High-Dose Hydrocortisone Treatment Does Not Affect Serum C-Reactive Protein (CRP) Concentrations in Healthy Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
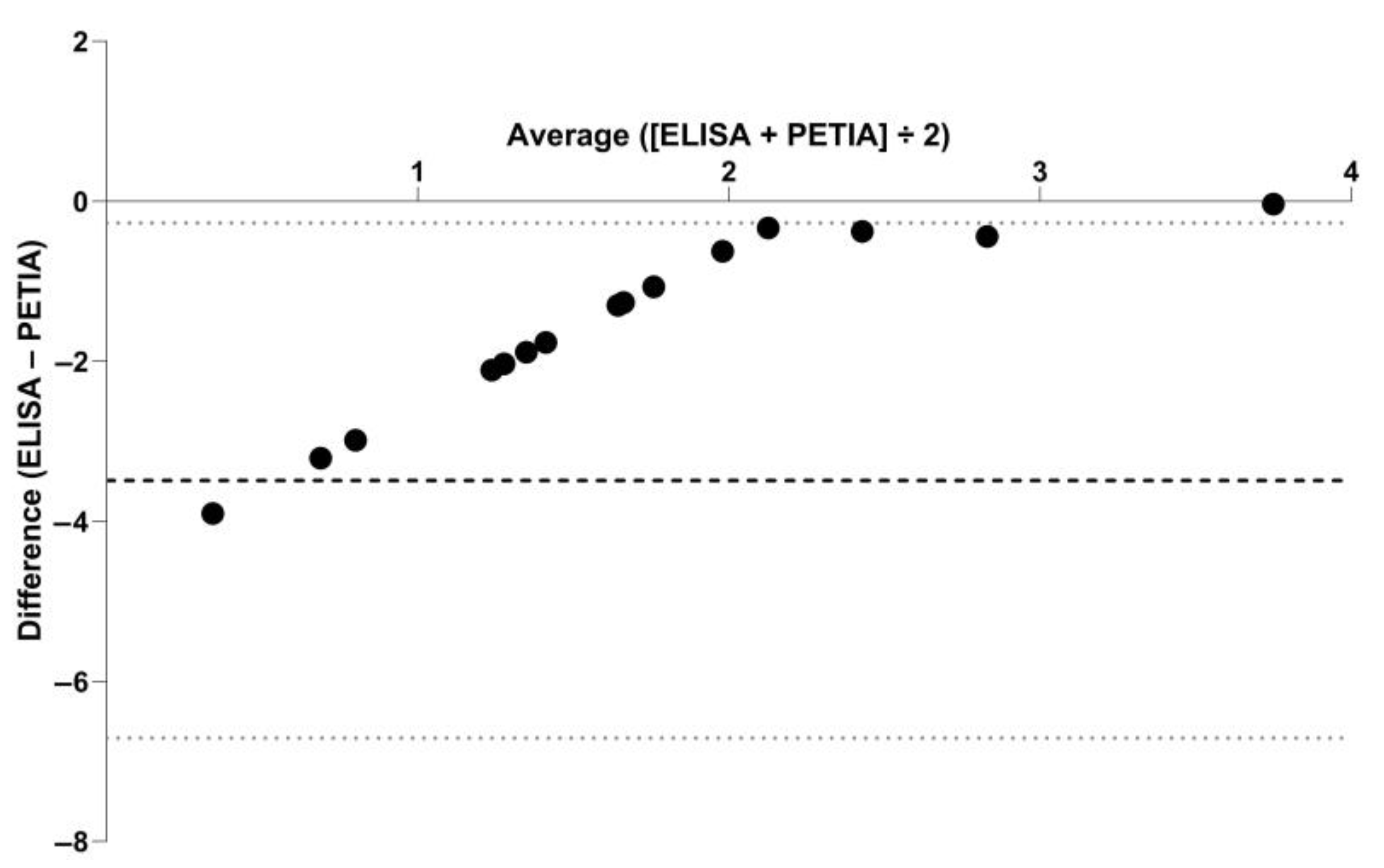
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Covin, M.A.; Steiner, J.M. Measurement and clinical applications of C-reactive protein in gastrointestinal diseases of dogs. Vet. Clin. Pathol. 2022, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, B.; Fürnrohr, B.G.; Vyse, T.J. C-reactive protein in rheumatology: Biology and genetics. Nat. Rev. Rheumatol. 2011, 7, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.D.; Sample, S.; Kohler, R.; Watson, K.; Muir, P.; Trepanier, L.A. Serum biomarkers of clinical and cytologic response in dogs with idiopathic immune-mediated polyarthropathy. J. Vet. Intern. Med. 2014, 28, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Crandell, J.; Morrison, J.A.; Deitz, K.; Pressel, M.; Ackermann, M.; Suchodolski, J.S.; Steiner, J.M.; Evans, R. Comparison of oral prednisone and prednisone combined with metronidazole for induction therapy of canine inflammatory bowel disease: A randomized-controlled trial. J. Vet. Intern. Med. 2010, 24, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, R.M.; Steiner, J.M. Clinical utility of currently available biomarkers in inflammatory enteropathies of dogs. J. Vet. Intern. Med. 2018, 32, 1495–1508. [Google Scholar] [CrossRef]
- Griebsch, C.; Arndt, G.; Raila, J.; Schweigert, F.J.; Kohn, B. C-reactive protein concentration in dogs with primary immune-mediated hemolytic anemia. Vet. Clin. Pathol. 2009, 38, 421–425. [Google Scholar] [CrossRef]
- Lowrie, M.; Penderis, J.; Eckersall, P.D.; McLaughlin, M.; Mellor, D.; Anderson, T.J. The role of acute phase proteins in diagnosis and management of steroid-responsive meningitis arteritis in dogs. Vet. J. 2009, 182, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Mysler, E.; Psioni, C.; Tate, P.; Tate, G. Influence of corticosteroids on C-reactive protein in patients with rheumatoid arthritis. Arthritis Res. Ther. 2004, 6, 57. [Google Scholar] [CrossRef]
- Ohno, K.; Yokoyama, Y.; Nakashima, K.; Setoguchi, A.; Fujino, Y.; Tsujimoto, H. C-reactive protein concentration in canine idiopathic polyarthritis. J. Vet. Med. Sci. 2006, 68, 1275–1279. [Google Scholar] [CrossRef]
