–553. [Google Scholar] [CrossRef]
- Meng, L.; Ely, J.J.; Stouffer, R.L.; Wolf, D.P. Rhesus monkeys produced by nuclear transfer. Biol. Reprod. 1997, 57, 454–459. [Google Scholar] [CrossRef] [Green Version]
- Hajian, M.; Jafarpour, F.; Aghamiri, S.M.; Varnosfaderani, S.R.; Esfahani, M.H.N. Effects of ovary storage temperature and embryo vitrification on somatic cell nuclear transfer outcomes in goats. Reprod. Fertil. 2020, 32, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Skrzyszowska, M.; Samiec, M. Generating cloned goats by somatic cell nuclear transfer—Molecular determinants and application to transgenics and biomedicine. Int. J. Mol. Sci. 2021, 22, 7490. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.A.; Wernery, U.; Hassan, F.A.H.; Wernery, R.; Skidmore, J.A. Production of the first cloned camel by somatic cell nuclear transfer. Biol. Reprod. 2021, 82, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.P.; Cibelli, J.B.; Diaz, F.; Moraes, C.T.; Farin, P.W.; Farin, C.E.; Hammer, C.J.; West, M.D.; Damiani, P.; Damiani, P. Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer. Cloning 2000, 2, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Dominko, T.; Mitalipova, M.; Haley, B.; Beyhan, Z.; Memili, E.; McKusick, B.; First, N.L. Bovine oocyte cytoplasm supports the development of embryos produced by nuclear transfer of somatic cell nuclei from various mammalian species. Biol. Reprod. 1999, 60, 1496–1502. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Dai, Y.; Du, W.; Zhao, C.; Wang, H.; Wang, L.; Li, R.; Liu, Y.; Wan, R.; Li, N. Cloned endangered species takin (Budorcas taxicolor) by inter-species nuclear transfer and comparison of the blastocyst development with yak (Bos grunniens) and bovine. Mol. Reprod. Dev. Inc. Gamete Res. 2006, 73, 189–195. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Dai, Y.; Du, W.; Zhao, C.; Wang, L.; Wang, H.; Li, R.; Liu, Y.; Wan, R.; et al. Nuclear reprogramming in embryos generated by the transfer of yak (Bos grunniens) nuclei into bovine oocytes and comparison with bovine–bovine SCNT and bovine IVF embryos. Theriogenology 2007, 67, 1331–1338. [Google Scholar] [CrossRef]
- Sansinena, M.J.; Hylan, D.; Hebert, K.; Denniston, R.S.; Godke, R.A. Banteng (Bos javanicus) embryos and pregnancies produced by interspecies nuclear transfer. Theriogenology 2005, 63, 1081–1091. [Google Scholar] [CrossRef]
- Hua, S.; Zhang, Y.; Song, K.; Song, J.; Zhang, Z.; Zhang, L.; Zhang, C.; Cao, J.; Ma, L. Development of bovine–ovine interspecies cloned embryos and mitochondria segregation in blastomeres during preimplantation. Anim. Reprod. Sci. 2008, 105, 245–257. [Google Scholar] [CrossRef]
