A Four-Year Survey of Hemoparasites from Nocturnal Raptors (Strigiformes) Confirms a Relation between Leucocytozoon and Low Hematocrit and Body Condition Scores of Parasitized Birds
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Laboratory Analysis
2.3. Statistical Analysis
3. Results
3.1. Study Population
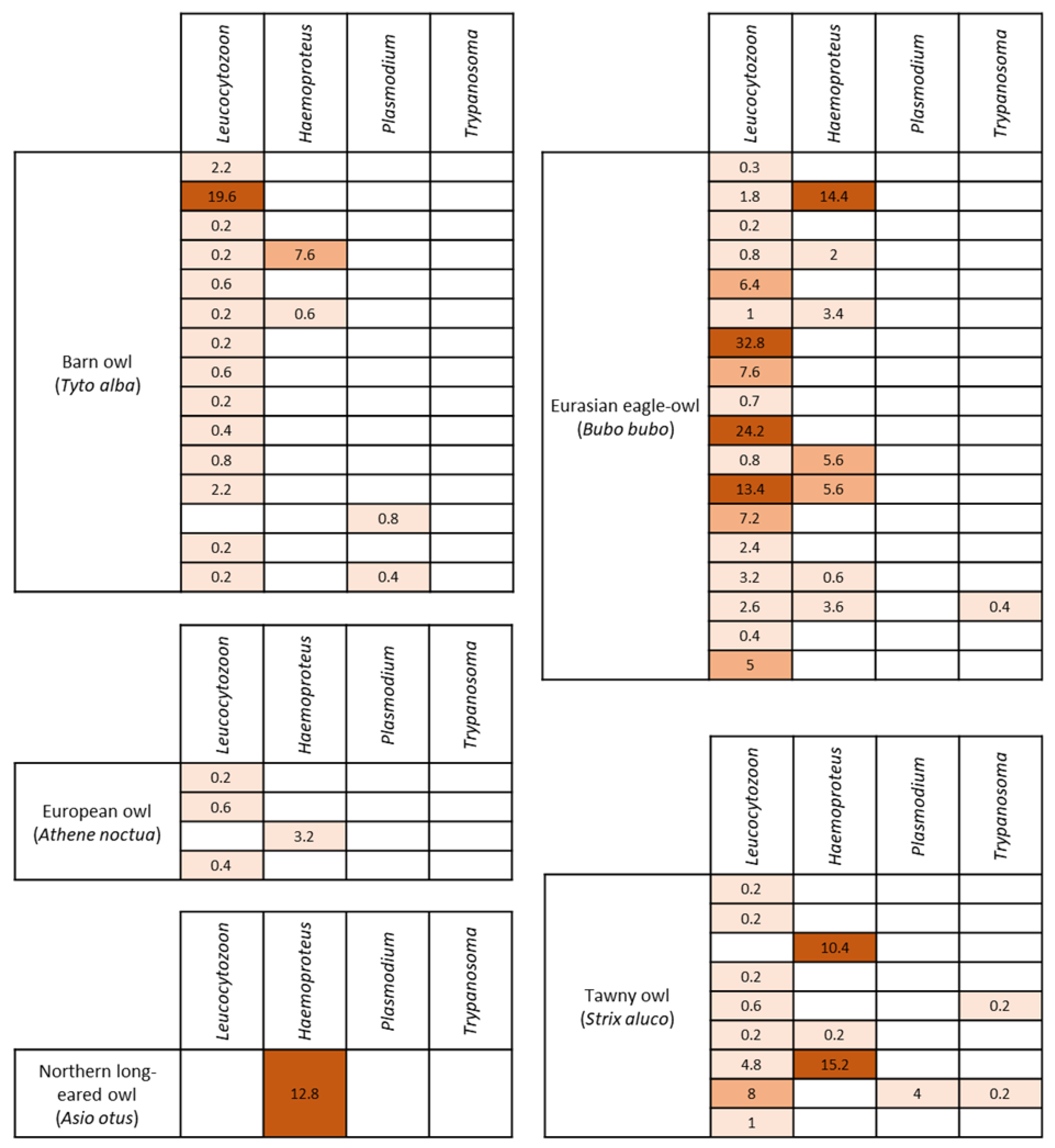
3.2. Hemoparasite Detection and Quantification
3.3. Analysis of Epidemiological Variables
3.4. Results of Hematological Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulin, R. Evolutionary ecology of parasites. In Evolutionary Ecology of Parasites; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Palm, H.W.; Theisen, S.; Pikalov, E.; Kleinertz, S. An Update: Manipulation of Fish Phenotype by Parasites. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128096338. [Google Scholar] [CrossRef]
- Rush, E.M.; Wernick, M.; Beaufrère, H.; Ammersbach, M.; Vergneau-Grosset, C.; Stacy, N.; Pendl, H.; Wellehan, J.F.X., Jr.; Warren, K.; LeSouef, A.; et al. Advances in clinical pathology and diagnostic medicine. In Current Therapy in Avian Medicine and Surgery; Saunders, W.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 461–530. ISBN 9781455746712. [Google Scholar] [CrossRef]
- Ishak, H.D.; Dumbacher, J.P.; Anderson, N.L.; Keane, J.J.; Valkiūnas, G.; Haig, S.M.; Tell, L.A.; Sehgal, R.N.M. Blood Parasites in Owls with Conservation Implications for the Spotted Owl (Strix occidentalis). PLoS ONE 2008, 3, e2304. [Google Scholar] [CrossRef]
- Scaglione, F.; Cannizzo, F.; Chiappino, L.; Sereno, A.; Ripepi, M.; Salamida, S.; Manuali, E.; Bollo, E. Plasmodium spp. In a captive raptor collection of a safaripark in northwest Italy. Res. Vet. Sci. 2016, 104, 123–125. [Google Scholar] [CrossRef]
- Coker, S.M.; Hernandez, S.M.; Kistler, W.M.; Curry, S.E.; Welch, C.N.; Barron, H.W.; Harsch, S.; Murray, M.H.; Yabsley, M.J. Diversity and prevalence of hemoparasites of wading birds in southern Florida, USA. Int. J. Parasitol. Parasites Wildl. 2017, 6, 220–225. [Google Scholar] [CrossRef]
- Van Hemert, C.; Meixell, B.W.; Smith, M.M.; Handel, C.M. Prevalence and diversity of avian blood parasites in a resident northern passerine. Parasites Vectors 2019, 12, 1–16. [Google Scholar] [CrossRef]