- Sato, T.; Ohno, K.; Tamamoto, T.; Oishi, M.; Kanemoto, H.; Fukushima, K.; Goto-Koshino, Y.; Takahashi, M.; Tsujimoto, H. Assessment of severity and changes in C-reactive protein concentration and various biomarkers in dogs with pancreatitis. J. Vet. Med. Sci. 2017, 79, 35–40. [Google Scholar] [CrossRef]
- Hillström, A.; Hagman, R.; Tvedten, H.; Kjelgaard-Hansen, M. Validation of a commercially available automated canine-specific immunoturbidimetric method for measuring canine C-reactive protein. Vet. Clin. Pathol. 2014, 43, 235–243. [Google Scholar] [CrossRef]
- Plickert, H.D.; Einspanier, R.; Arndt, G.; Brunnberg, L.; Kohn, B. Evaluation of a point-of-care test for canine C-reactive protein. Vet. Clin. Pathol. 2011, 40, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, N.; Suchodolski, J.S.; Steiner, J.M. Assessment of stability and determination of a reference range for canine C-reactive protein in serum. J. Vet. Intern. Med. 2006, 20, 791. [Google Scholar] [CrossRef]
- Kook, P.H.; Schellenberg, S.; Grest, P.; Reusch, C.E.; Corboz, L.; Glaus, T.M. Microbiological evaluation of gallbladder bile of healthy dogs and dogs with iatrogenic hypercortisolism: A pilot study. J. Vet. Intern. Med. 2010, 24, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, R.M.; Cranford, S.M.; Ambrus, A.; Grützner, N.; Schellenberg, S.; Ruaux, C.G.; Suchodolski, J.S.; Steiner, J.M. Validation of an enzyme-linked immunosorbent assay (ELISA) for the measurement of canine S100A12. Vet. Clin. Pathol. 2016, 45, 135–147. [Google Scholar] [CrossRef]
- Schellenberg, S.; Wenger, M.; Reusch, C.E.; Glaus, T.M. Course of hematological and biochemical changes during and after long-term hydrocortisone treatment in healthy Beagles. J. Vet. Int. Med. 2008, 22, 1476. [Google Scholar] [CrossRef][Green Version]
- Doumatey, A.P.; Zhou, J.; Adeyemo, A.; Rotimi, C. High sensitivity C-reactive protein (Hs-CRP) remains highly stable in long-term archived human serum. Clin. Biochem. 2014, 47, 315–318. [Google Scholar] [CrossRef]
