Impacts of Oral Florfenicol Medication and Residues on the Kidney and Liver of Nile Tilapia Oreochromis niloticus (L.)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Fish and Acclimation
2.3. Experimental Setup and Dosing Protocol
2.4. Florfenicol Diet Preparation and Dosing Administration
2.5. Tissue and Blood Collection
2.6. Oxidative Stress
2.7. Serum Biochemistry
2.8. Accrual and Depletion of Residues
2.9. Histopathology
2.10. Statistical Analysis
3. Results
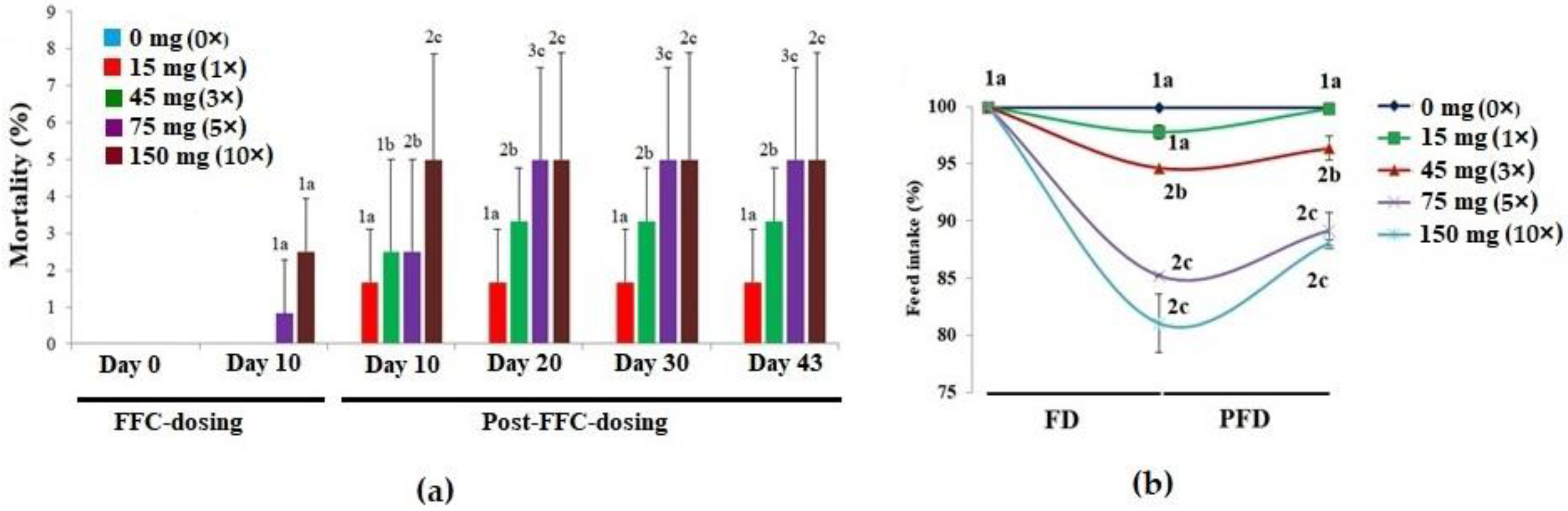
3.1. Mortalities, Feeding Behaviour and Feed Intake upon Florfenicol (FFC)-Dosing
3.2. Abnormalities in Florfenicol (FFC)-Dosed Oreochromis niloticus
3.3. LC-MS/MS Analysis
3.4. Oxidative Stress
3.4.1. Thiobarbituric Acid Reactive Substances (TBARS) as Malondialdehyde (MDA)
3.4.2. Ferric-Reducing Antioxidant Power (FRAP)
3.4.3. Total Nitric Oxide (TNO)
3.4.4. Glutathione-S-Transferase (GST) Activity
3.5. Serum Biochemistry
3.6. Kidney and Liver Histopathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, J.B.; Huang, D.R.; Zhong, M.; Liu, P.; Dong, J.D. Pharmacokinetics of florfenicol and behaviour of its metabolite florfenicol amine in orange-spotted grouper (Epinephelus coioides) after oral administration. J. Fish Dis. 2016, 39, 833–843. [Google Scholar] [CrossRef]
- Limbu, S.M.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. A global analysis on the systemic effects of antibiotics in cultured fish and their potential human health risk: A review. Rev. Aquac. 2021, 13, 1015–1059. [Google Scholar] [CrossRef]
- Patil, P.K.; Mishra, S.S.; Pradhan, P.K.; Manna, S.K.; Abraham, T.J.; Solanki, H.G.; Shahi, N.; Swain, P.; Sahoo, S.N.; Avunje, S.; et al. Usage pattern of chemicals, biologicals and veterinary medicinal products in Indian aquaculture. Rev. Aquac. 2022, 14, 2038–2063. [Google Scholar] [CrossRef]
- Rodrigues, S.; Antunes, S.C.; Nunes, B.; Correia, A.T. Histopathological effects in gills and liver of Sparus aurata following acute and chronic exposures to erythromycin and oxytetracycline. Environ. Sci. Pollut. Res. 2019, 26, 15481–15495. [Google Scholar] [CrossRef] [PubMed]
- Shiroma, L.S.; Soares, M.P.; Cardoso, I.L.; Ishikawa, M.M.; Jonsson, C.M.; Queiroz, S.C.N. Evaluation of health and environmental risks for juvenile tilapia (Oreochromis niloticus) exposed to florfenicol. Heliyon 2020, 6, e05716. [Google Scholar] [CrossRef] [PubMed]