- Thongphakdee, A.; Kobayashi, S.; Imai, K.; Inaba, Y.; Tasai, M.; Tagami, T.; Nirasawa, K.; Nagai, T.; Saito, N.; Techakumphu, M.; et al. Interspecies nuclear transfer embryos reconstructed from cat somatic cells and bovine ooplasm. J. Reprod. Dev. 2008, 54, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Thongphakdee, A.; Numchaisrika, P.; Omsongkram, S.; Chatdarong, K.; Kamolnorranath, S.; Dumnui, S.; Techakumphu, M. In vitro development of marbled cat embryos derived from interspecies somatic cell nuclear transfer. Reprod. Domest. Anim. 2006, 41, 219–226. [Google Scholar] [CrossRef]
- Selokar, N.L.; George, A.; Saha, A.P.; Sharma, R.; Muzaffer, M.; Shah, R.A.; Palta, P.; Chauhan, M.S.; Manik, R.S.; Singla, S.K. Production of interspecies handmade cloned embryos by nuclear transfer of cattle, goat, and rat fibroblasts to buffalo (Bubalus bubalis) oocytes. Anim. Reprod. Sci. 2011, 123, 279–282. [Google Scholar] [CrossRef]
- Takeda, K. Mitochondrial DNA transmission and confounding mitochondrial influences in cloned cattle and pigs. Reprod. Med. Biol. 2013, 12, 47–55. [Google Scholar] [CrossRef]
- Wittayarat, M.; Sato, Y.; Do, L.T.K.; Chatdarong, K.; Tharasanit, T.; Techakumphu, M.; Taniguchi, M.; Otoi, T. Epigenetic modulation on cat-cow interspecies somatic cell nuclear transfer embryos by treatment with trichostatin A. Anim. Sci. J. 2017, 88, 593–601. [Google Scholar] [CrossRef]
- Do, L.T.K.; Wittayarat, M.; Sato, Y.; Chatdarong, K.; Tharasanit, T.; Techakumphu, M.; Hirata, M.; Tanihara, F.; Taniguchi, M.; Otoi, T. Comparison of Blastocyst Development between Cat-Cow and Cat-Pig Interspecies Somatic Cell Nuclear Transfer Embryos Treated with Trichostatin A. Biol. Bull. 2021, 48, 107–117. [Google Scholar] [CrossRef]
- Chang, K.H.; Lim, J.M.; Kang, S.K.; Lee, B.C.; Moon, S.Y.; Hwang, W.S. Blastocyst formation, karyotype, and mitochondrial DNA of interspecies embryos derived from the nuclear transfer of human cord fibroblasts into enucleated bovine oocytes. Fertil. Steril. 2003, 80, 1380–1387. [Google Scholar] [CrossRef]
- Chen, Y.; He, Z.X.; Liu, A.; Wang, K.; Mao, W.W.; Chu, J.X.; Lu, Y.; Fang, Z.F.; Shi, Y.T.; Yang, Q.Z.; et al. Embryonic stem cells generated by nuclear transfer of human somatic nuclei into rabbit oocytes. Cell Res. 2003, 13, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Moulavi, F.; Hosseini, S.M.; Tanhaie-Vash, N.; Ostadhosseini, S.; Hosseini, S.H.; Hajinasrollah, M.; Asgharia, M.H.; Gourabi, H.; Shahverdi, A.; Vosough, A.D.; et al. Interspecies somatic cell nuclear transfer in Asiatic cheetah using nuclei derived from post-mortem frozen tissue in absence of cryo-protectant and in vitro matured domestic cat oocytes. Theriogenology 2017, 90, 197–203. [Google Scholar] [CrossRef]