- Carney, P.C.; Ruaux, C.G.; Suchodolski, J.S.; Steiner, J.M. Biological variability of C-reactive protein and specific pancreatic lipase immunoreactivity in apparently healthy dogs. J. Vet. Intern. Med. 2011, 25, 825–830. [Google Scholar] [CrossRef]
- Blum, C.A.; Nigro, N.; Schuetz, P.; Winzeler, B.; Arici, B.; Refardt, J.; Urwyler, S.A.; Briel, M.; Mueller, B.; Christ-Crain, M. Influence of glucocorticoids on markers of inflammation in community-acquired pneumonia. Endocr. Abstr. 2015, 37, EP5. [Google Scholar] [CrossRef]
- Raess, N.; Schuetz, P.; Cesana-Nigro, N.; Winzeler, B.; Urwyler, S.A.; Schaedelin, S.; Rodondi, N.; Blum, M.R.; Briel, M.; Mueller, B.; et al. Influence of prednisone on inflammatory biomarkers in community-acquired pneumonia: Secondary analysis of a randomized trial. J. Clin. Pharmacol. 2021, 61, 1406–1414. [Google Scholar] [CrossRef]
- Kuribayashi, T.; Shimada, T.; Matsumoto, M.; Kawato, K.; Honjyo, T.; Fukuyama, M.; Yamamoto, Y.; Yamamoto, S. Determination of serum C-reactive protein (CRP) in healthy beagle dogs of various ages and pregnant beagle dogs. Exp. Anim. 2003, 52, 387–390. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heilmann, R.M.; Grützner, N.; Kook, P.H.; Schellenberg, S.; Suchodolski, J.S.; Steiner, J.M. High-Dose Hydrocortisone Treatment Does Not Affect Serum C-Reactive Protein (CRP) Concentrations in Healthy Dogs. Vet. Sci. 2023, 10, 620. https://doi.org/10.3390/vetsci10100620
Heilmann RM, Grützner N, Kook PH, Schellenberg S, Suchodolski JS, Steiner JM. High-Dose Hydrocortisone Treatment Does Not Affect Serum C-Reactive Protein (CRP) Concentrations in Healthy Dogs. Veterinary Sciences. 2023; 10(10):620. https://doi.org/10.3390/vetsci10100620
Chicago/Turabian StyleHeilmann, Romy M., Niels Grützner, Peter H. Kook, Stefan Schellenberg, Jan S. Suchodolski, and Joerg M. Steiner. 2023. "High-Dose Hydrocortisone Treatment Does Not Affect Serum C-Reactive Protein (CRP) Concentrations in Healthy Dogs" Veterinary Sciences 10, no. 10: 620. https://doi.org/10.3390/vetsci10100620
APA StyleHeilmann, R. M., Grützner, N., Kook, P. H., Schellenberg, S., Suchodolski, J. S., & Steiner, J. M. (2023). High-Dose Hydrocortisone Treatment Does Not Affect Serum C-Reactive Protein (CRP) Concentrations in Healthy Dogs. Veterinary Sciences, 10(10), 620. https://doi.org/10.3390/vetsci10100620








