A Post-Operative Follow-Up of an Endangered Saltwater Fish Lensectomy for Cataract Management in a Public Aquarium: A Case Series
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Population
2.2. General and Ophthalmic Examinations
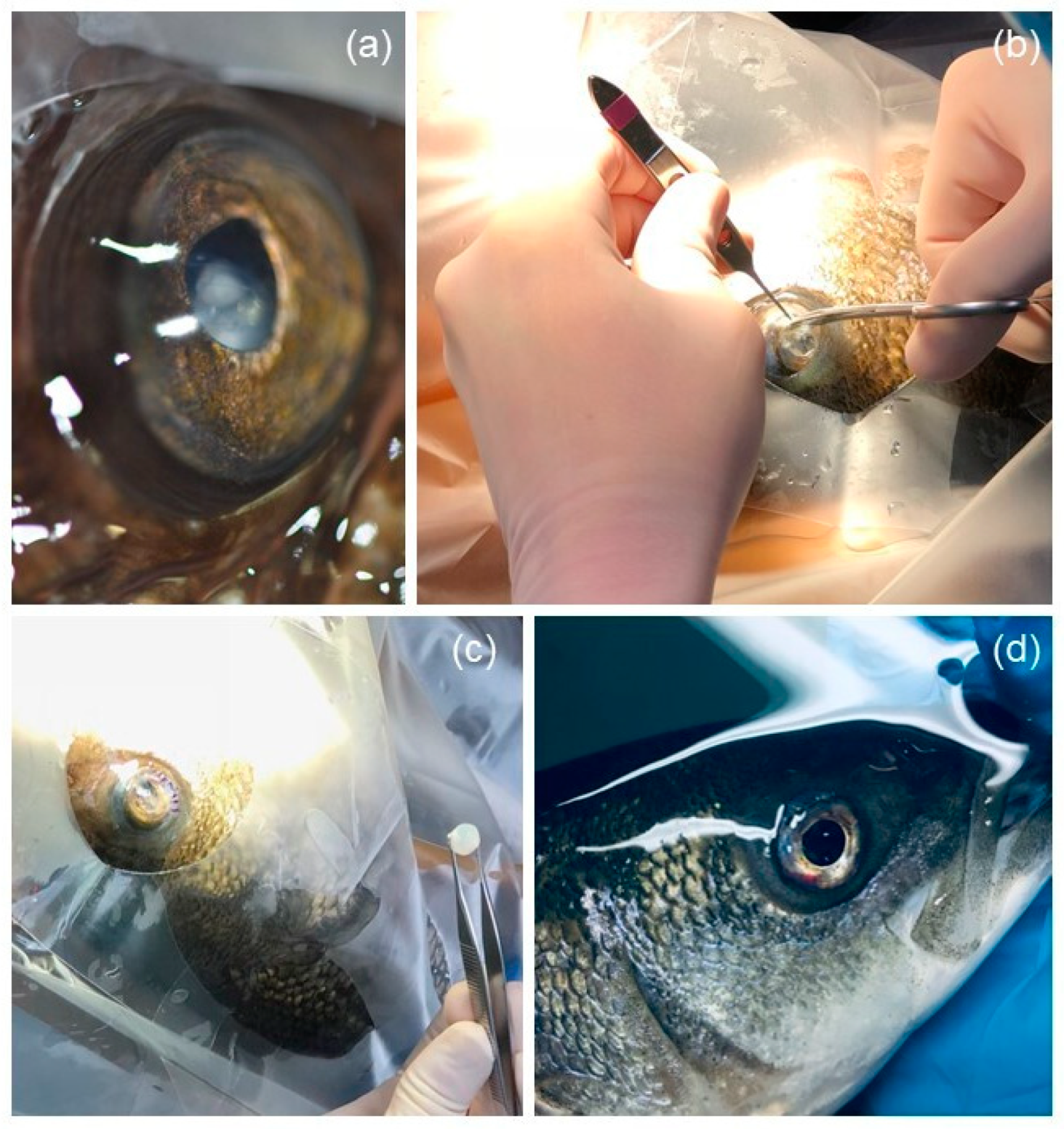
2.3. Surgical Procedure
2.4. Post-Operative Follow-Up
2.5. Statistics
3. Results
3.1. Study Population
3.2. Pre-Surgical Clinical Signs
3.3. Lensectomy
3.4. Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jurk, I. Ophthalmic disease of fish. Vet. Clin. N. Am. Exot. Anim. Pract. 2002, 5, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Adamovicz, L.; Lewbart, G.; Gilger, B. Phacoemulsification and aspiration for cataract management in a dollar sunfish, Lepomis marginatus (Holbrook)—A case report. J. Fish Dis. 2015, 38, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Hargis, W.J. Disorders of the eye in finfish. Annu. Rev. Fish Dis. 1991, 1, 95–117. [Google Scholar] [CrossRef]
- Jonassen, T.; Hamadi, M.; Remo, S.C.; Waagbo, R. An epidemiological study of cataracts in wild and farmed lumpfish (Cyclopterus lumpus L.) and the relation to nutrition. J. Fish Dis. 2017, 40, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Bardach, J.; Magnuson, J.; May, R.; Reinhart, J. Fish behavior and its use in the capture and culture of fishes. In Vision and the Feeding of Fishes, 1st ed.; Blaxter, J., Ed.; ICLARM: Manila, Philippines, 1980; pp. 32–56. [Google Scholar]
- Collin, S.; Hart, N. Vision and photoentrainment in fishes: The effects of natural and anthropogenic perturbation. Integr. Zool. 2015, 10, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Vergneau-Grosset, C.; Weber III, E.S. Fish Surgery. In Surgery of Exotic Animals, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 46–62. [Google Scholar]
- Recovery Strategy for the Striped Bass (Morone saxatilis), St. Lawrence Estuary Population, Canada. Species at Risk Act Recovery Strategy Series. Available online: https://www.sararegistry.gc.ca/virtual_sara/files/plans/rs_bar_raye_striped_bass_st.la_1011_eng.pdf (accessed on 22 June 2021).