- Gonzalez-Quevedo, C.; Pabón, A.; Rivera-Gutierrez, H.F. Prevalence of haemosporidians in a Neotropical endemic bird area. Avian Conserv. Ecol. 2016, 11, 7. [Google Scholar] [CrossRef]
- Morel, A.P.; Webster, A.; Prusch, F.; Anicet, M.; Marsicano, G.; Trainini, G.; Stocker, J.; Giani, D.; Bandarra, P.M.; da Rocha, M.I.S.; et al. Molecular detection and phylogenetic relationship of Haemosporida parasites in free-ranging wild raptors from Brazil. Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100521. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Fachet, K.; Dinkel, A.; Mackenstedt, U.; Woog, F. Carrion crows (Corvus corone) of southwest Germany: Important hosts for haemosporidian parasites. Malar. J. 2017, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shurulinkov, P.; Spasov, L.; Stoyanov, G.; Chakarov, N. Blood parasite infections in a wild population of ravens (Corvus corax) in Bulgaria. Malar. J. 2018, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Schumm, Y.R.; Wecker, C.; Marek, C.; Wassmuth, M.; Bentele, A.; Willems, H.; Reiner, G.; Quillfeldt, P. Blood parasites in Passeriformes in central Germany: Prevalence and lineage diversity of Haemosporida (Haemoproteus, Plasmodium and Leucocytozoon) in six common songbirds. PeerJ 2019, 6, e6259. [Google Scholar] [CrossRef]
- Nourani, L.; Djadid, N.D.; Rabiee, K.; Mezerji, M.S.; Shakiba, M.; Bakhshi, H.; Shokrollahi, B.; Farahani, R.K. Detection of haemosporidian parasites in wild and domestic birds in northern and central provinces of Iran: Introduction of new lineages and hosts. Int. J. Parasitol. Parasites Wildl. 2020, 13, 203–212. [Google Scholar] [CrossRef]
- Hanel, J.; Doležalová, J.; Stehlíková, Š.; Modrý, D.; Chudoba, J.; Synek, P.; Votýpka, J. Blood parasites in northern goshawk (Accipiter gentilis) with an emphasis to Leucocytozoon toddi. Parasitol. Res. 2016, 115, 263–270. [Google Scholar] [CrossRef]
- Krone, O.; Waldenström, J.; Valkiūnas, G.; Lessow, O.; Müller, K.; Iezhova, T.A.; Fickel, J.; Bensch, S. Haemosporidian Blood Parasites in European Birds of Prey and Owls. J. Parasitol. 2008, 94, 709–715. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kwak, D.; Kim, K.