Phospholipase C Zeta 1 (PLCZ1): The Function and Potential for Fertility Assessment and In Vitro Embryo Production in Cattle and Horses
Abstract
Simple Summary
Abstract
1. Introduction
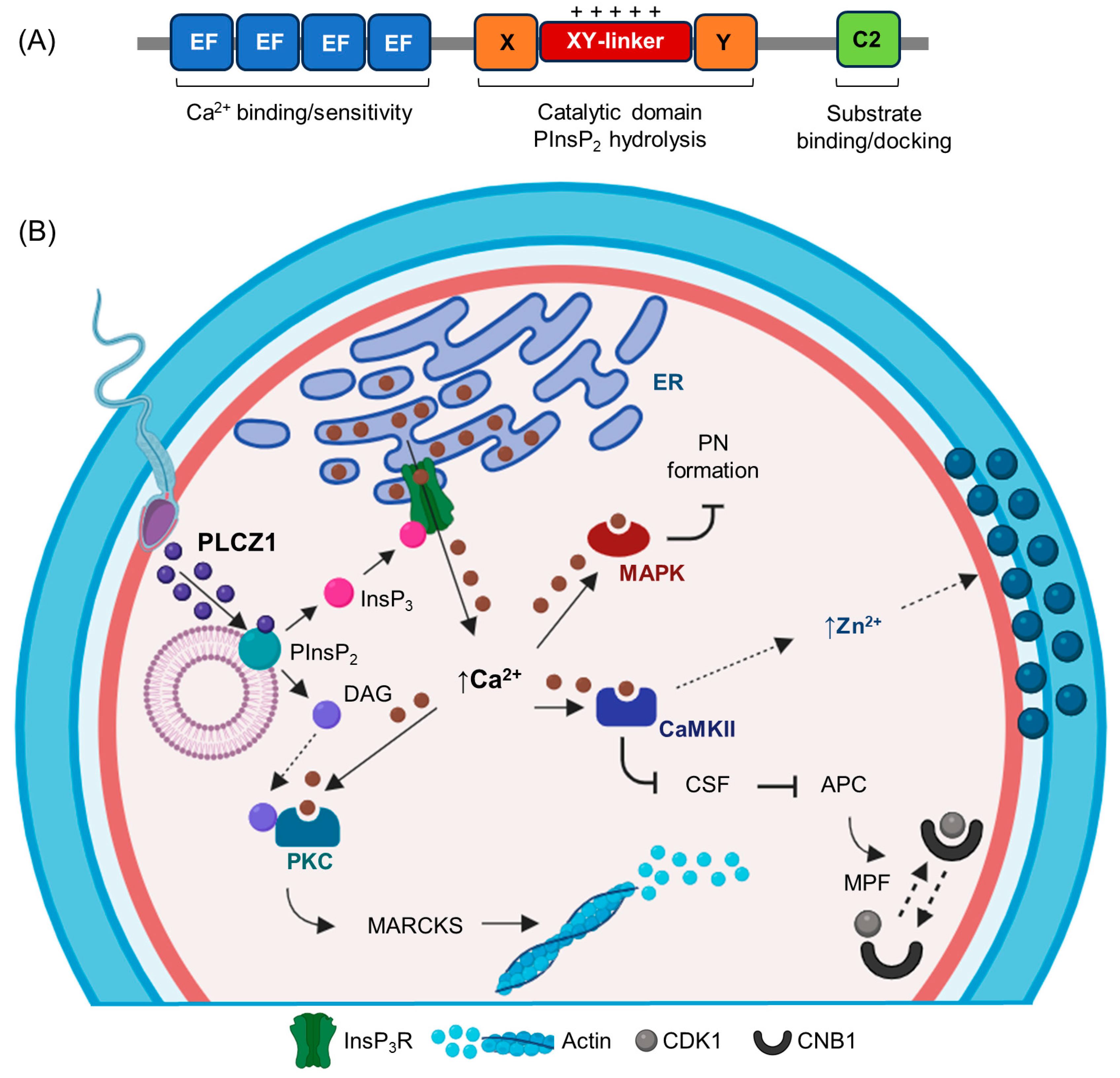
2. The Structure and Domain Organization of PLCZ1
3. The Oocyte Activation Function and Sperm Localization of PLCZ1
4. Equine PLCZ1
5. Bovine PLCZ1
6. The Evaluation of PLCZ1 as Diagnostic Tool to Predict Fertilization
7. The Potential Therapeutic Role of PLCZ1 for Artificial Oocyte Activation
| Species | Features Analyzed | Outcome |
|---|---|---|
| Human [33] | IF for PLCZ1 localization patterns Genomic analysis for PLCZ1 mutations Fertilization after ICSI | Clinical trial. <30% PLCZ1-positive sperm associated with fertilization failure. Gene mutations impaired sperm theca formation and PLCZ1. AOA improved fertilization in PLCZ1-negative sperm. Limited to couples with a history of ≤10% fertilization. |
| Human [19] | IF for PLCZ1 localization patterns Electron microscopy Sperm chromatin structure analysis FISH for aneuploidy assessment Fertilization after ICSI | Clinical trial. Multiparametric analysis. Sperm PLCZ1 is associated with fertilization. Gene mutations impaired sperm theca formation and PLCZ1. AOA improved fertilization in PLCZ1-reduced or -negative sperm. Limited to globozoospermic patients. |
| Human [32] | IF for PLCZ1 localization patterns Fertilization after ICSI | Clinical trial. Higher sperm PLCZ1 was associated with high fertilization rates. AOA improved fertilization in PLCZ1-reduced sperm. |
| Human [37] | IB for PLCZ1 abundance qPCR for mRNA PLCZ1 Genomic analysis for PLCZ1 mutations Fertilization after ICSI | Clinical trial. PLCZ1 mutations and impaired PLCZ1 protein was associated with male infertility. AOA improved fertilization in men carrying PLCZ1 mutations. |
| Human [31] | IB for PLCZ1 abundance IF for PLCZ1 localization patterns Genomic analysis for PLCZ1 mutations Fertilization after ICSI | Clinical trial. PLCZ1 absence related to fertilization failure. Gene mutations impaired PLCZ1 function. AOA improved fertilization in PLCZ1 mutations and resulted in birth. |
| Human [25] | IB for PLCZ1 abundance IF for PLCZ1 localization patterns Genomic analysis for PLCZ1 mutations Fertilization after ICSI | Experimental model. PLCZ1 absence related to fertilization failure. Gene mutations impaired PLCZ1 abundance, size, and function. AOA rescued oocyte activation using human PLCZ1-impaired sperm in a heterologous ICSI model. |
| Mouse [20] | IF for sperm exhibiting PLCZ1 Genomic analysis for PLCZ1 knockout Fertilization after ICSI and births | Experimental model. PLCZ1-knockout sperm does not activate oocytes. Activation by mRNA PLCZ1 or AOA rescued oocyte activation of PLCZ1-knockout sperm, resulting in births. |