- Carvalho, B.P.; Cunha, A.T.; Silva, B.D.; Sousa, R.V.; Leme, L.O.; Dode, M.A.; Melo, E.O. Production of transgenic cattle by somatic cell nuclear transfer (SCNT) with the human granulocyte colony-stimulation factor (hG-CSF). J. Anim. Sci. Biotechnol. 2019, 61, 61. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Guo, T.; Tong, X.; Luo, L.; Zhou, G.; Fu, Y.; Liu, Y. Experimental cloning of embryos through human-rabbit inter-species nuclear transfer. Front. Biol. 2007, 2, 80–84. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, Y.; Guo, Z.; Wang, F. Development of interspecies nuclear transfer embryos reconstructed with argali (Ovis ammon) somatic cells and sheep ooplasm. Cell Biol. Int. 2014, 38, 211–218. [Google Scholar] [CrossRef]
- Samiec, M.; Skrzyszowska, M. Can reprogrammingof overall epigenetic memory and specific parental genomicimprinting memory within donor cell-inherited nuclear ge-nome be a major hindrance for the somatic cell cloning ofmammals? A review. Ann. Anim. Sci. 2018, 18, 623–638. [Google Scholar] [CrossRef] [Green Version]
- Wiater, J.; Samiec, M.; Skrzyszowska, M.; Lipiński, D. Trichostatin A-assisted epigenomic modulation affects the expression profiles of not only recombinant human α1, 2-fucosyltransferase and α-galactosidase A enzymes but also Galα1→ 3Gal epitopes in porcine bi-transgenic adult cutaneous fibroblast cells. Int. J. Mol. Sci. 2021, 22, 1386. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Zhang, M.; Wang, D.; Ma, Y.; Wang, Y.; Wang, Y.; Huang, Y.; Zhang, Y. Transcriptional memory inherited from donor cells is a developmental defect of bovine cloned embryos. FASEB J. 2000, 34, 1637–1651. [Google Scholar] [CrossRef] [Green Version]
- Samiec, M.; Romanek, J.; Lipinski, D.; Opiela, J. Expression of pluripotency-related genes is highly dependenton trichostatin A-assisted epigenomic modulation of porcinemesenchymal stem cells analysed for apoptosis and subse-quently used for generating cloned embryos. Anim. Sci. J. 2019, 90, 1127–1141. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Y.; Qu, P.; Qing, S.; Qiao, F.; Zhang, Y.; Mager, J.; Wang, Y. Sperm-borne miR-449b in-fluences cleavage, epigenetic reprogramming and apoptosisof SCNT embryos in bovine. Sci. Rep. 2017, 7, 13403. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Qu, P.; Ma, X.; Qiao, F.; Ma, Y.; Qing, S.; Zhang, Y.; Wang, Y.; Cui, W. Tauroursodeoxycholicacid (TUDCA) alleviates endoplasmic reticulum stress of nuclear donor cells under serum starvation. PLoS ONE 2018, 13, e0196785. [Google Scholar]
