Development of a TaqMan-Probe-Based Multiplex Real-Time PCR for the Simultaneous Detection of Porcine Circovirus 2, 3, and 4 in East China from 2020 to 2022
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Primers and Probes
2.2. Viruses, Nucleic Acid, and Clinical Samples
2.3. DNA/RNA Extraction and Reverse Transcription
2.4. Construction of Recombinant Plasmids
2.5. Optimization of Multiplex qPCR Assay
2.6. Establishment of Standard Curves
2.7. Specificity of Multiplex qPCR Assay
2.8. Sensitivity of Multiplex qPCR Assay
2.9. Repeatability of Multiplex qPCR Assay
2.10. Clinical Sample Detection
3. Results
3.1. Optimization of qPCR Reaction Conditions
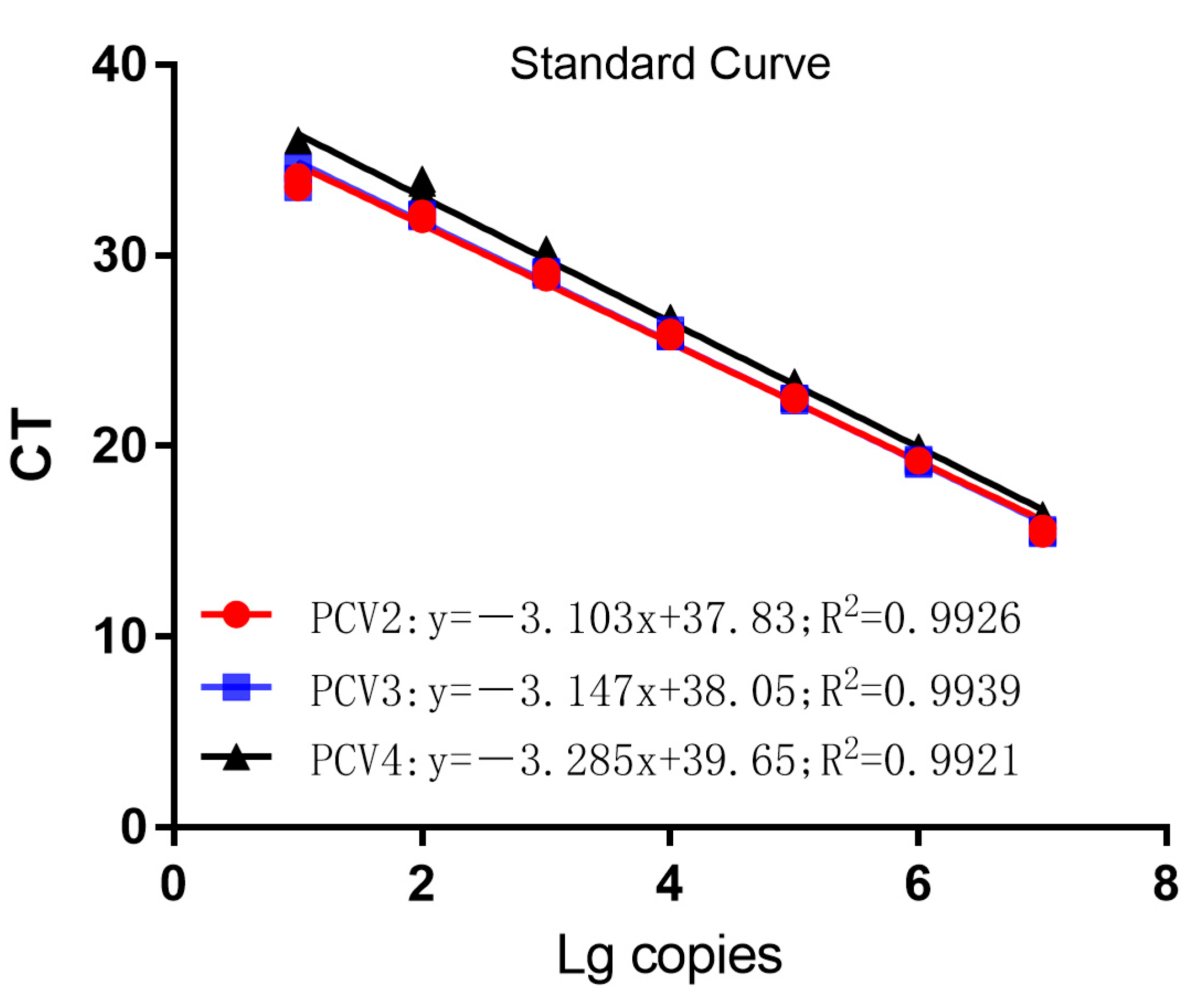
3.2. Establishment of Standard Curves
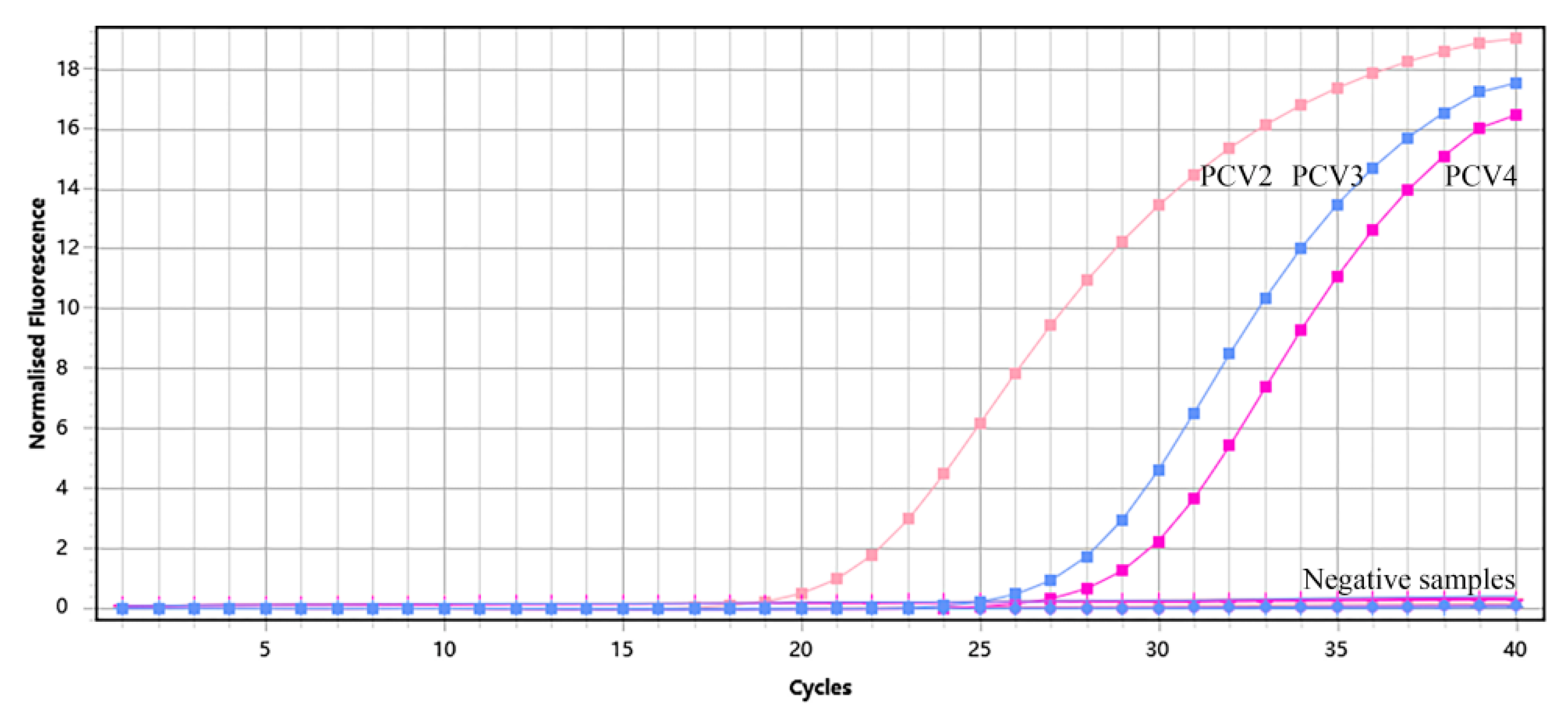
3.3. Specificity of Multiplex qPCR Assay
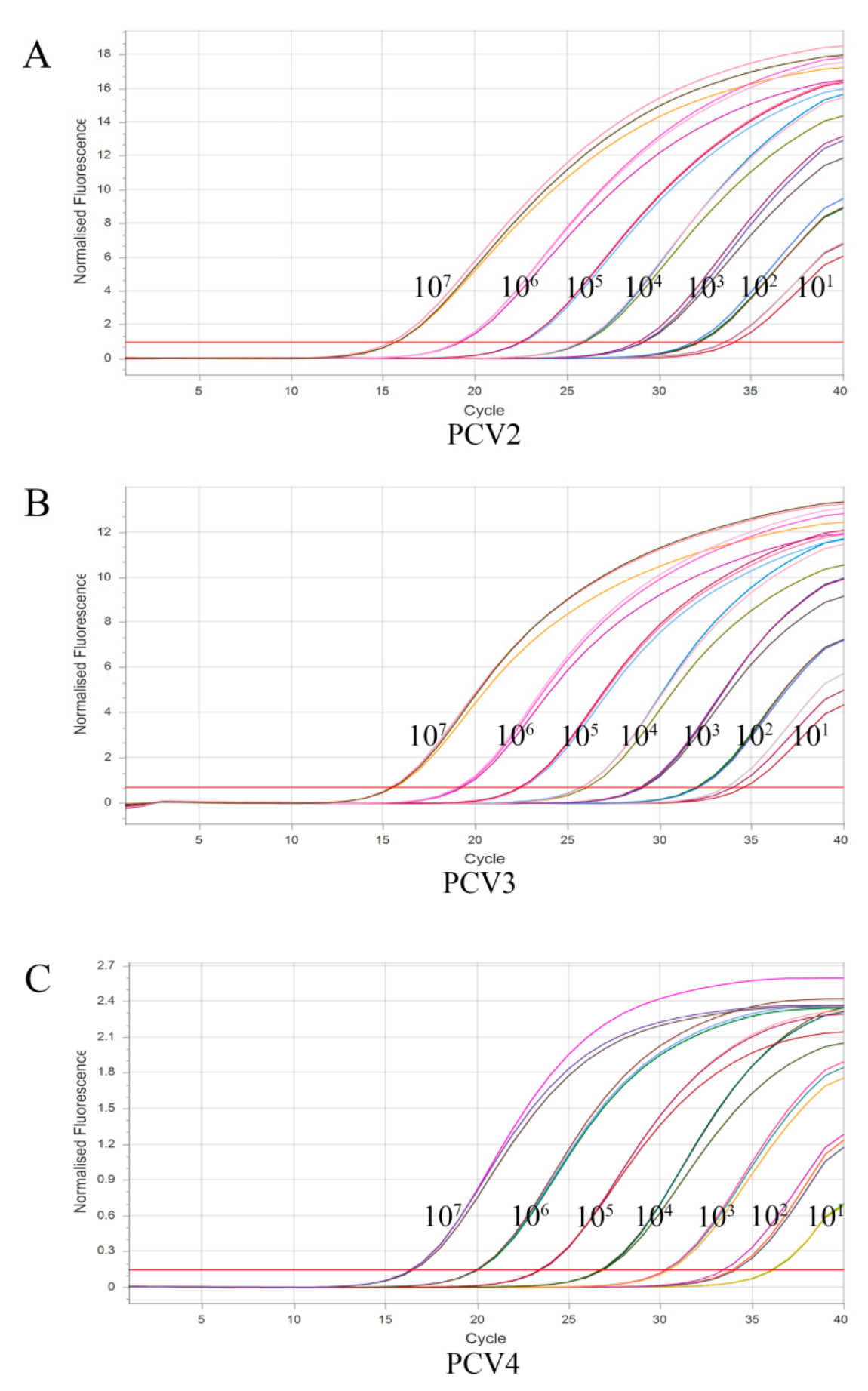
3.4. Sensitivity of Multiplex qPCR Assay
3.5. Repeatability of Multiplex Real-Time PCR Assay
3.6. Detection of Clinical Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Zhang, L.; Li, X.; Niu, G.; Ren, L. Recent Progress on Epidemiology and Pathobiology of Porcine Circovirus 3. Viruses 2021, 13, 1944. [Google Scholar] [CrossRef]
- Lagan, T.P.; Staines, A.; Gordon, A.; Sheridan, P.; McMenamy, M.; Duffy, C.; Collins, P.J.; Mooney, M.H.; Lemon, K. Co-infection status of novel parvovirus’s (PPV2 to 4) with porcine circovirus 2 in porcine respiratory disease complex and porcine circovirus-associated disease from 1997 to 2012. Transbound. Emerg. Dis. 2021, 68, 1979–1994. [Google Scholar] [CrossRef] [PubMed]
- Crowther, R.A.; Berriman, J.A.; Curran, W.L.; Allan, G.M.; Todd, D. Comparison of the structures of three circoviruses: Chicken anemia virus, porcine circovirus type 2, and beak and feather disease virus. J. Virol. 2003, 77, 13036–13041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tischer, I.; Rasch, R.; Tochtermann, G. Characterization of papovavirus-and picornavirus-like particles in permanent pig kidney cell lines. Zentralbl. Bakteriol. Orig. A 1974, 226, 153–167. [Google Scholar]
