Cleft Palate Syndrome in the Endangered Spectacled Flying Fox (Pteropus conspicillatus): Implications for Conservation and Comparative Research
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics and Data Collection
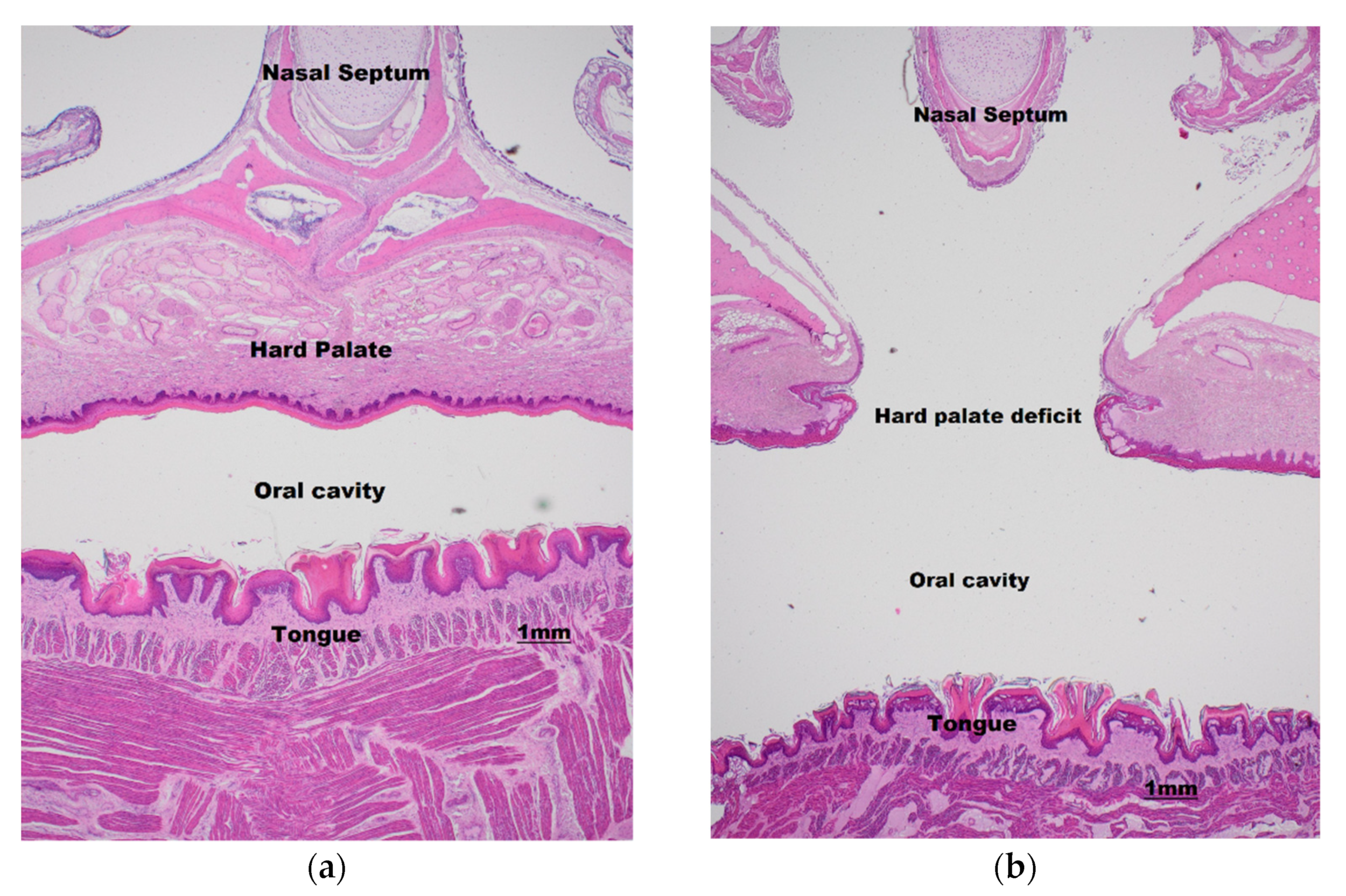
2.2. Defect Morphology, Cranial Measurements, and Histology
2.3. Cleft Palate Incidence
3. Results
3.1. Defect Morphology and Histology
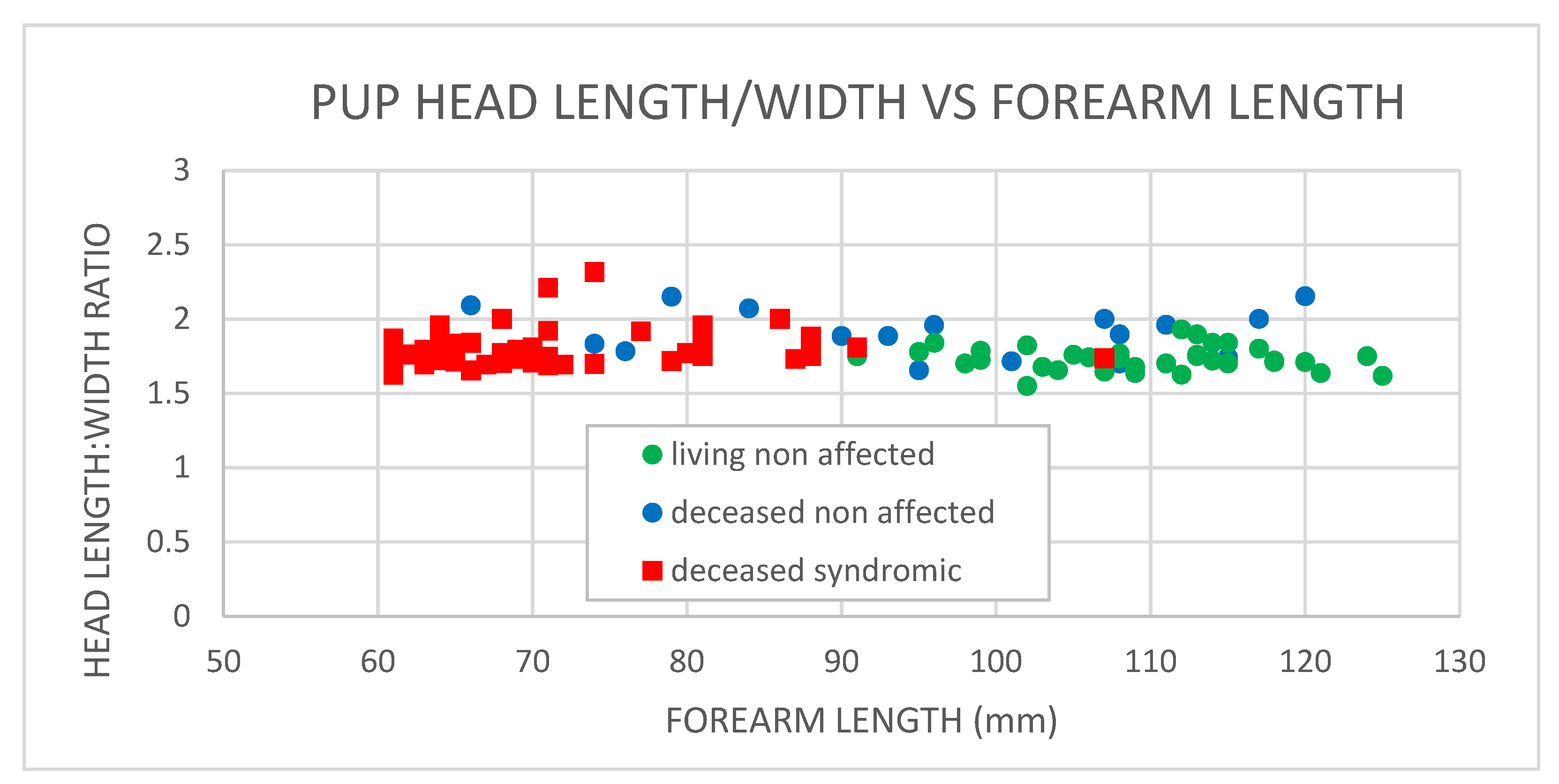