-T. The first clinical cases of Haemoproteus infection in a snowy owl (Bubo scandiacus) and a goshawk (Accipiter gentilis) at a zoo in the Republic of Korea. J. Vet. Med. Sci. 2018, 80, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Giorgiadis, M.; Guillot, J.; Duval, L.; Landau, I.; Quintard, B. Haemosporidian parasites from captive Strigiformes in France. Parasitol. Res. 2020, 119, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Attaran, H.; Luo, J.; Bo, W.; Nabavi, R.; He, H.X. Haemosporidian Blood Parasites in nestling birds of prey in Mongolia. bioRxiv 2021. [Google Scholar] [CrossRef]
- Gao, K.; Zhou, B.; Yang, L.-X.; Dong, L.; Huang, X.; Deng, W.-H. How Does Circadian Rhythm Shape Host-Parasite Associations? A Comparative Study on Infection Patterns in Diurnal and Nocturnal Raptors. Diversity 2021, 13, 338. [Google Scholar] [CrossRef]
- Baker, K.C.; Rettenmund, C.L.; Sander, S.J.; Rivas, A.E.; Green, K.C.; Mangus, L.; Bronson, E. Clinical effect of hemoparasite infections in snowy owls (Bubo scandiacus). J. Zoo Wildl. Med. 2018, 49, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Leppert, L.L.; Dufty, A.M.; Stock, S.; Oleyar, M.D.; Kaltenecker, G.S. Survey of Blood Parasites in Two Forest Owls, Northern Saw-whet Owls and Flammulated Owls, of Western North America. J. Wildl. Dis. 2008, 44, 475–479. [Google Scholar] [CrossRef]
- Valkiunas, G. Avian Malaria Parasites and Other Haemosporida; CRC Press: Boca Raton, FL, USA, 2005; p. 932. [Google Scholar]
- Chakarov, N.; Veiga, J.; Ruiz-Arrondo, I.; Valera, F. Atypical behavior of a black fly species connects cavity-nesting birds with generalist blood parasites in an arid area of Spain. Parasites Vectors 2021, 14, 257. [Google Scholar] [CrossRef]
- Maiorano, L.; Amori, G.; Capula, M.; Falcucci, A.; Masi, M.; Montemaggiori, A.; Pottier, J.; Psomas, A.; Rondinini, C.; Russo, D.; et al. Threats from Climate Change to Terrestrial Vertebrate Hotspots in Europe. PLoS ONE 2013, 8, e74989. [Google Scholar] [CrossRef]
- van Wijk, R.E.; Bauer, S.; Schaub, M. Repeatability of individual migration routes, wintering sites, and timing in a long-distance migrant bird. Ecol. Evol. 2016, 6, 8679–8685. [Google Scholar] [CrossRef] [PubMed]
- EN 32010L0063; Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. European Commission: Brussels, Belgium, 2010; pp. 33–79. Available online: http://data.europa.eu/eli/dir/2010/63/oj (accessed on 14 December 2022).