- Allan, G.; Meehan, B.; Todd, D.; Kennedy, S.; McNeilly, F.; Ellis, J.; Clark, E.G.; Harding, J.; Espuna, E.; Botner, A.; et al. Novel porcine circoviruses from pigs with wasting disease syndromes. Vet. Rec. 1998, 142, 467–468. [Google Scholar]
- Yao, J.; Qin, Y.; Zeng, Y.; Ouyang, K.; Chen, Y.; Huang, W.; Wei, Z. Genetic analysis of porcine circovirus type 2 (PCV2) strains between 2002 and 2016 reveals PCV2 mutant predominating in porcine population in Guangxi, China. BMC Vet. Res. 2019, 15, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palinski, R.; Piñeyro, P.; Shang, P.; Yuan, F.; Guo, R.; Fang, Y.; Byers, E.; Hause, B.M. A Novel Porcine Circovirus Distantly Related to Known Circoviruses Is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J. Virol. 2016, 91, e01879-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Bai, C.; Ge, K.; Li, Y.; Gao, W.; Jiang, S.; Wang, Y. Establishment of an SYBR Green-based real-time PCR assay for porcine circovirus type 4 detection. J. Virol. Methods 2020, 285, 113963. [Google Scholar] [CrossRef]
- Zhang, H.H.; Hu, W.Q.; Li, J.Y.; Liu, T.N.; Zhou, J.Y.; Opriessnig, T.; Xiao, C.T. Novel circovirus species identified in farmed pigs designated as Porcine circovirus 4, Hunan province, China. Transbound. Emerg. Dis. 2020, 67, 1057–1061. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Y.; Lian, X.; Sun, H.; Wang, J.; Liu, W.; Meng, G.; Li, P.; Zhu, D.; Jin, Y.; et al. Functional analysis of the interferon-stimulated response element of porcine circovirus type 2 and its role during viral replication in vitro and in vivo. Virol. J. 2012, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Fang, B.; Ma, J.; Liu, Y.; Bu, D.; Zhou, P.; Wang, H.; Jia, K.; Zhang, G. Insights into the epidemic characteristics and evolutionary history of the novel porcine circovirus type 3 in southern China. Transbound. Emerg. Dis. 2018, 65, e296–e303. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Su, M.; Guo, D.; Li, C.; Wei, S.; Feng, L.; Sun, D. Molecular detection and phylogenetic analysis of porcine circovirus type 3 in 21 Provinces of China during 2015–2017. Transbound. Emerg. Dis. 2019, 66, 1004–1015. [Google Scholar] [CrossRef]
- Segales, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Saade, G.; Deblanc, C.; Bougon, J.; Marois-Créhan, C.; Fablet, C.; Auray, G.; Belloc, C.; Leblanc-Maridor, M.; Gagnon, C.A.; Zhu, J.; et al. Coinfections and their molecular consequences in the porcine respiratory tract. Vet. Res. 2020, 51, 80. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Zhang, X.; Liu, X.; Ren, L. Co-Infection of Swine with Porcine Circovirus Type 2 and Other Swine Viruses. Viruses 2019, 11, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, T.G.; Giannitti, F.; Rossow, S.; Marthaler, D.; Knutson, T.P.; Li, L.; Deng, X.; Resende, T.; Vannucci, F.; Delwart, E. Detection of a novel circovirus PCV3 in pigs with cardiac and multi-systemic inflammation. Virol. J. 2016, 13, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruda, B.; Pineyro, P.; Derscheid, R.; Hause, B.; Byers, E.; Dion, K.; Long, D.; Sievers, C.; Tangen, J.; Williams, T.; et al. PCV3-associated disease in the United States swine herd. Emerg. Microbes Infect. 2019, 8, 684–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Zhang, Y.-H.; Tian, R.-B.; Hou, C.-Y.; Li, X.-S.; Zheng, L.-L.; Wang, L.-Q.; Chen, H.-Y. Prevalence and genetic analysis of porcine circovirus type 2 (PCV2) and type 3 (PCV3) between 2018 and 2020 in central China. Infect. Genet. Evol. 2021, 94, 105016. [Google Scholar] [CrossRef]
- Xu, T.; Hou, C.-Y.; Zhang, Y.-H.; Li, H.-X.; Chen, X.-M.; Pan, J.-J.; Chen, H.-Y. Simultaneous detection and genetic characterization of porcine circovirus 2 and 4 in Henan province of China. Gene 2022, 808, 145991. [Google Scholar] [CrossRef]