- Gouveia, C.; Huyser, C.; Egli, D.; Pepper, M.S. Lessons learned from somatic cell nuclear transfer. Int. J. Mol. Sci. 2020, 21, 2314. [Google Scholar] [CrossRef]
- Loi, P.; Modlinski, J.A.; Ptak, G. Interspecies somatic cell nuclear transfer: A salvage tool seeking first aid. Theriogenology 2011, 76, 217–228. [Google Scholar] [CrossRef]
- Loi, P.; Saragusty, J.; Ptak, G. Cloning the mammoth: A complicated task or just a dream? Adv. Exp. Med. Biol. 2014, 753, e489–e502. [Google Scholar]
- Folch, J.; Cocero, M.J.; Chesne, P.; Alabart, J.L.; Domínguez, V.; Cognie, Y.; Roche, A.; Fernández-Árias, A.; Martí, J.; Sánchez, P.; et al. First birth of an animal from an extinct subspecies (Capra pyrenaica pyrenaica) by cloning. Theriogenology 2009, 71, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H. Epigenetic reprogramming in mammalian species after SCNT-based cloning. Theriogenology 2016, 86, 80–90. [Google Scholar] [CrossRef]
- Loi, P.; Iuso, D.; Czernik, M.; Ogura, A. A new, dynamic era for somatic cell nuclear transfer? Trends Biotechnol. 2016, 34, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Smith, S.L.; Tian, X.C.; Lewin, H.A.; Renard, J.P.; Wakayama, T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat. Genet. 2007, 39, 295–302. [Google Scholar] [CrossRef]
- Kim, J. Chemosensitization prevents tolerance of Aspergillusfumigatus to antimycotic drugs. Biochem. Biophys. Res. Commun. 2008, 372, 71–266. [Google Scholar] [CrossRef]
- Gray, R.; Dobson, R. Extinct ibex is resurrected by cloning. Telegraph 2009, 71, 1026–1034. [Google Scholar]
- Ammari, A.A.; Amran, R.A.; Al Ghadi, M.G.; Alhimaidi, A.R. Morphometric Assessment of the Bovine Ovary for in vitro Matured Oocyte Quality to Determine Developmental Competence. Indian J. Anim. Res. 2022, 56, 557–562. [Google Scholar] [CrossRef]
- Ammari, A.A.; ALghadi, M.G.; Alhimaidi, A.R.; Amran, R.A. The role of passage numbers of donor cells in the development of Arabian Oryx–Cow interspecific somatic cell nuclear transfer embryos. Open Chem. 2022, 20, 342–349. [Google Scholar] [CrossRef]
- Amarnath, D.; Choi, I.; Moawad, A.R.; Wakayama, T.; Campbell, K.H. Nuclear-cytoplasmic incompatibility and inefficient development of pig-mouse cytoplasmic hybrid embryos. Reproduction 2011, 142, 295. [Google Scholar] [CrossRef] [Green Version]