| Mouse [50] | IF for sperm exhibiting PLCZ1 Gene knockout Fertilization after ICSI and births | Experimental model. PLCZ1-knockout sperm did not activate oocytes. Activation by mRNA PLCZ1 rescued oocyte activation of PLCZ1-knockout sperm resulting in births. |
| Mouse [133] | Oocyte activation test Fertilization after ICSI using chemically inactivated sperm and births | Experimental model. PLCZ1-inactivated sperm did not activate oocytes. Activation by equine mRNA PLCZ1 rescued oocyte activation of PLCZ1-inactivated sperm resulting in births. |
| Mouse [196] | IF for sperm exhibiting PLCZ1 Fertilization after ICSI using round spermatids | Experimental model. Activation by human mRNA PLCZ1 plus auxin-inducible degron technology improved oocyte activation of round spermatids resulting in births. |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dozortsev, D.; Qian, C.; Ermilov, A.; Rybouchkin, A.; De Sutter, P.; Dhont, M. Sperm-Associated Oocyte-Activating Factor Is Released from the Spermatozoon within 30 min after Injection as a Result of the Sperm-Oocyte Interaction. Hum. Reprod. 1997, 12, 2792–2796. [Google Scholar] [CrossRef] [PubMed]
- Stricker, S.A. Comparative Biology of Calcium Signaling during Fertilization and Egg Activation in Animals. Dev. Biol. 1999, 211, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Malcuit, C.; Kurokawa, M.; Fissore, R.A. Calcium Oscillations and Mammalian Egg Activation. J. Cell Physiol. 2006, 206, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.J.; Larman, M.G.; Saunders, C.M.; Hashimoto, K.; Swann, K.; Lai, F.A. Sperm Phospholipase Cζ from Humans and Cynomolgus Monkeys Triggers Ca2+ Oscillations, Activation and Development of Mouse Oocytes. Reproduction 2002, 124, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.M.; Larman, M.G.; Parrington, J.; Cox, L.J.; Royse, J.; Blayney, L.M.; Swann, K.; Lai, F.A. PLC Zeta: A Sperm-Specific Trigger of Ca(2+) Oscillations in Eggs and Embryo Development. Development 2002, 129, 3533–3544. [Google Scholar] [CrossRef] [PubMed]
- Knott, J.G.; Kurokawa, M.; Fissore, R.A. Release of the Ca2+ Oscillation-Inducing Sperm Factor during Mouse Fertilization. Dev. Biol. 2003, 260, 536–547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kouchi, Z.; Fukami, K.; Shikano, T.; Oda, S.; Nakamura, Y.; Takenawa, T.; Miyazaki, S. Recombinant Phospholipase Cζ Has High Ca2+ Sensitivity and Induces Ca2+ Oscillations in Mouse Eggs. Biochemistry 2004, 279, 10408–10412. [Google Scholar] [CrossRef] [PubMed]
- Swann, K.; Saunders, C.M.; Rogers, N.T.; Lai, F.A. PLCζ(Zeta): A Sperm Protein That Triggers Ca2+oscillations and Egg Activation in Mammals. Semin. Cell Dev. Biol. 2006, 17, 264–273. [Google Scholar] [CrossRef]