- Gaikowski, M.P.; Wolf, J.C.; Endris, R.G.; Gingerich, W.H. Safety of Aquaflor (florfenicol, 50% type A medicated article), administered in feed to channel catfish, Ictalurus punctatus. Toxicol. Pathol. 2003, 31, 689–697. [Google Scholar] [CrossRef]
- Gaikowski, M.P.; Wolf, J.C.; Schleis, S.M.; Tuomari, D.; Endris, R.G. Safety of florfenicol administered in feed to tilapia (Oreochromis sp.). Toxicol. Pathol. 2013, 41, 639–652. [Google Scholar] [CrossRef]
- Gaunt, P.S.; Gao, D.; Sun, F.; Endris, R. Efficacy of florfenicol for control of mortality caused by Flavobacterium columnare infection in channel catfish. J. Aquat. Anim. Health 2010, 22, 115–122. [Google Scholar] [CrossRef]
- Jarau, M.; MacInnes, J.I.; Lumsden, J.S. Erythromycin and florfenicol treatment of rainbow trout Oncorhynchus mykiss (Walbaum) experimentally infected with Flavobacterium psychrophilum. J. Fish Dis. 2019, 42, 325–334. [Google Scholar] [CrossRef]
- Bardhan, A.; Abraham, T.J.; Singha, J.; Saha, S.; Sarker, S.; Patil, P.K. The effects of extended feeding of florfenicol coated medicated diets on the safety, serum biomarkers and blood cells morphology of Nile tilapia Oreochromis niloticus (L.). Environ. Sci. Pollut. Res. 2022, 29, 39914–39927. [Google Scholar] [CrossRef]
- Bardhan, A.; Abraham, T.J.; Singha, J.; Sar, T.K.; Rajisha, R.; Krishna, E.K.N.; Kumar, K.A.; Patil, P.K. Histopathological aberrations and oxidative stress responses in Nile tilapia Oreochromis niloticus as influenced by dietary florfenicol and its metabolites. Aquaculture 2022, 559, 738447. [Google Scholar] [CrossRef]
- Bardhan, A.; Abraham, T.J.; Das, R.; Patil, P.K. Biological Responses of Nile tilapia Oreochromis niloticus as influenced by dietary florfenicol. Toxics 2022, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals). Guidelines of CPCSEA for Experimentation on Fishes; Animal Welfare Division, Ministry of Environment Forest and Climate Change, Government of India: New Delhi, India, 2021. Available online: https://GuidelinesofCPCSEAforExperimentationonFishes-2021.pdf (accessed on 2 July 2022).
- AVMA. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020; Available online: https://www.avma.org/sites/default/files/2020-01/2020-Euthanasia-Final-1-17-20.pdf (accessed on 2 July 2022).