3.2. Cranial Measurements
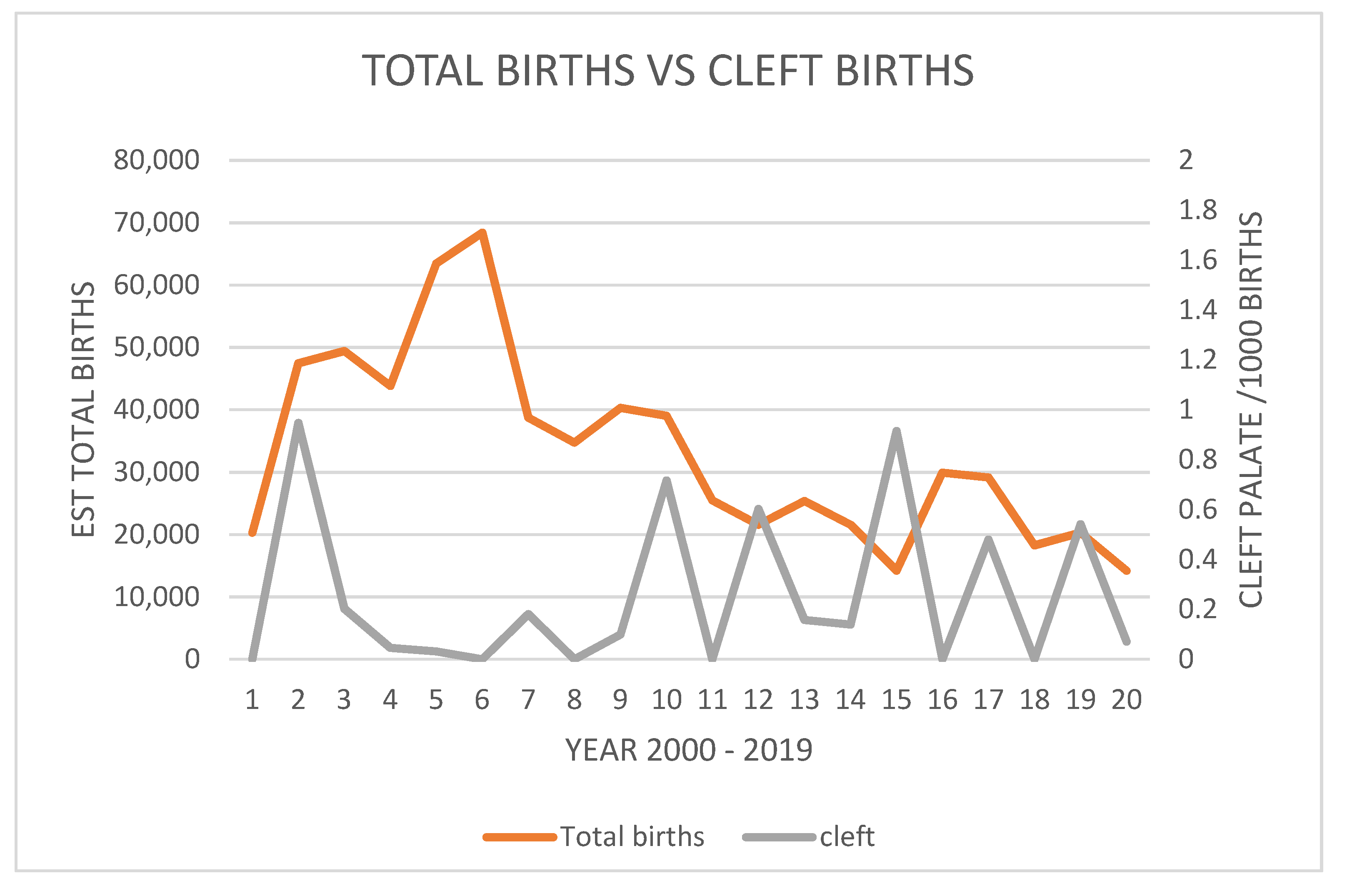
3.3. Cleft Palate Incidence
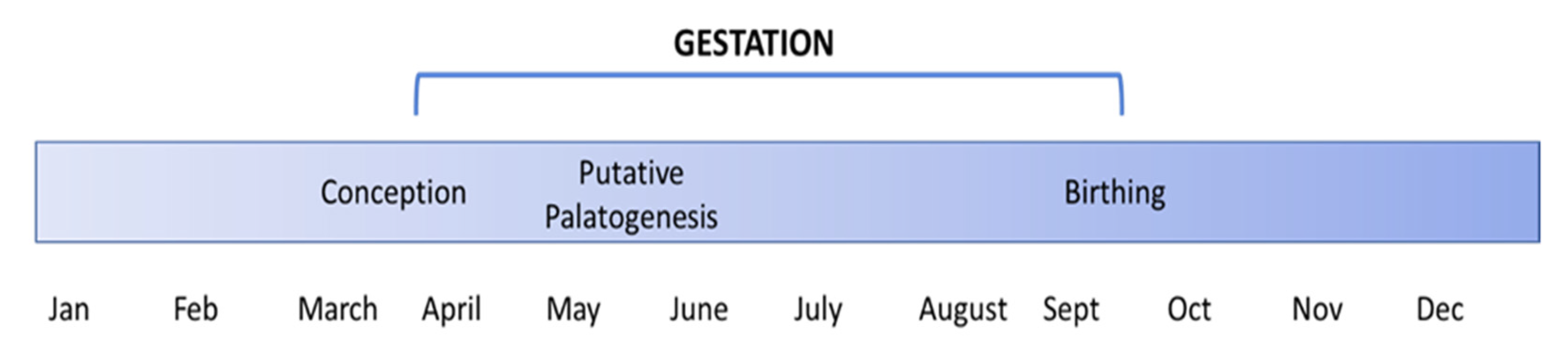
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volpato, L.E.; de Campos, A.T.; Aranha, A.M.; Borba, A.M.; Vieira, E.M.; Borges, A.H. Cleft lip and palate: Associated genetic and environmental factors. Sci. J. Dent. 2015, 2, 19–25. [Google Scholar]
- McLeod, N.M.H.; Arana-Urioste, M.L.; Saeed, N.R. Birth prevalence of cleft lip and palate in Sucre, Bolivia. Cleft Palate-Craniofacial J. 2004, 41, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.S.; Kohli, V.S. A comprehensive review of the genetic basis of cleft lip and palate. J. Oral Maxillofac. Pathol. 2012, 16, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Łobodzi’nska, A.; Gruszczynska, J.; Max, A.; Bartyzel, B.; Mikuła, M.; Mikula, I.; Grzegrzółka, B. Review article: Cleft palate in the domestic dog Canis lupus familiaris—Etiology, pathophysiology, diagnosis, prevention and treatment. Acta Sci. Pol. Zootech. 2014, 13, 5–28. [Google Scholar]
- Shupe, J.L.; James, L.F.; Binns, W.; Keeler, R.F. Cleft palate in cattle. Cleft Palate J. 1968, 5, 346–355. [Google Scholar]
- Mclean, J.; (Tolga Bat Rescue and Research Inc., Atherton, Qld, Australia). Personal communication, 2019.
