Bevacizumab Efficiently Inhibits VEGF-Associated Cellular Processes in Equine Umbilical Vein Endothelial Cells: An In Vitro Characterization
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drug Preparation
2.2. ELISA for EqUVEC VEGF Expression
2.3. Cell Culture
2.4. EqUVEC Cell Viability
2.5. EqUVEC Cell Proliferation
2.6. EqUVEC Tube Formation
2.7. EqUVEC Cell Migration
2.8. Statistics
3. Results
3.1. Bevacizumab Significantly Decreased EqUVEC VEGF Tissue Expression
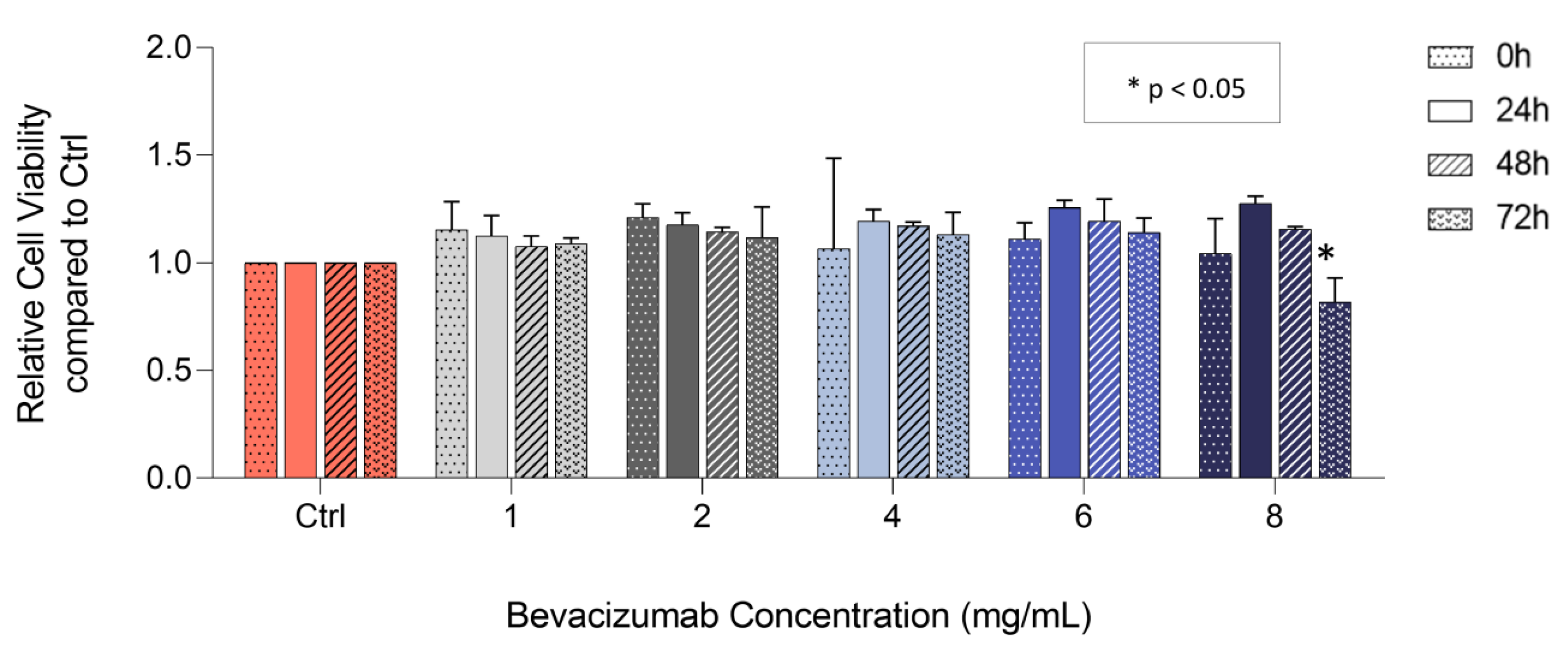
3.2. Bevacizumab Significantly Decreased EqUVEC Cell Viability at Higher Concentrations
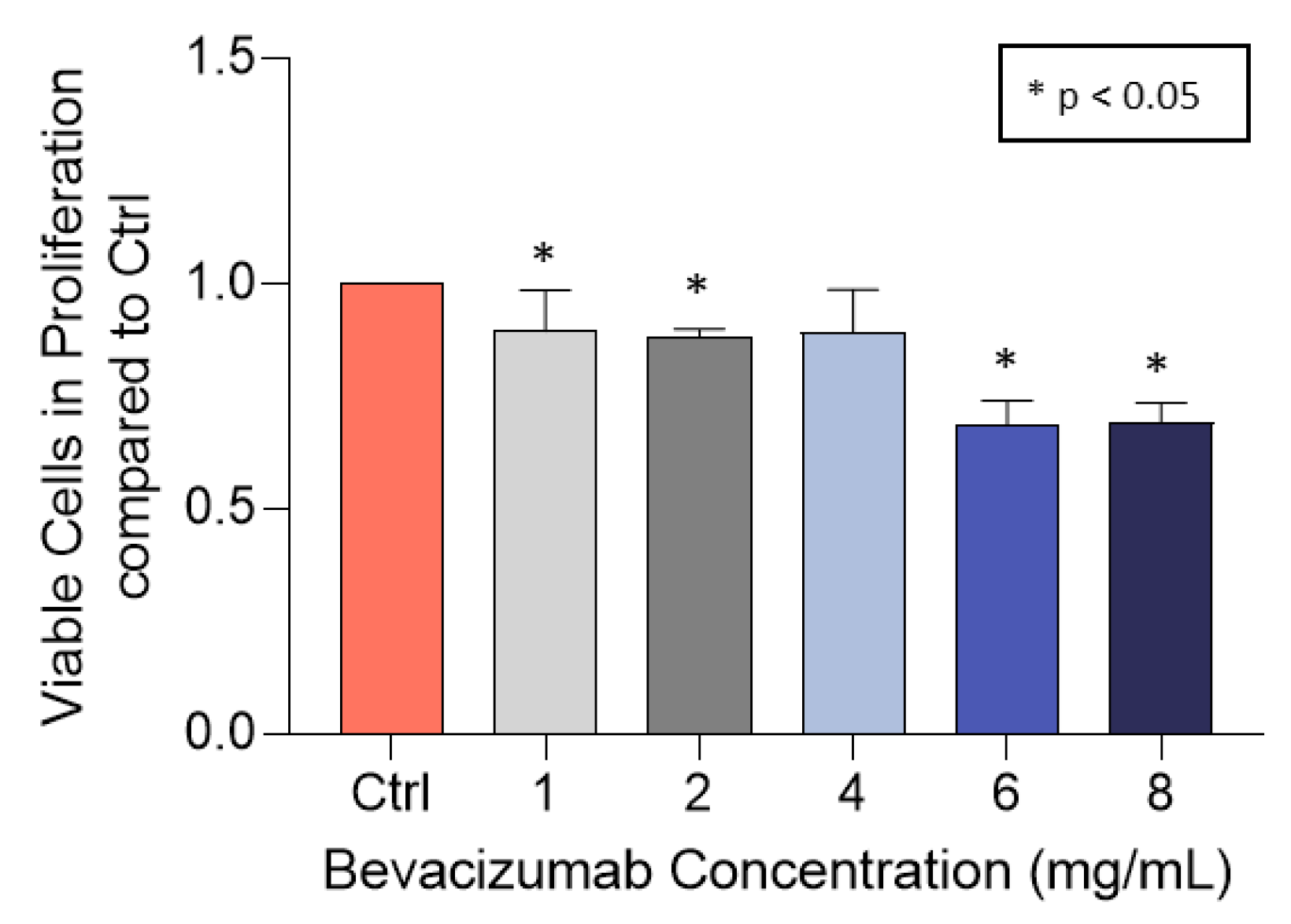
3.3. Bevacizumab Significantly Decreased EqUVEC Cell Proliferation
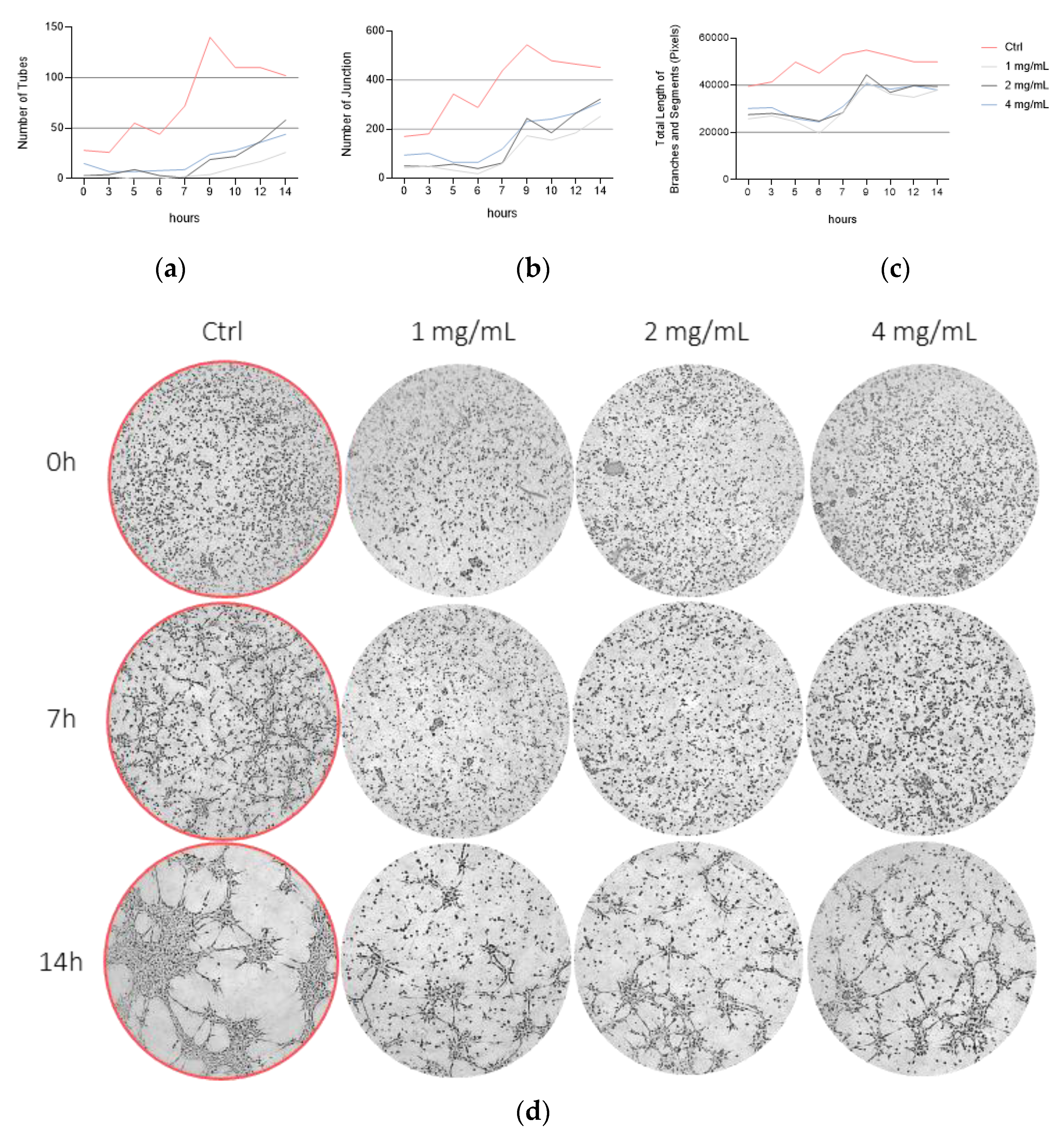
3.4. Bevacizumab Inhibited EqUVEC Tube Formation
3.5. Bevacizumab Significantly Inhibited EqUVEC Cell Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Gong, Y.; Liu, L.; Zhou, Y.; Fang, X.; Zhang, C.; Li, Y.; Li, J. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: A review. J. Appl. Toxicol. 2017, 37, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhu, Y.; Xing, C.; Li, S.; Bao, Z.; Lei, L.; Lin, D.; Wang, Y.; Chen, H.; Xu, X. An injectable thermosensitive hydrogel for dual delivery of diclofenac and Avastin® to effectively suppress inflammatory corneal neovascularization. Int. J. Pharm. 2022, 625, 122081. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis. Annu. Rev. Med. 2006, 57, 1–18. [Google Scholar] [CrossRef]
- Rieger, J.; Kaessmeyer, S.; Al Masri, S.; Hünigen, H.; Plendl, J. Endothelial cells and angiogenesis in the horse in health and disease—A review. Anat. Histol. Embryol. 2020, 49, 656–678. [Google Scholar] [CrossRef] [PubMed]