- Brubaker, J.L.; Karouna-Renier, N.K.; Chen, Y.; Jenko, K.; Sprague, D.T.; Henry, P.F.P. A noninvasive, direct real-time PCR method for sex determination in multiple avian species. Mol. Ecol. Resour. 2011, 11, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.; Sanchez, J.N. Creation and validation of a novel body condition scoring method for the magellanic penguin (Spheniscus magellanicus) in the zoo setting. Zoo Biol. 2015, 34, 538–546. [Google Scholar] [CrossRef]
- Samour, J. Avian Medicine; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Fudge, A.M.; Joseph, V. Disorders of avian leukocytes. In Laboratory Medicine: Avian and Exotic Pets; WB Saunders: Philadelphia, PA, USA, 2000; pp. 19–27. [Google Scholar]
- Tostes, R.; Dias, R.J.P.; Martinele, I.; Senra, M.V.X.; D’Agosto, M.; Massard, C.L. Multidisciplinary re-description of Plasmodium (Novyella) paranucleophilum in Brazilian wild birds of the Atlantic Forest kept in captivity. Parasitol. Res. 2017, 116, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, E.; Ferrer, D.; Molina, R.; Adlard, R.D. Prevalence of haematozoa in birds of prey in Catalonia, north-east Spain. Vet. Rec. 1999, 144, 632–636. [Google Scholar] [CrossRef]
- Tomé, R.; Santos, N.; Cardia, P.; Ferrand, N.; Korpimaki, E. Factors affecting the prevalence of blood parasites of Little Owls Athene noctua in southern Portugal. Ornis Fenn. 2005, 82, 63. [Google Scholar]
- Krone, O.; Priemer, J.; Streich, J.; Sommer, P.; Langgemach, T.; Lessow, O. Haemosporida of birds of prey and owls from Germany. Acta Protozool. 2001, 40, 281–290. [Google Scholar]
- Carlson, M.L.; Proudfoot, G.A.; Gentile, K.; Dispoto, J.; Weckstein, J.D. Haemosporidian prevalence in northern saw-whet owls Aegolius acadicus is predicted by host age and average annual temperature at breeding grounds. J. Avian Biol. 2018, 49, e01817. [Google Scholar] [CrossRef]
- Barino, G.T.M.; Rossi, M.F.; De Oliveira, L.; Junior, J.L.R.; D’Agosto, M.; Dias, R.J.P. Haemoproteus syrnii (Haemosporida: Haemoproteidae) in owls from Brazil: Morphological and molecular characterization, potential cryptic species, and exo-erythrocytic stages. Parasitol. Res. 2021, 120, 243–255. [Google Scholar] [CrossRef]
- Ilgūnas, M.; Himmel, T.; Harl, J.; Dagys, M.; Valkiūnas, G.; Weissenböck, H. Exo-Erythrocytic Development of Avian Haemosporidian Parasites in European Owls. Animals 2022, 12, 2212. [Google Scholar] [CrossRef]
- Pornpanom, P.; Chagas, C.R.F.; Lertwatcharasarakul, P.; Kasorndorkbua, C.; Valkiūnas, G.; Salakij, C. Molecular prevalence and phylogenetic relationship of Haemoproteus and Plasmodium parasites of owls in Thailand: Data from a rehabilitation centre. Int. J. Parasitol. Parasites Wildl. 2019, 9, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Levin, I.; Parker, G. Haemosporidian parasites: Impacts on avian hosts. In Fowler’s Zoo and Wild Animal Medicine Current Therapy; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011; Volume 7, p. 356. [Google Scholar]