- Arrêté Visant L’habitat Essentiel du Loup Tacheté (Anarhichas minor) [Critical Habitat of the Spotted Wolffish (Anarhichas minor) Order]. Available online: https://laws.justice.gc.ca/fra/reglements/DORS-2020-186/page-1.html (accessed on 22 June 2021).
- Béland, K.; Wong, E.; St-Cyr, J.; Lair, S. High occurrence rate of xanthomatosis and nephrocalcinosis in aquarium-housed Atlantic wolffish Anarhichas lupus and spotted wolffish A. minor. Dis. Aquat. Org. 2020, 139, 223–232. [Google Scholar] [CrossRef]
- COSEWIC Assessment and Status Report on the Spotted Wolffish Anarhichas minor in Canada. Available online: https://www.sararegistry.gc.ca/virtual_sara/files/cosewic/sr_spotted_wolffish_0501_e.pdf (accessed on 26 June 2023).
- COSEWIC Assessment and Status Report on the Atlantic Wolffish Anarhichas lupus in Canada. Available online: https://www.sararegistry.gc.ca/virtual_sara/files/cosewic/sr_atlantic_wolffish_1100_e.pdf (accessed on 26 June 2023).
- Harms, C.; Lewbart, G.; Swanson, C.; Kishimori, J.; Boylan, S. Behavioral and clinical pathology changes in koi carp (Cyprinus carpio) subjected to anesthesia and surgery with and without intra-operative analgesics. Comp. Med. 2005, 55, 221–226. [Google Scholar]
- Vergneau-Grosset, C.; Cruz-Benedetti, I. Fish sedation and anesthesia. Vet. Clin. N. Am. Exot. Anim. Pract. 2022, 25, 13–29. [Google Scholar]
- Raulic, J.; Beaudry, F.; Beauchamp, G.; Jalenques, M.; Summa, N.; Lair, S.; Youcef, W.; Vergneau-Grosset, C. Pharmacokinetic, pharmacodynamic, and toxicology study of robenacoxib in rainbow trout (Oncorhynchus mykiss). J. Zoo Wildl. Med. 2021, 52, 529–537. [Google Scholar] [CrossRef]
- Keeney, C.H.; Vorbach, B.; Clayton, L.; Seeley, K. Intraocular pressure in clinically normal brook trout (Salvelinus fontinalis) by means of rebound tonometry. J. Zoo Wildl. Med. 2019, 50, 107–110. [Google Scholar]
- Morone saxatilis—Striped Bass. Available online: https://www.fishbase.se/summary/353 (accessed on 20 October 2022).