- Amano, S. Requirement for Vascular Endothelial Growth Factor in Wound- and Inflammation-Related Corneal Neovascularization. Investig. Ophthalmol. Vis. Sci. 1998, 18–22. [Google Scholar]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Avery, R.L. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina 2006, 26, 352–354. [Google Scholar] [CrossRef]
- Bashshur, Z.F.; Bazarbachi, A.; Schakal, A.; Haddad, Z.A.; El Haibi, C.P.; Noureddin, B.N. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am. J. Ophthalmol. 2006, 142, 1–9. [Google Scholar] [CrossRef]
- Hosseini, H. Anti-VEGF therapy with bevacizumab for anterior segment eye disease. Cornea 2012, 31, 322–334. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Blakey, D.C.; Westwood, F.R.; Walker, M.; Hughes, G.D.; Davis, P.D.; Ashton, S.E.; Ryan, A.J. Antitumor activity of the novel vascular targeting agent ZD6126 in a panel of tumor models. Clin. Cancer Res. 2002, 8, 1974–1983.34. [Google Scholar]
- Vasudev, N.S.; Reynolds, A.R. Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. Angiogenesis 2014, 17, 471–494. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S. A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int. J. Biol. Macromol. 2022, 221, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Bodaan, C.J.; Wise, L.M.; Wakelin, K.A.; Stuart, G.S.; Real, N.C.; Mercer, A.A.; Riley, C.B.; Theoret, C. Short-term treatment of equine wounds with orf virus IL-10 and VEGF-E dampens inflammation and promotes repair processes without accelerating closure. Wound Repair Regen. 2016, 24, 966–980. [Google Scholar] [CrossRef]