- Magri, A.; Galuppi, R.; Fioravanti, M. Autochthonous Trypanosoma spp. in European Mammals: A Brief Journey amongst the Neglected Trypanosomes. Pathogens 2021, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Bairlein, F.; Iezhova, T.A.; Dolnik, O.V. Factors affecting the relapse of Haemoproteus belopolskyi infections and the parasitaemia of Trypanosoma spp. in a naturally infected European songbird, the blackcap, Sylvia atricapilla. Parasitol. Res. 2004, 93, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, M.; Weidinger, K.; Peske, L.; Volf, P.; Votýpka, J.; Vorisek, P. Trypanosomes and haemosporidia in the buzzard (Buteo buteo) and sparrowhawk (Accipiter nisus): Factors affecting the prevalence of parasites. Parasitol. Res. 2015, 114, 551–560. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; de la Hera, I.; Bensch, S.; Pérez-Tris, J. Evolution of seasonal transmission patterns in avian blood-borne parasites. Int. J. Parasitol. 2015, 45, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C.R.F.; Valkiūnas, G.; Guimarães, L.D.O.; Monteiro, E.F.; Guida, F.J.V.; Simões, R.F.; Rodrigues, P.T.; Luna, E.J.d.A.; Kirchgatter, K. Diversity and distribution of avian malaria and related haemosporidian parasites in captive birds from a Brazilian megalopolis. Malar. J. 2017, 16, 1–20. [Google Scholar] [CrossRef]
- Hisada, Y.; Saito, K.; Asakawa, M. Epidemiological Survey of Haemoproteus sp. Found Blakiston’s Owls (Ketupa blakistoni blakistoni) on Hokkaido Island, Japan. Jpn. J. Zoo Wildl. Med. 2004, 9, 85–89. [Google Scholar] [CrossRef]
- Karadjian, G.; Puech, M.-P.; Duval, L.; Chavatte, J.-M.; Snounou, G.; Landau, I. Haemoproteus syrnii in Strix aluco from France: Morphology, stages of sporogony in a hippoboscid fly, molecular characterization and discussion on the identification of Haemoproteus species. Parasite 2013, 20, 32. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Ozawa, K.; Kondo, H.; Echigoya, Y.; Shibuya, H.; Sato, Y.; Sehgal, R.N.M. A fatal case of a captive snowy owl (Bubo scandiacus) with Haemoproteus infection in Japan. Parasitol. Res. 2021, 120, 277–288. [Google Scholar] [CrossRef]
- Niedringhaus, K.D.; Fenton, H.M.; Cleveland, C.A.; Anderson, A.N.; Schwartz, D.; Alex, C.E.; Rogers, K.H.; Mete, A.; Yabsley, M.J. Case Series: Virulent hemosporidiosis infections in juvenile great horned owls (Bubo virginianus) from Louisiana and California, USA. Vet. Parasitol. Reg. Stud. Rep. 2018, 12, 49–54. [Google Scholar] [CrossRef]
- Apanius, V.; Kirkpatrick, C.E. Preliminary Report of Haemoproteus tinnunculi Infection in a Breeding Population of American Kestrels (Falco sparverius). J. Wildl. Dis. 1988, 24, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A.; Bensch, S.; Reviriego, M.I.; Balbontin, J.; De Lope, F. Effects of malaria double infection in birds: One plus one is not two. J. Evol. Biol. 2008, 21, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Otter, A. Fatal combined infection with Haemoproteus noctuae and Leucocytozoon ziemanni in juvenile snowy owls (Nyctea scandiaca). Vet. Rec. 1998, 143, 72–76. [Google Scholar] [CrossRef]