- Pujol, J.; Jalenques, M.; Lair, S.; Judith, F.; Vergneau-Grosset, C. Occurrence of germ cell neoplasia in male striped bass (Morone saxatilis) under human care in a public aquarium: Surgical treatment and outcome. in press.
- Gacic, Z.; Milosevic, M.; Mickovic, B.; Nikcevic, M.; Damjanovic, I. Effects of acute cooling on fish electroretinogram: A comparative study. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 184, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Gustavsen, K.; Paul-Murphy, J.; Weber, E.r.; Zwingenberger, A.; Dunker, F.; Dubielzig, R.; Reilly, C.; Murphy, C. Ocular anatomy of the black pacu (Colossoma macropomum): Gross, histologic, and diagnostic imaging. Vet. Ophthalmol. 2018, 21, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Somiya, H.; Tamura, T. Studies on the visual accommodation in fishes. J. Ichthyol. 1973, 20, 193–206. [Google Scholar]
- Douglas, R.; Hawryshyn, C. Behavioural studies of fish vision: An analysis of visual capabilities. In The Visual System of Fish, 1st ed.; Douglas, R., Djamgoz, M., Eds.; Springer: New York, NY, USA, 1990; pp. 373–418. [Google Scholar]
- Yi, N.; Park, S.; Jeong, M.; Kim, W.; Kim, S.; Chae, J.; Seo, K. Phacoemulsification and acryl foldable intraocular lens implantation in dogs: 32 cases. J. Vet. Sci. 2006, 7, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Nadelstein, B.; Bakal, R.; Lewbart, G. Orbital exenteration and placement of a prosthesis in fish. J. Am. Veter Med Assoc. 1997, 211, 603–606. [Google Scholar]
- Brooks, D.; Plummer, C.; Carastro, S.; Utter, M. Visual outcomes of phacoemulsification cataract surgery in horses: 1990–2013. Vet. Ophthalmol. 2014, 17, 117–128. [Google Scholar] [CrossRef]
- Wilkie, D.; Colitz, C. Update on veterinary cataract surgery. Curr. Opin. Ophthalmol. 2009, 20, 61–68. [Google Scholar] [CrossRef]
- Smith, P.; Brooks, D.; Lazarus, J.; Kubilis, P.; Gelatt, K. Ocular hypertension following cataract surgery in dogs: 139 cases (1992–1993). J. Am. Vet. Med. Assoc. 1996, 209, 105–111. [Google Scholar]
- Hurty, C.; Brazik, D.; Law, J.; Sakamoto, K.; Lewbart, G. Evaluation of the tissue reactions in the skin and body wall of koi (Cyprinus carpio) to five suture materials. Vet. Rec. 2002, 151, 324–328. [Google Scholar] [CrossRef]
- Klein, H.; Krohne, S.; Moore, G.; Stiles, J. Postoperative complications and visual outcomes of phacoemulsification in 103 dogs (179 eyes): 2006–2008. Vet. Ophthalmol. 2011, 14, 114–120. [Google Scholar] [CrossRef]
- Sigle, K.; Nasisse, M. Long-term complications after phacoemulsification for cataract removal in dogs: 172 cases (1995–2002). J. Am. Vet. Med. Assoc. 2006, 228, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Lois, N.; Wong, D. Pseudophakic retinal detachment. Surv. Ophthalmol. 2003, 48, 467–487. [Google Scholar] [PubMed]
- Padros, F.; Knudsen, R.; Blasco-Costa, I. Histopathological characterisation of retinal lesions associated to Diplostomum species (Platyhelminthes: Trematoda) infection in polymorphic Arctic charr Salvelinus alpinus. Int. J. Parasitol. Parasites Wildl. 2018, 7, 68–74. [Google Scholar] [CrossRef]