- Turlewicz-Podbielska, H.; Augustyniak, A.; Pomorska-Mol, M. Novel Porcine Circoviruses in View of Lessons Learned from Porcine Circovirus Type 2-Epidemiology and Threat to Pigs and Other Species. Viruses 2022, 14, 261. [Google Scholar] [CrossRef]
- Law, J.W.F.; Ab Mutalib, N.S.; Chan, K.G.; Lee, L.H. Rapid Methods for the Detection of Foodborne Bacterial Pathogens: Principles, Applications, Advantages and Limitations. Front. Microbiol. 2015, 5, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, L.; Noll, L.; Stoy, C.; Porter, E.; Fu, J.; Feng, Y.; Peddireddi, L.; Liu, X.; Dodd, K.A.; et al. Development of a real-time PCR assay for detection of African swine fever virus with an endogenous internal control. Transbound. Emerg. Dis. 2020, 67, 2446–2454. [Google Scholar] [CrossRef] [PubMed]
- Tischer, I.; Mields, W.; Wolff, D.; Vagt, M.; Griem, W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch. Virol. 1986, 91, 271–276. [Google Scholar] [CrossRef]
- Hamel, A.L.; Lin, L.L.; Nayar, G.P. Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J. Virol. 1998, 72, 5262–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaumann, F.; Correa-Fiz, F.; Franzo, G.; Sibila, M.; Núñez, J.I.; Segalés, J. Current Knowledge on Porcine circovirus 3 (PCV-3): A Novel Virus With a Yet Unknown Impact on the Swine Industry. Front. Vet. Sci. 2018, 5, 315. [Google Scholar] [CrossRef] [Green Version]
- Alomar, J.; Saporiti, V.; Perez, M.; Gonçalvez, D.; Sibila, M.; Segalés, J. Multisystemic lymphoplasmacytic inflammation associated with PCV-3 in wasting pigs. Transbound. Emerg. Dis. 2021, 68, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Lin, C.N.; Ooi, P.T. What do we know about porcine circovirus 3 (PCV3) diagnosis so far?: A review. Transbound. Emerg. Dis. 2021, 68, 2915–2935. [Google Scholar] [CrossRef]