- A Harasym, C. West Nile virus and hemoparasites in captive snowy owls (Bubo scandiacus)—Management strategies to optimize survival. Can. Vet. J. 2008, 49, 1136. [Google Scholar] [PubMed]
- Ziman, M.; Colagross-Schouten, A.; Griffey, S.; Stedman, B. Haemoproteus spp. and Leukocytozoon spp. in a Captive Raptor Population. J. Wildl. Dis. 2004, 40, 137–140. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Samour, J.H.; D’Aloia, M.A.; Howlett, J.C. Normal haematology of captive saker falcons (Falco cherrug). Comp. Haematol. Int. 1996, 6, 50–52. [Google Scholar] [CrossRef]
- Wiegmann, A.; Springer, A.; Rinaud, T.; Ottensmann, M.; Legler, M.; Krüger, O.; Fehr, M.; Chakarov, N.; Strube, C. The prevalence of Leucocytozoon spp. in nestlings of three wild raptor species including implications on haematological and blood chemistry values. Int. J. Parasitol. Parasites Wildl. 2021, 16, 236–243. [Google Scholar] [CrossRef]
- Clark, N.J.; Wells, K.; Dimitrov, D.; Clegg, S.M. Co-infections and environmental conditions drive the distributions of blood parasites in wild birds. J. Anim. Ecol. 2016, 85, 1461–1470. [Google Scholar] [CrossRef]

| Bird Species | Overall Positivity | Leucocytozoon | Haemoproteus | Trypanosoma | Plasmodium | ||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence | Parasitemia | Prevalence | Parasitemia | Prevalence | Parasitemia | Prevalence | Parasitemia | ||
| Barn owl (Tyto alba) | 15/90; 16.7% (10.3–25.7%) | 14/90; 15.5% (9.5–24.4%) | 0.3 [0.2, 2.9] | 2/90; 2.2% (0.6–7.7%) | 4.1 [0.6, 7.6] | 0/90; 0% - | - | 2/90; 2.2% (0.6–7.7%) | 0.6 [0.4, 0.8] |
| Eurasian eagle-owl (Bubo bubo) | 18/19; 94.7% (75.3–99.1%) | 18/19; 94.7% (75.3–99.1%) | 2.5 [0.8, 6.4] | 8/19; 42.1% (23.1–63.7%) | 3.6 [2, 5.6] | 1/19; 5.3% (0.9–24.6%) | 0.4 [0.4, 0.4] | 0/19; 0% - | - |
| Tawny owl (Strix aluco) | 9/10; 90% (59.6–98.2%) | 8/10; 80% (49.0–94.3%) | 0.4 [0.2, 2.9] | 3/10; 30% (10.7–60.3%) | 10.4 [0.2, 15.2] | 2/10; 20% (5.6–50.9%) | 0.2 [0.2, 0.2] | 1/10; 10% (1.8–40.4%) | 4 [4, 4] |
| European owl (Athene noctua) | 4/10; 40% (16.8–68.7%) | 3/10; 30% (10.7–60.3%) | 0.5 [0.4, 0.6] | 1/10; 10% (1.8–40.4%) | 3.2 [3.2, 3.2] | 0/10; 0% - | - | 0/10; 0% - | - |
| Eurasian Scops-owl (Otus scops) | 0/4; 0% - | 0/4; 0% - | - | 0/4; 0% - | - | 0/4; 0% - | - | 0/4; 0% - | - |
| Northern long-eared owl (Asio otus) | 1/1; 100% (20.6–100%) | 0/1; 0% - | - | 1/1; 100% (20.6–100%) | 12.8 [12.8, 12.8] | 0/1; 0% - | - | 0/1; 0% - | - |
| Overall positivity | 47/134; 35.1% (27.5–43.5%) | 43/134; 32.1% (24.8–40.4%) | - | 15/134; 11.2% (6.9–17.6%) | - | 3/134; 2.2% (0.7–6.4%) | - | 3/134; 2.2% (0.7–6.4%) | - |
| Blood Parameters | Barn Owl (Tyto alba) | Eurasian Eagle-Owl (Bubo bubo) | Tawny Owl (Strix aluco) | European Owl (Athene noctua) | Eurasian Scops-Owl (Otus scops) | Northern Long-Eared Owl (Asio otus) |
|---|---|---|---|---|---|---|
| n = 90 | n = 19 | n = 10 | n = 10 | n = 4 | n = 1 | |
| Hematocrit (%) | 38 [35, 42] | 41 [35, 45] | 34 [31, 41] | 37 [30, 45] | 32.5 [28.5, 39] | 39 - |