- Brooks, A.C.; Menzies-Gow, N.; Bailey, S.R.; Cunningham, F.M.; Elliott, J. Endotoxin-induced HIF-1alpha stabilisation in equine endothelial cells: Synergistic action with hypoxia. Inflamm. Res. 2010, 59, 689–698. [Google Scholar] [CrossRef]
- Kovac, M.; Litvin, Y.A.; Aliev, R.O.; Zakirova, E.Y.; Rutland, C.S.; Kiyasov, A.P.; Rizvanov, A.A. Gene Therapy Using Plasmid DNA Encoding VEGF164 and FGF2 Genes: A Novel Treatment of Naturally Occurring Tendinitis and Desmitis in Horses. Front. Pharmacol. 2018, 9, 978. [Google Scholar] [CrossRef]
- Martano, M.; Power, K.; Restucci, B.; Pagano, I.; Altamura, G.; Borzacchiello, G.; Maiolino, P. Expression of vascular endothelial growth factor (VEGF) in equine sarcoid. BMC Vet. Res. 2018, 14, 266. [Google Scholar] [CrossRef]
- Moore, C.P.; Halenda, R.M.; Grevan, V.L.; Collins, B.K. Post traumatic keratouveitis in horses. Equine Vet. J. 1998, 30, 366–372. [Google Scholar] [CrossRef]
- Müller, K.; Ellenberger, C.; Hoppen, H.-O.; Schoon, H.-A. Immunohistochemical study of angiogenesis and angiogenic factors in equine granulosa cell tumours. Res. Vet. Sci. 2012, 92, 471–477. [Google Scholar] [CrossRef]
- Wakelin, K.A. Viral Proteins as Novel Therapeutics in Chronic Horse Wounds. Bachelor Thesis, University of Otago, Otago, New Zealand, 2015. [Google Scholar]
- Wise, L.M.; Bodaan, C.J.; Stuart, G.S.; Real, N.C.; Lateef, Z.; Mercer, A.A.; Riley, C.B.; Theoret, C.L. Treatment of limb wounds of horses with orf virus IL-10 and VEGF-E accelerates resolution of exuberant granulation tissue, but does not prevent its development. PLoS ONE 2018, 13, e0197223. [Google Scholar] [CrossRef] [PubMed]