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Genet. 2011, 33, 247–255. [Google Scholar] [CrossRef]
- Grunstra, N.D.S.; Zachos, F.E.; Herdina, A.N.; Fischer, B.; Pavlicev, M.; Mitteroecker, P. Humans as inverted bats: A comparative approach to the obstetric conundrum. Am. J. Hum. Biol. 2019, 31, 2. [Google Scholar] [CrossRef]
- Ng, J.H.J.; Tachedjian, M.; Deakin, J.; Wynne, J.W.; Cui, J.; Haring, V.; Broz, I.; Chen, H.; Belov, K.; Wang, L.F.; et al. Evolution and comparative analysis of the bat MHC-I region. Sci. Rep. 2016, 6, 21256. [Google Scholar] [CrossRef]
- Hoeksema, N.; Wiesmann, M.; Kiliaan, A.; Hagoort, P.; Vernes, S. Bats and the comparative neurobiology of vocal learning. In Proceedings of the Evolution of Language International Conference, Brussels, Belgium, 14–17 April 2020. [Google Scholar]
- Cox, L.L.; Cox, T.C.; Moreno Uribe, L.M.; Zhu, Y.; Richter, C.T.; Nidey, N.; Standley, J.M.; Deng, M.; Blue, E.; Chong, J.X.; et al. Mutations in the Epithelial Cadherin-p120-Catenin Complex Cause Mendelian Non-Syndromic Cleft Lip with or without Cleft Palate. Am. J. Hum. Genet. 2018, 102, 1143–1157. [Google Scholar] [CrossRef]
- Bliek, B.J.; Steegers-Theunissen, R.P.; Blok, L.J.; Santegoets, L.A.; Lindemans, J.; Oostra, B.A.; Steegers, E.A.; de Klein, A. Genome-wide pathway analysis of folate-responsive genes to unravel the pathogenesis of orofacial clefting in man. Birth Defects Research. A Clin. Mol. Teratol. 2008, 82, 627–635. [Google Scholar] [CrossRef]
- Plamondon, J.A.; Harris, M.J.; Mager, D.L.; Gagnier, L.; Juriloff, D.M. The clf2 gene has an epigenetic role in the multifactorial etiology of cleft lip and palate in the A/WySn mouse strain. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 716–727. [Google Scholar] [CrossRef]
- Spritz, R.A. The genetics and epigenetics of orofacial clefts. Curr. Opin. Pediatr. 2001, 13, 556–560. [Google Scholar] [CrossRef]
- Seelan, R.S.; Mukhopadhyay, P.; Pisano, M.M.; Greene, R.M. Developmental Epigenetics of the Murine Secondary Palate. ILAR J. 2012, 55, 3–4. [Google Scholar] [CrossRef][Green Version]
- Park-Wyllie, L.; Mazzotta, P.; Pastuszak, A.; Moretti, M.E.; Beique, L.; Hunnisett, L.; Friesen, M.H.; Jacobson, S.; Kasapinovic, S.; Chang, D.; et al. Birth defects after maternal exposure to corticosteroids: Prospective cohort study and meta-analysis of epidemiological studies. Teratology 2000, 62, 385–392. [Google Scholar] [CrossRef]
- Panter, K.P.; Keeler, R.F.; James, L.F.; Thomas, D. Impact of plant toxins on fetal and neonatal development: A review. Bunch J. Range Manag. 1992, 45, 52–57. [Google Scholar] [CrossRef]
- Shaw, G.M.; Nelson, V.; Lovannisci, D.M.; Finnell, R.H.; Lammer, E.J. Maternal Occupational Chemical Exposures and Biotransformation Genotypes as Risk Factors for Selected Congenital Anomalies. Am. J. Epidemiol. 2003, 157, 475–484. [Google Scholar] [CrossRef]
- Romitti, P.A.; Herring, A.M.; Dennis, L.K.; Wong-Gibbons, D.L. Meta-Analysis: Pesticides and Orofacial Clefts. Cleft Palate–Craniofacial J. 2007, 44, 4. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, F.; Huang, J.; Xin, H. Evaluation of the Association of Zoonotic Ljungan Virus with Perinatal Deaths and Fetal Malformation. Birth Defects Res. (Part C) 2015, 105, 81–85. [Google Scholar] [CrossRef]