- Melo, L.M.; Silva, S.B.; Magalhães, L.C.; Cortez, J.V.; Kumar, S.; Duarte, J.M.; Rola, L.D.; Chaves, M.S.; Freitas, V.J. The use of somatic cell nuclear transfer to obtain interspecific cloned embryos from brown brocket deer karyoplast and bovine cytoplast: Embryo development and nuclear gene expression. Theriogenol. Wild 2022, 1, 100001. [Google Scholar] [CrossRef]
- Srirattana, K.; Matsukawa, K.; Akagi, S.; Tasai, M.; Tagami, T.; Nirasawa, K.; Nagai, T.; Kanai, Y.; Parnpai, R.; Takeda, K. Constant transmission of mitochondrial DNA in intergeneric cloned embryos reconstructed from swamp buffalo fibroblasts and bovine ooplasm. Anim. Sci. J. 2011, 82, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Bishop, C.E.; Treff, N.R.; Walker, S.J.; Sandler, V.M.; Becker, S.; Klimanskaya, I.; Wun, W.S.; Dunn, R.; Hall, R.M. Reprogramming of human somatic cells using human and animal oocytes. Cloning Stem Cells 2009, 11, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Beyhan, Z.; Rodriguez, R.M.; Ross, P.J.; Iager, A.E.; Kaiser, G.G.; Chen, Y.; Cibelli, J.B. Bovine ooplasm partially remodels primate somatic nuclei following somatic cell nuclear transfer. Cloning Stem Cells 2009, 11, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Lagutina, I.; Lazzari, G.; Duchi, R.; Turini, P.; Tessaro, I.; Brunetti, D.; Colleoni, S.; Crotti, G.; Galli, C. Comparative aspects of somatic cell nuclear transfer with conventional and zona-free method in cattle, horse, pig, and sheep. Theriogenology 2007, 67, 90–98. [Google Scholar] [CrossRef]
- Holt, W.V.; Pickard, A.R.; Prather, R.S. Wildlife conservation and reproductive cloning. Reprodction 2004, 127, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, Y.; Du, W.; Zhang, L.; Yu, S.; Dai, Y.; Zhao, C.; Li, N. Aberrant gene expression in organs of bovine clones that die within two days after birth. Biol. Reprod. 2005, 72, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Ty, L.V.; Hanh, N.V.; Uoc, N.T.; Duc, N.G.; Thanh, N.T.; Bui, L.C.; Huu, Q.X.; Nguyen, B.X. Preliminary results of cell cryobanking and embryo production of black bear (Ursus thibetanus) by interspecies somatic cell nuclear transfer. Theriogenology 2003, 59, 290, (abstract). [Google Scholar]
- Kitiyanant, Y.; Saikhun, J.; Chaisalee, B.; White, K.L.; Pavasuthipaisit, K. Somatic cell cloning in buffalo (Bubalus bubalis): Effects of interspecies cytoplasmic recipients and activation procedures. Cloning Stem Cells 2001, 3, 97–104. [Google Scholar] [CrossRef]