- Muellerleile, L.-M.; Bernkopf, M.; Wambacher, M.; Nell, B. Topical bevacizumab for the treatment of corneal vascularization in dogs: A case series. Vet. Ophthalmol. 2021, 24, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ-A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of In Vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- Ozel, I.; Duerig, I.; Domnich, M.; Lang, S.; Pylaeva, E.; Jablonska, J. The Good, the Bad, and the Ugly: Neutrophils, Angiogenesis, and Cancer. Cancers 2022, 14, 536. [Google Scholar] [CrossRef] [PubMed]
- Stryker, Z.I.; Rajabi, M.; Davis, P.J.; Mousa, S.A. Evaluation of Angiogenesis Assays. Biomedicines 2019, 7, 37. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Asena, L.; Dursun Altınörs, D. Topical Bevacizumab for the Treatment of Ocular Surface Squamous Neoplasia. J. Ocul. Pharmacol. Ther. 2015, 31, 487–490. [Google Scholar] [CrossRef]
- Faramarzi, A.; Feizi, S. Subconjunctival Bevacizumab Injection for Ocular Surface Squamous Neoplasia. Cornea 2013, 32, 998–1001. [Google Scholar] [CrossRef]
- Finger, P.T.; Chin, K.J. Refractory squamous cell carcinoma of the conjunctiva treated with subconjunctival ranibizumab (Lucentis): A two-year study. Ophthalmic Plast. Reconstr. Surg. 2012, 28, 85–89. [Google Scholar] [CrossRef]