- Hayman, D.T.S. Bats as viral reservoirs. Annu. Rev. Virol. 2016, 3, 77–99. [Google Scholar] [CrossRef]
- O’Leary, D.R.; Kuhn, S.; Kniss, K.; Hinckley, A.F.; Rasmussen, S.A.; Pape, W.J.; Kightlinger, K.; Beecham, B.D.; Miller, T.K.; Neitzel, D.F.; et al. Birth Outcomes Following West Nile Virus Infection of Pregnant Women in the United States: 2003–2004. Pediatrics 2006, 117, 3. [Google Scholar] [CrossRef]
- Kane, S.E.; Holler, L.D.; Braun, L.J.; Neill, J.D.; Young, D.B.; Ridpath, J.F.; Chase, C.C.L. Bovine viral diarrhea virus outbreak in a beef cow herd in South Dakota. JAVMA 2015, 246, 12. [Google Scholar] [CrossRef] [PubMed]
- Divya, D.V.; Prasad, M.S.; Radhakrishna, A.N.; Reddy, S.P.; Pratyusha, K.; Santosh Kumar, K.V.K.; Sandeep, R.V. The Serological Evidence of Cytomegalovirus Infection as a Potent Aetiological Factor for Cleft Lip/Palate, Mental Retardation and Deafness. J. Clin. Diagn. Res. 2017, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, R.; Sethi, S.; Khanal, M. Isolated Unilateral Soft Palate Palsy Following Tonsillopharyngitis Caused by Epstein-Barr Virus Infection. Cleft Palate–Craniofacial J. 2017, 54, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.; Oliveira, I.; Sousa, P.; Doriqui, M.; Fiamoncini, E.; Ferreira Júnior, O.; Consolaro, A. Cleft LIP and palate in babies with microcephaly by zika virus: Would there be an etiopathogenic relationship? Int. J. Oral Maxillofac. Surg. 2019, 48, 21. [Google Scholar] [CrossRef]
- Mclean, J.; Johnson, A.; Woods, D.; Muller, R.; Blair, D.; Buettner, P. Growth rates of, and milk feeding schedules for, juvenile spectacled flying-foxes (Pteropus conspicillatus) reared for release at a rehabilitation centre in north Queensland, Australia. Aust. J. Zool. 2019, 66, 201–213. [Google Scholar] [CrossRef]
- Buettner, P.G.; Westcott, D.A.; Maclean, J.; Brown, L.; McKeown, A.; Johnson, A.; Wilson, K.; Blair, D.; Luly, J.; Skerratt, L.; et al. Tick Paralysis in Spectacled Flying-Foxes (Pteropus conspicillatus) in North Queensland, Australia: Impact of a Ground-Dwelling Ectoparasite Finding an Arboreal Host. PLoS ONE 2013, 8, e73078. [Google Scholar] [CrossRef]
- Fox, S.; Luly, J.; Mitchell, C.; Maclean, J.; Westcott, D. Demographic indications of decline in the spectacled flying fox (Pteropus conspicillatus) on the Atherton Tablelands of northern Queensland. Wildl. Res. 2008, 35, 417–424. [Google Scholar] [CrossRef]
- Bush, J.O.; Jiang, R. Palatogenesis: Morphogenetic and molecular mechanisms of secondary palate development. Development 2012, 139, 231–243. [Google Scholar] [CrossRef]
- Meng, L.; Bian, Z.; Torensma, R.; Von den Hoff, J.W. Biological mechanisms in palatogenesis and cleft palate. J. Dent. Res. 2009, 88, 22–33. [Google Scholar] [CrossRef]
- Hall, L.; Richards, G.; Saunders, L. Flying Foxes, Fruit and Blossom Bats of Australia; Krieger Publishing: Malabar, FL, USA, 2000. [Google Scholar] [CrossRef]