- Ha, Z.; Yu, C.; Xie, C.; Wang, G.; Zhang, Y.; Hao, P.; Li, J.; Li, Z.; Li, Y.; Rong, F.; et al. Retrospective surveillance of porcine circovirus 4 in pigs in Inner Mongolia, China, from 2016 to 2018. Arch. Virol. 2021, 166, 1951–1959. [Google Scholar] [CrossRef]
- Sun, W.; Du, Q.; Han, Z.; Bi, J.; Lan, T.; Wang, W.; Zheng, M. Detection and genetic characterization of porcine circovirus 4 (PCV4) in Guangxi, China. Gene 2021, 773, 145384. [Google Scholar] [CrossRef]
- Tian, R.B.; Zhao, Y.; Cui, J.T.; Zheng, H.; Xu, T.; Hou, C.; Wang, Z.; Li, X.; Zheng, L.; Chen, H. Molecular detection and phylogenetic analysis of Porcine circovirus 4 in Henan and Shanxi Provinces of China. Transbound. Emerg. Dis. 2021, 68, 276–282. [Google Scholar] [CrossRef]
- Opriessnig, T.; Karuppannan, A.K.; Castro, A.; Xiao, C.-T. Porcine circoviruses: Current status, knowledge gaps and challenges. Virus Res. 2020, 286, 198044. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Xiao, Y.; Li, X.; Li, S.; Xie, N.; Yan, X.; Li, X.; Zhu, J. Development and application of a quadruplex real-time PCR assay for differential detection of porcine circoviruses (PCV1 to PCV4) in Jiangsu province of China from 2016 to 2020. Transbound. Emerg. Dis. 2021, 68, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.J.; Huang, Y.; Guo, Y.T.; Wang, L.; Zhang, Y.; Zhou, L.; Ge, X.; Han, J.; Guo, X.; Yang, H. Prevalence and Evolution Analysis of Porcine Circovirus 3 in China from 2018 to 2022. Animals 2022, 12, 1588. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, Z.; Li, Y.; Ding, Z.; Zeng, Q.; Wan, T.; Wu, H. Detection of Porcine Circovirus 1/2/3 and Genetic Analysis of Porcine Circovirus 2 in Wild Boar from Jiangxi Province of China. Animals 2022, 12, 2021. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, T.; Wen, J.; Yang, L.; Lai, S.; Sun, X.; Xu, Z.; Zhu, L. Prevalence and phylogenetic analysis of porcine circovirus type 2 (PCV2) and type 3 (PCV3) in the Southwest of China during 2020–2022. Front. Vet. Sci. 2022, 9, 1042792. [Google Scholar] [CrossRef]