- Nomikos, M.; Blayney, L.M.; Larman, M.G.; Campbell, K.; Rossbach, A.; Saunders, C.M.; Swann, K.; Lai, F.A. Role of Phospholipase C-ζ Domains in Ca2+-Dependent Phosphatidylinositol 4,5-Bisphosphate Hydrolysis and Cytoplasmic Ca2+ Oscillations. J. Biol. Chem. 2005, 280, 31011–31018. [Google Scholar] [CrossRef]
- Swann, K.; Windsor, S.; Campbell, K.; Elgmati, K.; Nomikos, M.; Zernicka-Goetz, M.; Amso, N.; Lai, F.A.; Thomas, A.; Graham, C. Phospholipase C-ζ-Induced Ca2+ Oscillations Cause Coincident Cytoplasmic Movements in Human Oocytes that Failed to Fertilize after Intracytoplasmic Sperm Injection. Fertil. Steril. 2012, 97, 742–747. [Google Scholar] [CrossRef]
- Nomikos, M.; Yu, Y.; Elgmati, K.; Theodoridou, M.; Campbell, K.; Vassilakopoulou, V.; Zikos, C.; Livaniou, E.; Amso, N.; Nounesis, G.; et al. Phospholipase Cζ Rescues Failed Oocyte Activation in a Prototype of Male Factor Infertility. Fertil. Steril. 2013, 99, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Saunders, C.M.; Lai, F.A.; Swann, K. Preimplantation Development of Mouse Oocytes Activated by Different Levels of Human Phospholipase C Zeta. Hum. Reprod. 2008, 23, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Fissore, R.A. Release of Phospholipase C ζ and [Ca2+]i Oscillation-Inducing Activity during Mammalian Fertilization. Reproduction 2007, 134, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M. Novel Signalling Mechanism and Clinical Applications of Sperm-Specific PLCζ. Biochem. Soc. Trans. 2015, 43, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Kline, D.; Kline, J.T. Repetitive Calcium Transients and the Role of Calcium in Exocytosis and Cell Cycle Activation in the Mouse Egg. Dev. Biol. 1992, 149, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Ducibella, T.; Huneau, D.; Angelichio, E.; Xu, Z.; Schultz, R.M.; Kopf, G.S.; Fissore, R.; Madoux, S.; Ozil, J.-P. Egg-to-Embryo Transition Is Driven by Differential Responses to Ca2+ Oscillation Number. Dev. Biol. 2002, 250, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Yassine, S.; Escoffier, J.; Martinez, G.; Coutton, C.; Karaouzéne, T.; Zouari, R.; Ravanat, J.L.; Metzler-Guillemain, C.; Lee, H.C.; Fissore, R.; et al. Dpy19l2-Deficient Globozoospermic Sperm Display Altered Genome Packaging and DNA Damage That Compromises the Initiation of Embryo Development. Mol. Hum. Reprod. 2014, 21, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Z.; Jia, W.; Hou, M.; Zhang, X. Actl7a Deficiency in Mice Leads to Male Infertility and Fertilization Failure. Biochem. Biophys. Res. Commun. 2022, 623, 154–161. [Google Scholar] [CrossRef]