- Tait, J.; Perotto-Baldivieso, H.L.; McKeown, A.; Westcott, D.A. Are flying foxes coming to town? Urbanisation of the Spectacled flying-fox (Pteropus conspicillatus) in Australia. PLoS ONE 2014, 9, e109810. [Google Scholar] [CrossRef]
- Moura, E.; Pimpão, C.T. Veterinary Dysmorphology. In A Bird’s-Eye View of Veterinary Medicine; Perez-Marin, C.C., Ed.; InTech: London, UK, 2012; pp. 71–98. [Google Scholar] [CrossRef]
- Fox, S.; Waycott, M.; Dunshea, G. Isolation and characterisation of polymorphic microsatellite loci in the vulnerable spectacled flying fox, Pteropus conspicillatus. Conserv. Genet. 2007, 8, 1013–1016. [Google Scholar] [CrossRef]
- Fox, S.; Waycott, M.; Blair, D.; Luly, J. An assessment of regional genetic differentiation in the spectacled flying fox (Pteropus conspicillatus Gould). In People Landscapes: Archaeological and Biogeographic Approaches to Landscapes; Haberle, S.G., David, B., Eds.; ANU Press: Canberra, Australia, 2012; pp. 459–472. [Google Scholar]
- Braybrook, C.; Warry, G.; Howell, G.; Mandryko, V.; Arnason, A.; Bjornsson, A.; Ross, M.T.; Moore, G.E.; Stanier, P. Physical and transcriptional mapping of the X-linked cleft palate and ankyloglossia (CPX) critical region. Hum. Genet. 2001, 108, 537–545. [Google Scholar] [CrossRef]
- Koifman, A.; Blaser, S.; Chitayat, D. The Genetics of Facial Cleft. In Prenatal Diagnosis of Orofacial Malformations; Tonni, G., Sepulveda, W., Wong, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Scialli, A.R. Stress and birth defects. Relias Media 2001, 3, 65–69. [Google Scholar]
- Queensland Department of Environment and Resource Management. Spectacled Flying Fox Recovery Plan; Queensland Department of Environment and Resource Management: Queensland, Australia, 2010. [Google Scholar]
- Rosenzweig, S.; Blaustein, F.M. Cleft palate in A/J mice resulting from restraint and deprivation of food and water. Teratology 1970, 3, 47–52. [Google Scholar] [CrossRef]
- Montenegro, M.A.; Palomino, H.; Palomino, H.M. The influence of earthquake-induced stress on human facial clefting and its simulation in mice. Arch. Oral Biol. 1995, 40, 33–37. [Google Scholar] [CrossRef]
- Edson, D.; Field, H.; McMichael, L.; Jordan, D.; Kung, N.; Mayer, D.; Smith, C. Flying-Fox Roost Disturbance and Hendra Virus Spillover Risk. PLoS ONE 2015, 10, e0125881. [Google Scholar] [CrossRef]
- McMichael, L.; Edson, D.; Smith, C.; Mayer, D.; Smith, I.; Kopp, S.; Meers, J.; Field, H. Physiological stress and Hendra virus in flying-foxes (Pteropus spp.), Australia. PLoS ONE 2017, 12, e0182171. [Google Scholar] [CrossRef]
- Churchill, S. Australian Bats; Reed: New Holland, Australia, 1998; pp. 84–85. [Google Scholar]
- Westcott, D.A.; Kreitals, N. Stable Isotope Assessment of the Dietary Contribution of Different Habitats to the Spectacled Flying-Fox: A Preliminary Report; Report to the Queensland Environmental Protection Agency; Queensland Environmental Protection Agency: Queensland, Australia, 2008. [Google Scholar]
- McMichael, L.; Edson, D.; McKeown, A.; Sánchez, C.; Mayer, D.; Kopp, S.; Meers, J.; Field, H. Hematology and Plasma Biochemistry of Wild Spectacled Flying Foxes (Pteropus conspicillatus) in Australia. J. Wildl. Dis. 2019, 55, 449–454. [Google Scholar] [CrossRef]