- Paul, S.; Stone, D.U. Intralesional bevacizumab use for invasive ocular surface squamous neoplasia. J. Ocul. Pharmacol. Ther. 2012, 28, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.C.; Chin, K.J.; Finger, P.T. Subconjunctival ranibizumab for squamous cell carcinoma of the conjunctiva with corneal extension. Br. J. Ophthalmol. 2009, 93, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, E.A. Equine Ocular Adnexal and Nasolacrimal Disease. In Equine Ophthalmology, 2nd ed.; Gilger Brian, C., Ed.; Elsevier: Philadelphia, PA, USA, 2011; pp. 133–180. [Google Scholar]
- Lavach, J.D.; Severin, G.A. Neoplasia of the equine eye, adnexa, and orbit: A review of 68 cases. J. Am. Vet. Med. Assoc. 1977, 170, 202–203. [Google Scholar] [PubMed]
- Sykora, S.; Brandt, S. Papillomavirus infection and squamous cell carcinoma in horses. Vet. J. 2017, 223, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Muellerleile, L.-M.; Buxbaum, B.; Nell, B.; Fux, D.A. In-Vitro binding analysis of anti-human vascular endothelial growth factor antibodies bevacizumab and aflibercept with canine, feline, and equine vascular endothelial growth factor. Res. Vet. Sci. 2019, 124, 233–238. [Google Scholar] [CrossRef]
- Sadick, H.; Schäfer, E.; Weiss, C.; Rotter, N.; Müller, C.E.; Birk, R.; Sadick, M.; Häussler, D. An In Vitro study on the effect of bevacizumab on endothelial cell proliferation and VEGF concentration level in patients with hereditary hemorrhagic telangiectasia. Exp. Ther. Med. 2022, 24, 555. [Google Scholar] [CrossRef]
- Technical Bulletin. CellTiter 96® Aqueous One Solution Cell Proliferation Assay; Promega Corporation: Madison, WI, USA. Available online: https://www.promega.com/-/media/files/resources/protocols/technical-bulletins/0/celltiter-96-aqueous-one-solution-cell-proliferation-assay-system-protocol.pdf (accessed on 21 October 2023).