- Roberts, R.J. Fish Pathology; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Davis, D.J.; Klug, J.; Hankins, M.; Doerr, H.M.; Monticelli, S.R.; Song, A.; Gillespie, C.H.; Bryda, E.C. Effects of clove oil as a euthanasia agent on blood collection efficiency and serum cortisol levels in Danio rerio. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 564–567. [Google Scholar] [PubMed]
- Bowker, J.D.; Carty, D.; Bowman, M.P. The safety of Aquaflor (50% florfenicol) administered in feed to fingerling yellow perch. N. Am. J. Aquac. 2013, 75, 517–523. [Google Scholar] [CrossRef]
- Elia, A.C.; Pacini, N.; Fioravanti, M.L.; Dörr, A.J.M.; Zaccaroni, A.; Parmeggiani, A.M.; Gustinelli, A.; Mordenti, O.; Abete, M.C.; Prearo, M. Assessment of detoxifying markers for florfenicol in rainbow trout liver. J. Aquat. Anim. Health 2016, 28, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Han, Z.; Li, C.; Lv, L.; Cheng, Z.; Liu, S. Florfenicol induces more severe hemotoxicity and immunotoxicity than equal doses of chloramphenicol and thiamphenicol in Kunming mice. Immunopharmacol. Immunotoxicol. 2016, 38, 472–485. [Google Scholar] [CrossRef]
- Sumithra, T.G.; Sharma, K.S.; Gangadharan, S.; Suresh, G.; Prasad, V.; Amala, P.V.; Sayooj, P.; Gop, A.P.; Anil, M.K.; Patil, P.K.; et al. Dysbiosis and restoration dynamics of the gut microbiome following therapeutic exposure to florfenicol in snubnose pompano (Trachinotus blochii) to aid in sustainable aquaculture production strategies. Front. Microbiol. 2022, 13, 881275. [Google Scholar] [CrossRef]
- Saba, A.B.; Ola-Davies, O.; Oyeyemi, M.O.; Ajala, O. The toxic effects of prolonged administration of chloramphenicol on the liver and kidney of rats. Afr. J. Biomed. Res. 2000, 3, 133–137. [Google Scholar]
- Yang, F.; Yang, F.; Wang, G.; Kong, T.; Wang, H.; Zhang, C. Effects of water temperature on tissue depletion of florfenicol and its metabolite florfenicol amine in crucian carp (Carassius auratus gibelio) following multiple oral doses. Aquaculture 2020, 515, 734542. [Google Scholar] [CrossRef]
- Biswal, A.; Srivastava, P.P.; Krishna, G.; Paul, T.; Pal, P.; Gupta, S.; Varghese, T.; Jayant, M. An integrated biomarker approach for explaining the potency of exogenous glucose on transportation induced stress in Labeo rohita fingerlings. Sci. Rep. 2021, 11, 5713. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.; Wang, M.; Wu, Y.; Sun, Y.; Su, H.; Deng, J. Mixture toxicity effects of chloramphenicol, thiamphenicol, florfenicol in Daphnia magna under different temperatures. Ecotoxicology 2021, 30, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.V.; Paschoal, J.A.R.; Barone, R.S.C.; Cyrino, J.E.P.; Rath, S. Depletion study and estimation of withdrawal periods for florfenicol and florfenicol amine in pacu (Piaractus mesopotamicus). Aquac. Res. 2018, 49, 111–119. [Google Scholar] [CrossRef]
- Sathya, A.; Prabhu, T.; Ramalingam, S. Structural, biological and pharmaceutical importance of antibiotic agent chloramphenicol. Heliyon 2020, 6, e03433. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.F.; Shien, J.H.; Chang, S.K.; Chou, C.C. Florfenicol as a modulator enhancing antimicrobial activity: Example using combination with thiamphenicol against Pasteurella multocida. Front. Microbiol. 2016, 7, 389. [Google Scholar] [CrossRef] [PubMed]