- Miller, L.C.; Fleming, D.S.; Lager, K.M. Comparison of the Transcriptome Response within the Swine Tracheobronchial Lymphnode Following Infection with PRRSV, PCV-2 or IAV-S. Pathogens 2020, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Wu, X.; Zhang, L.; Xin, C.; Liu, Y.; Shi, J.; Peng, Z.; Xu, S.; Fu, F.; Yu, J.; et al. The occurrence of porcine circovirus 3 without clinical infection signs in Shandong Province. Transbound. Emerg. Dis. 2017, 64, 1337–1341. [Google Scholar] [CrossRef]
- Du, S.L.; Xu, F.; Lin, Y.; Wang, Y.; Zhang, Y.; Su, K.; Li, T.; Li, H.; Song, Q. Detection of Porcine Circovirus Type 2a and Pasteurella multocida Capsular Serotype D in Growing Pigs Suffering from Respiratory Disease. Vet. Sci. 2022, 9, 528. [Google Scholar] [CrossRef]
| Pathogen | Primers and Probes | Sequence (5′ end to 3′ End) | Length (bp) | Gene | Position |
|---|---|---|---|---|---|
| PCV2 | PCV2-F | TTACACGGATATTGTATTCCTGGTCG | 295 | Cap | 1089–1383 a |
| PCV2-R | GTGGGCTCCAGTGCTGTTATTCTA | ||||
| PCV2-QF | AGTCTCAGCCACAGCTGATT | 128 | 1175–1302 a | ||
| PCV2-QR | TCCTCCCGCCATACCAT | ||||
| PCV2-Probe | Cy5-AGCCCTTCTCCTACCACTCCCGCT-BHQ2 | ||||
| PCV3 | PCV3-F | ACAAAGAGGCCAGCGAGTA | 381 | Rep | 470–850 b |
| PCV3-R | CATCCAGAATAACAGCACCC | ||||
| PCV3-QF | CGGATTCTGACGGAGACG | 125 | 568–692 b | ||
| PCV3-QR | TCACGCGGTTTACCCAACCC | ||||
| PCV3-Probe | FAM-GCTATGGGCGGGGTTTGCGT-TAMRA | ||||
| PCV4 | PCV4-QF | GTCCACACCTGCACAAAGTT | 120 | Cap | 1114–1233 c |
| PCV4-QR | CCTCCACTTCCAGCCTAACA | ||||
| PCV4-Probe | Texas Red-AGGTCCTGGTCCGCCATGCT-BHQ2 | ||||
| β-Actin | ACTB-QF | CCCTGGAGAAGAGCTACGAG | 175 | Wang [22] | |
| ACTB-QR | AGGTCCTTCCTGATGTCCAC | ||||
| ACTB-Probe | HEX-CGGCAACGAGCGCTTCCGGT-BHQ1 | ||||
| PCV2 (CY5) | ||||||
| Probe concentration (nM) | Primer concentration (nM) | |||||
| 200 | 400 | 600 | 800 | 1000 | 1200 | |
| 200 | 25.79 | 25.81 | 25.66 | 25.71 | 25.96 | 25.63 |
| 400 | 26.05 | 25.54 | 24.96 | 25.62 | 25.25 | 25.77 |
| 600 | 25.76 | 25.80 | 25.99 | 24.79 | 24.75 | 24.66 |