- Färkkilä, A.; Pihlajoki, M.; Tauriala, H.; Bützow, R.; Leminen, A.; Unkila-Kallio, L.; Heikinheimo, M.; Anttonen, M. Serum vascular endothelial growth factor A (VEGF) is elevated in patients with ovarian granulosa cell tumor (GCT), and VEGF inhibition by bevacizumab induces apoptosis in GCT In Vitro. J. Clin. Endocrinol. Metab. 2011, 96, E1973-81. [Google Scholar] [CrossRef]
- Koenig, Y.; Bock, F.; Horn, F.; Kruse, F.; Straub, K.; Cursiefen, C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes. Arch. Clin. Exp. Ophthalmol. 2009, 247, 1375–1382. [Google Scholar] [CrossRef]
- Lynch, S.S.; Cheng, C.M. Bevacizumab for neovascular ocular diseases. Ann. Pharmacother. 2007, 41, 614–625. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, H.; Blobe, G.C.; Theuer, C.P.; Hurwitz, H.I.; Nixon, A.B. Effects of the combination of TRC105 and bevacizumab on endothelial cell biology. Investig. New Drugs 2014, 32, 851–859. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Sun, J.-G.; Yao, J.; Shan, K.; Liu, B.-H.; Yao, M.-D.; Ge, H.-M.; Jiang, Q.; Zhao, C.; Yan, B. Effect of nanoencapsulation using poly (lactide-co-glycolide) (PLGA) on anti-angiogenic activity of bevacizumab for ocular angiogenesis therapy. Biomed. Pharmacother. 2018, 107, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Baudin, B.; Bruneel, A.; Bosselut, N.; Vaubourdolle, M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat. Protoc. 2007, 2, 481–485. [Google Scholar] [CrossRef] [PubMed]



| 0 h | 24 h | 48 h | 72 h | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bevacizumab Concentration | VEGF (pg/mL) | 95% CI 1 | IQR 2 | p-Value | VEGF (pg/mL) | 95% CI 1 | IQR 2 | p- Value | VEGF (pg/mL) | 95% CI 1 | IQR 2 | p- Value | VEGF (pg/mL) | 95% CI 1 | IQR 2 | p-Value |
| Control | 7.01 | 6.86–7.16 | 0.13 | 7.81 | 7.71–7.91 | 0.09 | 8.34 | 8.22–8.46 | 0.10 | 9.32 | 9.03–9.61 | 0.23 | ||||
| 1 mg/mL | 7.30 | 7.14–7.46 | 0.12 | 0.19 | 7.05 | 6.68–7.42 | 0.30 | 0.03 * | 7.60 | 7.48–7.72 | 0.09 | <0.01 * | 7.63 | 7.43–7.83 | 0.16 | 0.02 * |
| 2 mg/mL | 7.09 | 6.36–7.82 | 0.64 | 0.85 | 7.33 | 7.14–7.52 | 0.16 | 0.05 * | 7.75 | 7.66–7.84 | 0.08 | 0.03 * | 7.78 | 7.52–8.04 | 0.22 | 0.01 * |