- Remen, M.; Imsland, A.K.; Stefansson, S.O.; Jonassen, T.M.; Foss, A. Interactive effects of ammonia and oxygen on growth and physiological status of juvenile Atlantic cod (Gadus morhua). Aquaculture 2008, 274, 292–299. [Google Scholar] [CrossRef]
- Kelly, D.J.; Cox, A.J.; Tolcos, M.; Cooper, M.E.; Wilkinson-Berka, J.L.; Gilbert, R.E. Attenuation of tubular apoptosis by blockade of the renin-angiotensin system in diabetic Ren-2 rats. Kidney Int. 2002, 61, 31–39. [Google Scholar] [CrossRef][Green Version]
- Casado, F.; Mudunuru, S.A.; Nasr, R. A case of hypokalemia possibly induced by Nafcillin. Antibiotics 2018, 7, 108. [Google Scholar] [CrossRef]
- Collett, S.R. Nutrition and wet litter problems in poultry. Anim. Feed. Sci. Technol. 2012, 173, 65–75. [Google Scholar] [CrossRef]
- Sreejai, R.; Jaya, D.S. Studies on the changes in lipid peroxidation and antioxidants in fishes exposed to hydrogen sulfide. Toxicol. Int. 2010, 17, 71. [Google Scholar]
- Linares, V.; Alonso, V.; Albina, M.L.; Bellés, M.; Sirvent, J.J.; Domingo, J.L.; Sánchez, D.J. Lipid peroxidation and antioxidant status in kidney and liver of rats treated with sulfasalazine. Toxicology 2009, 256, 152–156. [Google Scholar] [CrossRef]
- Alessio, H.M. Lipid peroxidation in healthy and diseased models: Influence of different types of exercise. In Handbook of Oxidants and Antioxidants in Exercise; Elsevier: Houston, TX, USA, 2000. [Google Scholar]
- Chatterjee, A.; Bhattacharya, R.; Chatterjee, S.; Saha, N.C. Acute toxicity of organophosphate pesticide profenofos, pyrethroid pesticide λ cyhalothrin and biopesticide azadirachtin and their sublethal effects on growth and oxidative stress enzymes in benthic oligochaete worm, Tubifex tubifex. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 242, 108943. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, Z.; Gao, B.; Liu, P.; Li, J. Effects of florfenicol on the antioxidant status, detoxification system and biomolecule damage in the swimming crab (Portunus trituberculatus). Ecotoxicol. Environ. Saf. 2017, 143, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Amer, A.A.; Elbialy, Z.I.; Gouda, A.H. Effects of including triticale on growth performance, digestive enzyme activity, and growth-related genes of Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 528, 735568. [Google Scholar] [CrossRef]
- Sukhovskaya, I.V.; Borvinskaya, E.V.; Smirnov, L.P.; Kochneva, A.A. Role of glutathione in the functioning of the system of antioxidant protection in fish. Inland Water Biol. 2017, 10, 97–102. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, H.; Thompson, D.C.; Shertzer, H.G.; Nebert, D.W.; Vasiliou, V. Glutathione defense mechanism in liver injury: Insights from animal models. Food Chem. Toxicol. 2013, 60, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, S.; Ebrahimpour, A.; Hamid, A.A.; Manap, M.Y.A.; Anwar, F.; Saari, N. The improvement of the endogenous antioxidant property of stone fish (Actinopyga lecanora) tissue using enzymatic proteolysis. BioMed Res. Int. 2013, 2013, 849529. [Google Scholar] [CrossRef] [PubMed]