- Malin, K.; Witkowska-Piłaszewicz, O.; Papis, K. The many problems of somatic cell nuclear transfer in reproductive cloning of mammals. Theriogenology 2022, 189, 246–254. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Petkov, S.; Purup, S.; Hyttel, P.; Callesen, H. Passagenumber of porcine embryonic germ cells affects epigeneticstatus and blastocyst rate following somatic cell nucleartransfer. Anim. Reprod. Sci. 2014, 147, 39–46. [Google Scholar] [CrossRef]
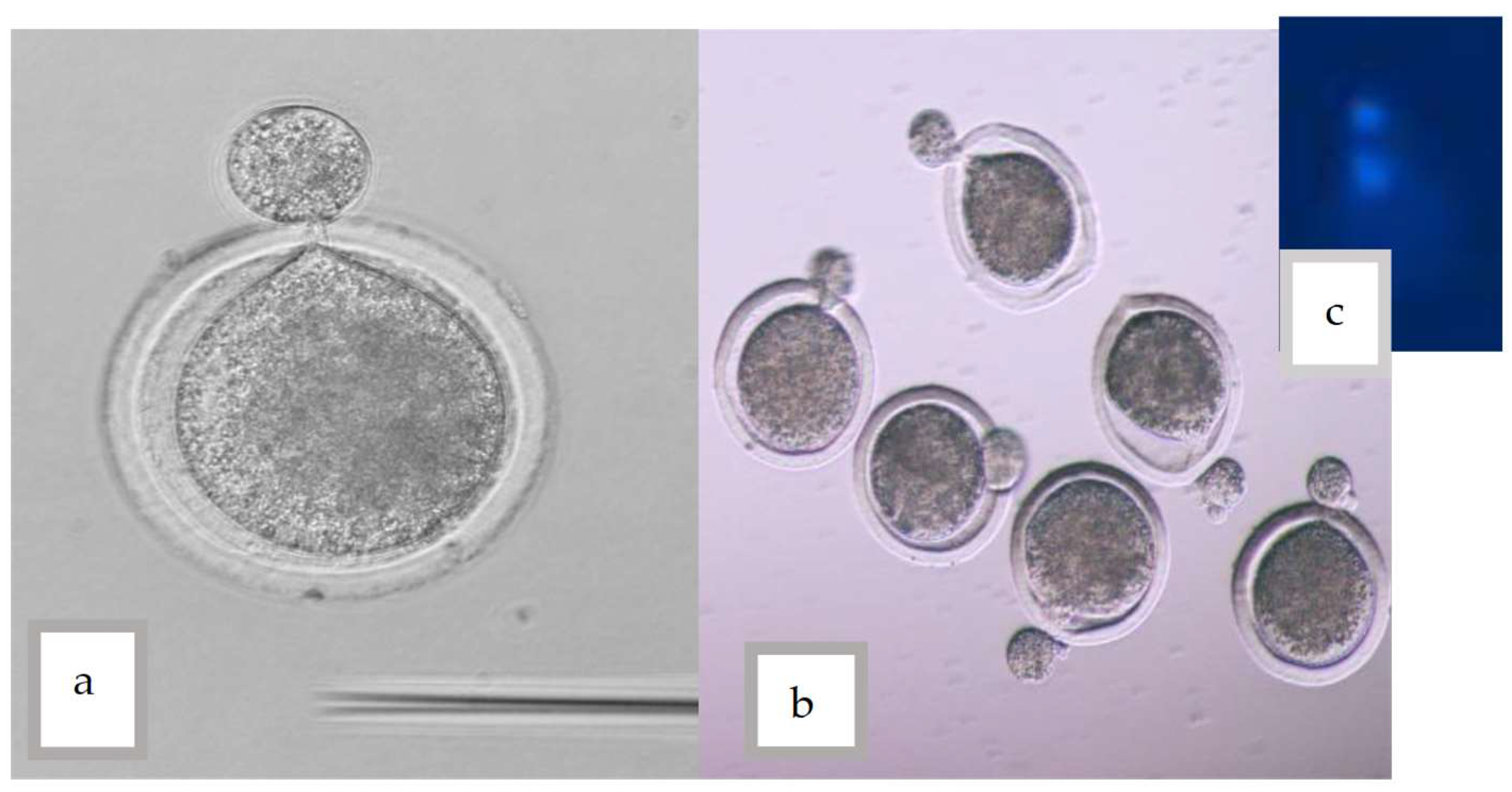
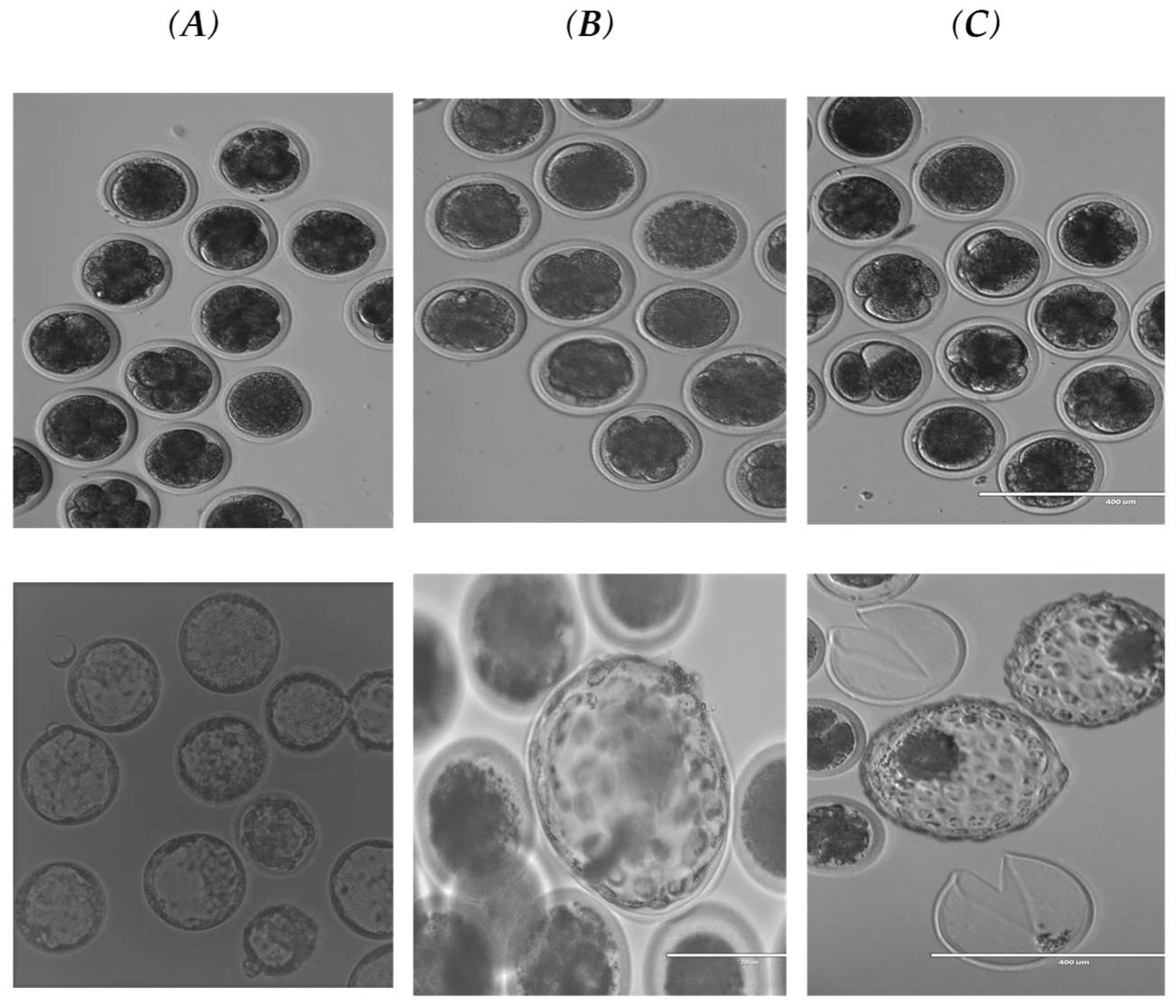
| Group | No. of Oocytes No, % | With FB No, % | Without FB No, % | No. of Enucleation No, % | No. of Activation No, % | No. of Culture Embryo% | Cleavage Rate % No, % | Blastocyst Rate % No, % |
|---|---|---|---|---|---|---|---|---|
| Oryx interspecific SCNT | 810/1832 = 44.21% | 536/810 = 66.17% | 274/810 = 33.83% | 439/536 = 81.90% | 241/439 54.90% | 150/241 = 62.24% | 65/241 = 26.97% | 6/65 = 9.23% |
| Cow SCNT | 716/1832 = 39.09% | 528/716 = 73.74% | 188/716 = 26.26% | 451/528 = 85.41% | 299/451 = 66.30% | 230/299 = 76% | 96 (33%) | 8/96 = 8.33% |
| Cow IVF | 306/1832 = 16.7% | - | - | - | - | 306 | 130 (42%) | 74 (57%) |
| Type | Oocytes No. | With FB No. & % | 1 Cell No. & % | 2 Cells No. & % | 4 Cells No. & % | 8 Cells No. & % | 16 Cells No. & % |
|---|---|---|---|---|---|---|---|
| Oryx interspecific SCNT | 810 | 536/810 = 66.17% | 110/536 = 20.52% | 21/65 = 32.31% | 19/65 = 29.23% | 13/65 = 20% | 2/65 = 3.08% |
| Cow SCNT | 716 | 522/716 = 72.91% | 23/522 = 4.41% | 26/93 = 27.96% | 6/93 = 6.45% | 21/93 = 22.58% | 18/93 = 19.35% |
| IVF cow | 306 | - | 80/306 = 26.14% | 13/107 12.15% | 8/107 = 7.48% | 8/107 = 7.48% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammari, A.A.; ALGhadi, M.G.; Amran, R.A.; Al Malahi, N.M.; Alhimaidi, A.R. Interspecific Nuclear Transfer Blastocysts Reconstructed from Arabian Oryx Somatic Cells and Domestic Cow Ooplasm. Vet. Sci. 2023, 10, 17. https://doi.org/10.3390/vetsci10010017
Ammari AA, ALGhadi MG, Amran RA, Al Malahi NM, Alhimaidi AR. Interspecific Nuclear Transfer Blastocysts Reconstructed from Arabian Oryx Somatic Cells and Domestic Cow Ooplasm. Veterinary Sciences. 2023; 10(1):17. https://doi.org/10.3390/vetsci10010017
Chicago/Turabian StyleAmmari, Aiman A., Muath G. ALGhadi, Ramzi A. Amran, Nawal M. Al Malahi, and Ahmad R. Alhimaidi. 2023. "Interspecific Nuclear Transfer Blastocysts Reconstructed from Arabian Oryx Somatic Cells and Domestic Cow Ooplasm" Veterinary Sciences 10, no. 1: 17. https://doi.org/10.3390/vetsci10010017