| 4 mg/mL | 7.56 | 7.19–7.93 | 0.32 | 0.15 | 7.49 | 7.22–7.94 | 0.23 | 0.11 | 8.20 | 7.90–8.50 | 0.27 | 0.58 | 7.47 | 7.27–7.67 | 0.16 | 0.02 * |
| 6 mg/mL | 6.67 | 6.40–6.94 | 0.24 | 0.12 | 7.02 | 6.82–7.22 | 0.18 | 0.02 * | 7.58 | 7.56–7.60 | 0.02 | <0.01 * | 7.99 | 7.61–8.37 | 0.30 | 0.02 * |
| 8 mg/mL | 6.80 | 6.44–7.17 | 0.28 | 0.30 | 7.05 | 6.80–7.30 | 0.21 | 0.05 * | 7.23 | 7.11–7.36 | 0.11 | 0.01 * | 7.81 | 7.43–8.19 | 0.34 | 0.02 * |
| 24 h | 48 h | 72 h | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bevacizumab Concentration | Relative VEGF Level | 95% CI 1 | IQR 2 | p-Value | Relative VEGF Level | 95% CI 1 | IQR 2 | p-Value | Relative VEGF Level | 95% CI 1 | IQR 2 | p-Value |
| Control | 1.00 | 1.00 | 1.00 | |||||||||
| 1 mg/mL | 0.90 | 0.85–0.95 | 0.04 | 0.06 | 0.91 | 0.89–0.93 | 0.01 | 0.01 * | 0.82 | 0.79–0.85 | 0.02 | <0.01 * |
| 2 mg/mL | 0.94 | 0.91–0.97 | 0.02 | 0.04 * | 0.93 | 0.92–0.94 | 0.01 | 0.01 * | 0.83 | 0.80–0.87 | 0.03 | 0.01 * |
| 4 mg/mL | 0.96 | 0.92–0.99 | 0.03 | 0.15 | 0.98 | 0.94–1.02 | 0.04 | 0.46 | 0.80 | 0.77–0.83 | 0.02 | <0.01 * |
| 6 mg/mL | 0.90 | 0.87–0.93 | 0.03 | 0.02 * | 0.91 | 0.90–0.91 | <0.01 | <0.01 * | 0.86 | 0.80–0.91 | 0.05 | 0.02 * |
| 8 mg/mL | 0.90 | 0.87–0.94 | 0.03 | 0.03 * | 0.87 | 0.85–0.89 | 0.02 | <0.01 * | 0.84 | 0.78–0.89 | 0.05 | 0.02 * |
| 24 h | 48 h | 72 h | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bevacizumab Concentration | Relative VEGF Level | 95% CI 1 | IQR 2 | Relative VEGF Level | 95% CI 1 | IQR 2 | Relative VEGF Level | 95% CI 1 | IQR 2 |
| Control | 1.00 | 1.00 | 1.00 | ||||||
| 1 mg/mL | 1.13 | 1.08–1.17 | 0.04 | 1.08 | 1.06–1.10 | 0.02 | 1.09 | 1.08–1.10 | 0.01 |
| 2 mg/mL | 1.18 | 1.15–1.20 | 0.02 | 1.14 | 1.13–1.15 | 0.01 | 1.12 | 1.06–1.18 | 0.05 |
| 4 mg/mL | 1.20 | 1.17–1.22 | 0.02 | 1.17 | 1.16–1.18 | 0.01 | 1.13 | 1.09–1.18 | 0.04 |
| 6 mg/mL | 1.26 | 1.24–1.27 | 0.01 | 1.19 | 1.15–1.24 | 0.04 | 1.14 | 1.11–1.17 | 0.03 |
| 8 mg/mL | 1.28 | 1.26–1.29 | 0.01 | 1.16 | 1.15–1.16 | 0.00 | 0.82 * | 0.77–0.87 | 0.04 |
| 72 h | ||||
|---|---|---|---|---|
| Bevacizumab Concentration | Relative VEGF Level | 95% CI 1 | IQR 2 | p-Value |
| Control | 1.00 | |||
| 1 mg/mL | 0.89 | 0.85–0.94 | 0.04 | 0.04 * |