- Sevcikova, M.; Modra, H.; Blahova, J.; Dobsikova, R.; Plhalova, L.; Zitka, O.; Hynek, D.; Kizek, R.; Skoric, M.; Svobodova, Z. Biochemical, haematological and oxidative stress responses of common carp (Cyprinus carpio L.) after sub-chronic exposure to copper. Vet. Med. 2016, 61, 35–50. [Google Scholar] [CrossRef]
- Mikulikova, I.; Modrá, H.; Blahova, J.; Marsalek, P.; Groch, L.; Siroka, Z.; Kruzikova, K.; Jarkovsky, J.; Littnerová, S.; Svobodova, Z. The effects of Click 500 SC (terbuthylazine) on common carp Cyprinus carpio under (sub) chronic conditions. Neuroendocrinol. Lett. 2011, 32, 15–24. [Google Scholar]
- Farjad, E.; Momeni, H.R. Silymarin ameliorates oxidative stress and enhances antioxidant defense system capacity in cadmium-treated mice. Cell J. 2018, 20, 422. [Google Scholar]
- Xu, H.; Yang, M.; Qiu, W.; Pan, C.; Wu, M. The impact of endocrine-disrupting chemicals on oxidative stress and innate immune response in zebrafish embryos. Environ. Toxicol. Chem. 2013, 32, 1793–1799. [Google Scholar] [CrossRef]
- Carlström, M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 2021, 17, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, C.; Cui, Y.; Li, S.; Jin, G.; Shi, W.; Bao, Y. Florfenicol causes excessive lipid peroxidation and apoptosis induced renal injury in broilers. Ecotoxicol. Environ. Saf. 2021, 207, 111282. [Google Scholar] [CrossRef] [PubMed]
- Seely, J.C.; Francke, S.; Mog, S.R.; Frazier, K.S.; Hard, G.C. Renal Papillary Rarefaction: An Artifact Mimicking Papillary Necrosis. Toxicol. Pathol. 2019, 47, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Phosphate toxicity and vascular mineralization. In Phosphate and Vitamin D in Chronic Kidney Disease; Karger: Basel, Switzerland, 2013; pp. 74–85. [Google Scholar]
- Reda, R.M.; Ibrahim, R.E.; Ahmed, E.N.G.; El-Bouhy, Z.M. Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. Egypt J. Aquat. Res. 2013, 39, 241–248. [Google Scholar] [CrossRef]
- Wolf, J.C.; Baumgartner, W.A.; Blazer, V.S.; Camus, A.C.; Engelhardt, J.A.; Fournie, J.W.; Frasca, S., Jr.; Groman, D.B.; Kent, M.L.; Khoo, L.H.; et al. Nonlesions, misdiagnoses, missed diagnoses, and other interpretive challenges in fish histopathology studies: A guide for investigators, authors, reviewers, and readers. Toxicol. Pathol. 2015, 43, 297–325. [Google Scholar] [CrossRef]
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Inglis, V.; Richards, R.H.; Varma, K.J.; Sutherland, I.H.; Brokken, E.S. Florfenicol in Atlantic salmon, Salmo salar L., parr: Tolerance and assessment of efficacy against furunculosis. J. Fish Dis. 1991, 14, 343–351. [Google Scholar] [CrossRef]
- Straus, D.L.; Bowker, J.D.; Bowman, M.P.; Carty, D.; Mitchell, A.J.; Farmer, B.D. Safety of aquaflor-medicated feed to sunshine bass. N. Am. J. Aquac. 2012, 74, 1–7. [Google Scholar] [CrossRef]






| Groups | Experimental Period | Liver | Kidney | Gills | Spleen | Peritoneum | Skin |
|---|---|---|---|---|---|---|---|
| 3× | FD | Hepatomegaly | NC | NC | Splenomegaly | NC | Mucous secretion |
| PFD | Hepatomegaly (30) | NC | NC | NC | NC | NC | |
| 5× | FD | Hepatomegaly | Watery | NC | Splenomegaly | NC | Mucous secretion |