| 800 | 25.41 | 25.31 | 25.42 | 25.87 | 25.23 | 25.60 |
| 1000 | 26.07 | 25.81 | 25.53 | 25.45 | 25.23 | 25.41 |
| PCV3 (FAM) | ||||||
| Probe concentration (nM) | Primer concentration (nM) | |||||
| 200 | 400 | 600 | 800 | 1000 | 1200 | |
| 200 | 28.33 | 28.68 | 28.75 | 28.59 | 28.66 | 28.91 |
| 400 | 28.76 | 28.61 | 28.66 | 28.67 | 28.69 | 28.51 |
| 600 | 28.73 | 28.92 | 28.69 | 28.53 | 27.96 | 27.88 |
| 800 | 29.00 | 28.22 | 28.52 | 29.00 | 28.93 | 27.95 |
| 1000 | 28.29 | 27.43 | 28.80 | 28.92 | 28.43 | 28.12 |
| PCV4 (Texas Red) | ||||||
| Probe concentration (nM) | Primer concentration (nM) | |||||
| 200 | 400 | 600 | 800 | 1000 | 1200 | |
| 200 | 29.01 | 29.17 | 29.27 | 29.15 | 29.00 | 29.17 |
| 400 | 29.07 | 29.00 | 28.75 | 28.60 | 28.78 | 28.65 |
| 600 | 28.94 | 28.27 | 28.40 | 28.34 | 28.28 | 28.25 |
| 800 | 28.95 | 27.83 | 28.18 | 28.08 | 28.11 | 28.00 |
| 1000 | 27.67 | 27.47 | 27.51 | 27.61 | 27.66 | 27.54 |
| Sample Number | Pathogen | Ct Value a | ||
|---|---|---|---|---|
| FAM | Cy5 | Texas Red | ||
| 1 | PCV2 | 21.39 ± 0.11 | - | - |
| 2 | PCV3 | - | 26.34 ± 0.15 | - |
| 3 | PCV4 | - | - | 29.04 ± 0.12 |
| 4 | PCV1 | - | - | - |
| 5 | ASFV | - | - | - |
| 6 | PRV | - | - | - |
| 7 | CSFV | - | - | - |
| 8 | JEV | - | - | - |
| 9 | PDCoV | - | - | - |
| 10 | PRRSV | - | - | - |
| 11 | TGEV | - | - | - |
| 12 | PEDV | - | - | - |
| Standard Plasmid | Concentration (Copies/μL) | Intra-Assay | Inter-Assay | ||||
|---|---|---|---|---|---|---|---|
| Mean | S.D. | CV (%) | Mean | S.D. | CV (%) | ||
| PCV2 | 107 | 15.24 | 0.102 | 0.67 | 15.28 | 0.061 | 0.40 |
| 106 | 19.24 | 0.094 | 0.49 | 19.24 | 0.061 | 0.32 | |
| 105 | 23.52 | 0.188 | 0.80 | 23.56 | 0.038 | 0.16 | |
| 104 | 26.13 | 0.037 | 0.14 | 26.29 | 0.226 | 0.86 | |
| PCV3 | 107 | 15.24 | 0.102 | 0.67 | 15.31 | 0.103 | 0.67 |
| 106 | 19.22 | 0.083 | 0.43 | 19.25 | 0.046 | 0.24 | |
| 105 | 23.54 | 0.184 | 0.78 | 23.71 | 0.247 | 1.04 | |