- Park, Y.; Kim, J.; Jeong, M.; Kim, S.; Yoon, J.; Seo, K. A Retrospective study on the association between vitreous degeneration and cataract in dogs. Vet. Ophthalmol. 2015, 18, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Bharksuwana, E.; Maher, W. Curare, an aid in the surgery of cataract. Bull. N. Y. Acad. Med. 1976, 52, 212–215. [Google Scholar] [CrossRef]
- Moore, D.; McLellan, G.; Dubielzig, R. A study of the morphology of canine eyes enucleated or eviscerated due to complications following phacoemulsification. Vet. Ophthalmol. 2003, 6, 219–226. [Google Scholar] [CrossRef]
- Catanzariti, J.; Salomez, E.; Bruandet, J.; Thevenon, A. Visual deficiency and scoliosis. Spine J. 2001, 26, 48–52. [Google Scholar] [CrossRef]
- Vergneau-Grosset, C.; University of Montreal, Montreal, QC, Canada. Personal comunication, 2018.
- Secchi, A.; Fregona, I.; D’Ermo, F. Lysophosphatidyl choline in the aqueous humor during ocular inflammation. Br. J. Ophthalmol. 1979, 63, 768–770. [Google Scholar] [CrossRef]


| Fish Number | Specie | Sex (Method of Determination) | Body Weight (kg) | Cataract | Bilateral Lensectomy |
|---|---|---|---|---|---|
| 1 | Morone saxatilis | Male (ultrasound) | 6.5 | Bilateral | No |
| 2 | Morone saxatilis | Male (gonadectomy) | 1.4 | Bilateral | Yes |
| 3 | Morone saxatilis | Male (necropsy) | 1.7 | Bilateral | No |
| 4 | Morone saxatilis | Unknown | 1.7 | Bilateral | Yes |
| 5 | Morone saxatilis | Male (gonadectomy) | 1.6 | Bilateral | No |
| 6 | Morone saxatilis | Male (necropsy) | 1.5 | Bilateral (one luxated lens) | Yes |
| 7 | Morone saxatilis | Unknown | 1.3 | Unilateral | No |
| 8 | Anarhichas lupus | Male (necropsy) | 4.5 | Bilateral | Yes |
| 9 | Anarhichas lupus | Unknown | 1.5 | Unilateral | No |
| 10 | Anarhichas minor | Male (necropsy) | 2.5 | Bilateral (one luxated lens) | No |
| 11 | Anarhichas minor | Unknown | 1.4 | Unilateral | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujol, J.; Lamglait, B.; Vanore, M.; Rousseau, C.; Vergneau-Grosset, C. A Post-Operative Follow-Up of an Endangered Saltwater Fish Lensectomy for Cataract Management in a Public Aquarium: A Case Series. Vet. Sci. 2023, 10, 611. https://doi.org/10.3390/vetsci10100611
Pujol J, Lamglait B, Vanore M, Rousseau C, Vergneau-Grosset C. A Post-Operative Follow-Up of an Endangered Saltwater Fish Lensectomy for Cataract Management in a Public Aquarium: A Case Series. Veterinary Sciences. 2023; 10(10):611. https://doi.org/10.3390/vetsci10100611
Chicago/Turabian StylePujol, Julie, Benjamin Lamglait, Maria Vanore, Catherine Rousseau, and Claire Vergneau-Grosset. 2023. "A Post-Operative Follow-Up of an Endangered Saltwater Fish Lensectomy for Cataract Management in a Public Aquarium: A Case Series" Veterinary Sciences 10, no. 10: 611. https://doi.org/10.3390/vetsci10100611