| PFD | Hepatomegaly (30) | NC | Mucous secretion (20) | Splenomegaly (10) | NC | Mucous secretion (10) | |
| 10× | FD | Hepatomegaly | Watery | NC | Splenomegaly | Blackish | Mucous secretion |
| PFD | Hepatomegaly (30) | NC | Mucous secretion (20) | Splenomegaly (10) | NC | Mucous secretion (10) |
| Organ | 1× | 3× | 5× | 10× | ||||
|---|---|---|---|---|---|---|---|---|
| 10FD | 43PFD | 10FD | 43PFD | 10FD | 43PFD | 10FD | 43PFD | |
| Kidney | ||||||||
| DG | 3.03 ± 0.08 1a | 2.12 ± 0.07 2b | 3.14 ± 0.04 1ad | 2.37 ± 0.11 2c | 3.18 ± 0.04 1d | 2.43 ± 0.10 2c | 3.26 ± 0.04 1d | 2.70 ± 0.14 2e |
| DE | 1.38 ± 0.11 1a | 0.48 ± 0.24 2b | 1.54 ± 0.10 1c | 0.97 ± 0.10 2d | 1.64 ± 0.11 1e | 1.11 ± 0.07 2f | 1.74 ± 0.10 1e | 1.37 ± 0.08 2a |
| HS | 1.41 ± 0.07 1a | 0.63 ± 0.29 2b | 1.58 ± 0.14 1c | 1.07 ± 0.14 2d | 1.64 ± 0.10 1c | 1.12 ± 0.07 2e | 1.77 ± 0.10 1f | 1.75 ± 0.10 1f |
| V | 1.28 ± 0.10 1a | 1.03 ± 0.13 2b | 1.44 ± 0.10 1c | 1.26 ± 0.11 2a | 1.58 ± 0.13 1d | 1.37 ± 0.11 2c | 1.49 ± 0.07 1e | 1.40 ± 0.10 1e |
| NA | 1.18 ± 0.11 1ac | 0.73 ± 0.21 2b | 1.28 ± 0.10 1ade | 1.14 ± 0.10 2c | 1.31 ± 0.07 1def | 1.27 ± 0.11 1d | 1.38 ± 0.07 1ef | 1.33 ± 0.07 1f |
| GL | 1.09 ± 0.07 1ac | 0.43 ± 0.15 2b | 1.17 ± 0.10 1a | 1.05 ± 0.13 2c | 1.18 ± 0.07 1a | 1.15 ± 0.10 1a | 1.32 ± 0.07 1d | 1.29 ± 0.07 1d |
| M | 1.12 ± 0.07 1ad | 0.97 ± 0.10 2b | 1.21 ± 0.07 1ac | 1.11 ± 0.07 2d | 1.24 ± 0.07 1c | 1.12 ± 0.11 2d | 1.38 ± 0.08 1e | 1.30 ± 0.07 1e |
| Liver | ||||||||
| GV | 4.59 ± 0.05 1a | 3.46 ± 0.04 2b | 4.67 ± 0.02 1c | 3.54 ± 0.04 2b | 4.69 ± 0.02 1ad | 3.59 ± 0.09 2e | 4.73 ± 0.03 1d | 4.60 ± 0.05 2a |
| CV | 1.31 ± 0.07 1ac | 1.27 ± 0.10 1a | 1.42 ± 0.07 1bc | 1.28 ± 0.13 2c | 1.44 ± 0.10 1bc | 1.30 ± 0.14 2a | 1.59 ± 0.09 1d | 1.39 ± 0.07 2c |
| CD | 1.04 ± 0.14 1a | 1.00 ± 0.10 1a | 1.11 ± 0.09 1a | 1.09 ± 0.07 1a | 1.23 ± 0.08 1b | 1.17 ± 0.10 2b | 1.45 ± 0.10 1c | 1.31 ± 0.06 2c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardhan, A.; Abraham, T.J.; Singha, J.; Rajisha, R.; Krishna, E.K.N.; Panda, S.K.; Patil, P.K. Impacts of Oral Florfenicol Medication and Residues on the Kidney and Liver of Nile Tilapia Oreochromis niloticus (L.). Vet. Sci. 2023, 10, 36. https://doi.org/10.3390/vetsci10010036
Bardhan A, Abraham TJ, Singha J, Rajisha R, Krishna EKN, Panda SK, Patil PK. Impacts of Oral Florfenicol Medication and Residues on the Kidney and Liver of Nile Tilapia Oreochromis niloticus (L.). Veterinary Sciences. 2023; 10(1):36. https://doi.org/10.3390/vetsci10010036
Chicago/Turabian StyleBardhan, Avishek, Thangapalam Jawahar Abraham, Jasmine Singha, Ravindran Rajisha, Edaparambil Krishnappan Nanitha Krishna, Satyen Kumar Panda, and Prasanna Kumar Patil. 2023. "Impacts of Oral Florfenicol Medication and Residues on the Kidney and Liver of Nile Tilapia Oreochromis niloticus (L.)" Veterinary Sciences 10, no. 1: 36. https://doi.org/10.3390/vetsci10010036
APA StyleBardhan, A., Abraham, T. J., Singha, J., Rajisha, R., Krishna, E. K. N., Panda, S. K., & Patil, P. K. (2023). Impacts of Oral Florfenicol Medication and Residues on the Kidney and Liver of Nile Tilapia Oreochromis niloticus (L.). Veterinary Sciences, 10(1), 36. https://doi.org/10.3390/vetsci10010036