- Rogers, J.M.; Kavlock, R.J. Developmental toxicology. In Casarett & Doull’s Toxicology, 5th ed.; Klaassen, C.D., Ed.; McGraw-Hill: New York, NY, USA, 1996; pp. 301–331. [Google Scholar]
- McMichael, L.; Edson, D.; McLaughlin, A.; Mayer, D.; Kopp, S.; Meers, J.; Field, H. Haematology and Plasma Biochemistry of Wild Black Flying-Foxes, (Pteropus alecto) in Queensland, Australia. PLoS ONE 2015, 10, e0125741. [Google Scholar] [CrossRef]
- Schaer, J.; McMichael, L.; Gordon, A.N.; Russell, D.; Matuschewski, K.; Perkins, S.L.; Field, H.; Power, M. Phylogeny of Hepatocystis parasites of Australian flying foxes reveals distinct parasite clade. Int. J. Parasitol. 2018, 7, 207–212. [Google Scholar] [CrossRef]
- Boyles, A.L.; Abee, L.; Wilcox, A.J.; Taylor, J.A.; Shi, M.; Weinberg, C.R.; Meyer, K.; Fredriksen, Å.; Ueland, P.M.; Johansen, A.M.; et al. Oral facial clefts and gene polymorphisms in metabolism of folate/one-carbon and vitamin A: A pathway-wide association study. Genet. Epidemiol. 2009, 33, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.C.; Ridpath, J.F.; Walker, J.B.; Hietala, S.K. An outbreak of late-term abortions, premature births, and congenital deformities associated with a Bovine viral diarrhea virus 1 subtype b that induces thrombocytopenia. J. Vet. Diagn. Investig. 2010, 22, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Osario, J.E.; Schoepp, R.J.; Yuill, T.M. Effects of La Crosse virus infection on pregnant and domestic rabbits and Mongolian gerbils. Am. J. Trop. Med. Hyg. 1996, 55, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Kenney, A.D.; Dowdle, J.A.; Bozzacco, L.; McMichael, T.M.; St Gelais, C.; Panfil, A.R.; Sun, Y.; Schlesinger, L.S.; Anderson, M.Z.; Green, P.L.; et al. Human Genetic Determinants of Viral Diseases. Annu. Rev. Genet. 2017, 51, 241–263. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McMichael, L.; Mclean, J.; Taylor, J.; Martinez, Y.; Meers, J. Cleft Palate Syndrome in the Endangered Spectacled Flying Fox (Pteropus conspicillatus): Implications for Conservation and Comparative Research. Vet. Sci. 2023, 10, 38. https://doi.org/10.3390/vetsci10010038
McMichael L, Mclean J, Taylor J, Martinez Y, Meers J. Cleft Palate Syndrome in the Endangered Spectacled Flying Fox (Pteropus conspicillatus): Implications for Conservation and Comparative Research. Veterinary Sciences. 2023; 10(1):38. https://doi.org/10.3390/vetsci10010038
Chicago/Turabian StyleMcMichael, Lee, Jennefer Mclean, Jim Taylor, Yissu Martinez, and Joanne Meers. 2023. "Cleft Palate Syndrome in the Endangered Spectacled Flying Fox (Pteropus conspicillatus): Implications for Conservation and Comparative Research" Veterinary Sciences 10, no. 1: 38. https://doi.org/10.3390/vetsci10010038
APA StyleMcMichael, L., Mclean, J., Taylor, J., Martinez, Y., & Meers, J. (2023). Cleft Palate Syndrome in the Endangered Spectacled Flying Fox (Pteropus conspicillatus): Implications for Conservation and Comparative Research. Veterinary Sciences, 10(1), 38. https://doi.org/10.3390/vetsci10010038