| 2 mg/mL | 0.88 | 0.87–0.89 | 0.01 | <0.01 * |
| 4 mg/mL | 0.89 | 0.85–0.94 | 0.04 | 0.04 * |
| 6 mg/mL | 0.68 | 0.66–0.71 | 0.02 | <0.01 * |
| 8 mg/mL | 0.69 | 0.67–0.71 | 0.02 | <0.01 * |
| 0 h | 24 h | 48 h | 72 h | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bevacizumab Concentration | Area (%) | 95% CI 1 | IQR 2 | p-Value | Area (%) | 95% CI 1 | IQR 2 | p-Value | Area (%) | 95% CI 1 | IQR 2 | p-Value | Area (%) | 5% CI 1 | IQR 2 | p-Value |
| Control | 54.20 | 47.44–60.96 | 7.12 | 13.23 | 10.51–15.95 | 3.85 | 0.13 | 0.05–0.21 | 5.56 | 0.00 | - | - | ||||
| 1 mg/mL | 49.00 | 44.91–53.08 | 2.35 | 0.30 | 29.74 | 27.09–32.4 | 7.49 | <0.01 * | 10.57 | 8.99–12.15 | 3.21 | <0.01 * | 1.49 | 0.95–2.02 | 0.80 | <0.01 * |
| 2 mg/mL | 47.35 | 44.97–49.74 | 3.84 | 0.17 | 35.27 | 31.94–38.61 | 5.32 | <0.01 * | 19.93 | 17.05–22.8 | 8.41 | <0.01 * | 4.83 | 3.97–5.69 | 0.87 | <0.01 * |
| 4 mg/mL | 47.51 | 44.42–50.60 | 6.35 | 0.20 | 41.40 | 39.23–43.56 | 3.85 | <0.01 * | 29.64 | 27.28–32.00 | 4.58 | <0.01 * | 16.30 | 13.07–19.53 | 7.75 | <0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lessiak, U.; Pratscher, B.; Tichy, A.; Nell, B. Bevacizumab Efficiently Inhibits VEGF-Associated Cellular Processes in Equine Umbilical Vein Endothelial Cells: An In Vitro Characterization. Vet. Sci. 2023, 10, 632. https://doi.org/10.3390/vetsci10110632
Lessiak U, Pratscher B, Tichy A, Nell B. Bevacizumab Efficiently Inhibits VEGF-Associated Cellular Processes in Equine Umbilical Vein Endothelial Cells: An In Vitro Characterization. Veterinary Sciences. 2023; 10(11):632. https://doi.org/10.3390/vetsci10110632
Chicago/Turabian StyleLessiak, Ulrike, Barbara Pratscher, Alexander Tichy, and Barbara Nell. 2023. "Bevacizumab Efficiently Inhibits VEGF-Associated Cellular Processes in Equine Umbilical Vein Endothelial Cells: An In Vitro Characterization" Veterinary Sciences 10, no. 11: 632. https://doi.org/10.3390/vetsci10110632
APA StyleLessiak, U., Pratscher, B., Tichy, A., & Nell, B. (2023). Bevacizumab Efficiently Inhibits VEGF-Associated Cellular Processes in Equine Umbilical Vein Endothelial Cells: An In Vitro Characterization. Veterinary Sciences, 10(11), 632. https://doi.org/10.3390/vetsci10110632