| WBC count (cells/mm3) | 21,000 [13,000, 28,800] | 14,935 [9260, 18,995] | 10,250 [10,000, 16,750] | 14,750 [12,750, 8750] | 28,375 [16,960, 36,625] | 12,000 - |
| Heterophils (%) | 52 [40, 65] | 66 [61, 82] | 55 [27, 60] | 52 [43, 59] | 65 [50–67] | 16 - |
| Eosinophils (%) | 4 [0, 10] | 2 [0, 5] | 3 [0, 5] | 1 [0, 2] | 1 [0–4] | 62 - |
| Basophils (%) | 0 [0, 1] | 0 [0, 1] | 1 [1, 2] | 0 [0, 0] | 0 [0, 1] | 2 - |
| Lymphocytes (%) | 40 [24, 49] | 21 [14, 30] | 39 [33, 40] | 42 [26, 54] | 30 [20–35] | 14 - |
| Monocytes (%) | 2 [1, 4] | 2 [1, 5] | 5 [5, 7] | 3 [2, 4] | 3 [2–15] | 6 - |
| Blood Parameters | Parasitised n = 15 | Non-Parasitised n = 69 | p-Value |
|---|---|---|---|
| Haematocrit (%) | 35 [30, 39] | 39 [35, 43] | p = 0.007 * |
| WBC count (cells/mm3) | 35,275 [20,750, 40,200] | 20,500 [11,625, 26,875] | p = 0.003 ** |
| Heterophils (%) | 61 [41, 76] | 50.5 [40, 62] | p = 0.124 * |
| Eosinophils (%) | 1 [0, 9] | 4 [1, 10] | p = 0.216 ** |
| Basophils (%) | 0 [0, 1] | 0 [0, 1] | p = 0.591 ** |
| Lymphocytes (%) | 29 [20, 40] | 41 [28, 49] | p = 0.110 * |
| Monocytes (%) | 3 [0, 5] | 2 [1, 4] | p = 0.435 ** |
| H/L ratio | 2.1 [1.4, 3.8] | 1.3 [0.7, 2.2] | p = 0.054 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Maldonado, B.; Mencía-Gutiérrez, A.; Andreu-Vázquez, C.; Fernández, R.; Pastor-Tiburón, N.; Alvarado, A.; Carrero, A.; Fernández-Novo, A.; Esperón, F.; González, F. A Four-Year Survey of Hemoparasites from Nocturnal Raptors (Strigiformes) Confirms a Relation between Leucocytozoon and Low Hematocrit and Body Condition Scores of Parasitized Birds. Vet. Sci. 2023, 10, 54. https://doi.org/10.3390/vetsci10010054
Martín-Maldonado B, Mencía-Gutiérrez A, Andreu-Vázquez C, Fernández R, Pastor-Tiburón N, Alvarado A, Carrero A, Fernández-Novo A, Esperón F, González F. A Four-Year Survey of Hemoparasites from Nocturnal Raptors (Strigiformes) Confirms a Relation between Leucocytozoon and Low Hematocrit and Body Condition Scores of Parasitized Birds. Veterinary Sciences. 2023; 10(1):54. https://doi.org/10.3390/vetsci10010054
Chicago/Turabian StyleMartín-Maldonado, Bárbara, Aida Mencía-Gutiérrez, Cristina Andreu-Vázquez, Rocío Fernández, Natalia Pastor-Tiburón, Alberto Alvarado, Alicia Carrero, Aitor Fernández-Novo, Fernando Esperón, and Fernando González. 2023. "A Four-Year Survey of Hemoparasites from Nocturnal Raptors (Strigiformes) Confirms a Relation between Leucocytozoon and Low Hematocrit and Body Condition Scores of Parasitized Birds" Veterinary Sciences 10, no. 1: 54. https://doi.org/10.3390/vetsci10010054
APA StyleMartín-Maldonado, B., Mencía-Gutiérrez, A., Andreu-Vázquez, C., Fernández, R., Pastor-Tiburón, N., Alvarado, A., Carrero, A., Fernández-Novo, A., Esperón, F., & González, F. (2023). A Four-Year Survey of Hemoparasites from Nocturnal Raptors (Strigiformes) Confirms a Relation between Leucocytozoon and Low Hematocrit and Body Condition Scores of Parasitized Birds. Veterinary Sciences, 10(1), 54. https://doi.org/10.3390/vetsci10010054