| 104 | 26.11 | 0.024 | 0.09 | 26.34 | 0.308 | 1.17 | |
| PCV4 | 107 | 15.21 | 0.063 | 0.42 | 15.28 | 0.052 | 0.34 |
| 106 | 19.15 | 0.026 | 0.14 | 19.17 | 0.073 | 0.38 | |
| 105 | 24.29 | 0.107 | 0.44 | 24.43 | 0.140 | 0.57 | |
| 104 | 26.44 | 0.042 | 0.16 | 26.90 | 0.522 | 1.94 | |
| Pathogen Type | Positive Samples a | Infection Rate (%) |
|---|---|---|
| PCV2 | 189 | 35.33 |
| PCV3 | 216 | 40.37 |
| PCV4 | 177 | 33.08 |
| PCV2 + PCV3 | 166 | 31.03 |
| PCV2 + PCV4 | 161 | 30.09 |
| PCV3 + PCV4 | 165 | 30.84 |
| PCV2 + PCV3 + PCV4 | 151 | 28.22 |
| β-Actin | 535 | 100 |
| In total | 535 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Liu, H.; Chen, J.; Zhang, J.; Li, X.; Long, Y.; Jiang, Y.; Li, W.; Zhou, B. Development of a TaqMan-Probe-Based Multiplex Real-Time PCR for the Simultaneous Detection of Porcine Circovirus 2, 3, and 4 in East China from 2020 to 2022. Vet. Sci. 2023, 10, 29. https://doi.org/10.3390/vetsci10010029
Zou J, Liu H, Chen J, Zhang J, Li X, Long Y, Jiang Y, Li W, Zhou B. Development of a TaqMan-Probe-Based Multiplex Real-Time PCR for the Simultaneous Detection of Porcine Circovirus 2, 3, and 4 in East China from 2020 to 2022. Veterinary Sciences. 2023; 10(1):29. https://doi.org/10.3390/vetsci10010029
Chicago/Turabian StyleZou, Jianwen, Huaicheng Liu, Jing Chen, Jin Zhang, Xiaohan Li, Yunfeng Long, Yan Jiang, Wenliang Li, and Bin Zhou. 2023. "Development of a TaqMan-Probe-Based Multiplex Real-Time PCR for the Simultaneous Detection of Porcine Circovirus 2, 3, and 4 in East China from 2020 to 2022" Veterinary Sciences 10, no. 1: 29. https://doi.org/10.3390/vetsci10010029
APA StyleZou, J., Liu, H., Chen, J., Zhang, J., Li, X., Long, Y., Jiang, Y., Li, W., & Zhou, B. (2023). Development of a TaqMan-Probe-Based Multiplex Real-Time PCR for the Simultaneous Detection of Porcine Circovirus 2, 3, and 4 in East China from 2020 to 2022. Veterinary Sciences, 10(1), 29. https://doi.org/10.3390/vetsci10010029