- Cheung, S.; Parrella, A.; Tavares, D.; Keating, D.; Xie, P.; Rosenwaks, Z.; Palermo, G.D. Single-Center Thorough Evaluation and Targeted Treatment of Globozoospermic Men. Reprod. Physiol. Dis. 2021, 38, 2073–2086. [Google Scholar] [CrossRef]
- Hirose, N.; Kikuchi, Y.; Kageyama, A.; Sugita, H.; Sakurai, M.; Kawata, Y.; Terakawa, J.; Wakayama, T.; Ito, J.; Kashiwazaki, N. Successful Production of Offspring Derived from Phospholipase C Zeta-Deficient Sperm by Additional Artificial Activation. Life 2023, 13, 980. [Google Scholar] [CrossRef]
- Wang, T.; Cao, B.; Cai, Y.; Chen, S.; Wang, B.; Yuan, Y.; Zhang, Q. PLCZ11 Deficiency Decreased Fertility in Male Mice Which Is Associated with Sperm Quality Decline and Abnormal Cytoskeleton in Epididymis. Int. J. Mol. Sci. 2023, 24, 314. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M.; Elgmati, K.; Theodoridou, M.; Calver, B.L.; Nounesis, G.; Swann, K.; Lai, F.A. Phospholipase Cζ Binding to PtdIns(4,5)P2 Requires the XY-Linker Region. J. Cell Sci. 2011, 124, 2582–2590. [Google Scholar] [CrossRef]
- Yu, Y.; Nomikos, M.; Theodoridou, M.; Nounesis, G.; Lai, F.A.; Swann, K. PLC Causes Ca2+ Oscillations in Mouse Eggs by Targeting Intracellular and Not Plasma Membrane PI(4,5)P2. Mol. Biol. Cell 2012, 23, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Heytens, E.; Schmitt-John, T.; Moser, J.M.; Jensen, N.M.; Soleimani, R.; Young, C.; Coward, K.; Parrington, J.; De Sutter, P. Reduced Fertilization after ICSI and Abnormal Phospholipase C Zeta Presence in Spermatozoa from the Wobbler Mouse. Reprod. Biomed. Online 2010, 21, 742–749. [Google Scholar] [CrossRef][Green Version]
- Heytens, E.; Parrington, J.; Coward, K.; Young, C.; Lambrecht, S.; Yoon, S.Y.; Fissore, R.A.; Hamer, R.; Deane, C.M.; Ruas, M.; et al. Reduced Amounts and Abnormal Forms of Phospholipase C Zeta (PLCζ) in Spermatozoa from Infertile Men. Hum. Reprod. 2009, 24, 2417–2428. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M.; Elgmati, K.; Theodoridou, M.; Calver, B.L.; Cumbes, B.; Nounesis, G.; Nounesis, G.; Swann, K.; Lai, F.A. Male Infertility-Linked Point Mutation Disrupts the Ca2+ Oscillation-Inducing and PIP(2) Hydrolysis Activity of Sperm PLCζ. Biochem. J. 2011, 434, 211–217. [Google Scholar] [CrossRef]
- Nomikos, M.; Stamatiadis, P.; Sanders, J.R.; Beck, K.; Calver, B.L.; Buntwal, L.; Lofty, M.; Sideratou, Z.; Swann, K.; Lai, F.A. Male Infertility-Linked Point Mutation Reveals a Vital Binding Role for the C2 Domain of Sperm PLCζ. Biochem. J. 2017, 474, 1003–1016. [Google Scholar] [CrossRef]
- Kashir, J.; Konstantinidis, M.; Jones, C.; Heindryckx, B.; De Sutter, P.; Parrington, J.; Wells, D.; Coward, K. Characterization of Two Heterozygous Mutations of the Oocyte Activation Factor Phospholipase C Zeta (PLCζ) from an Infertile Man by Use of Minisequencing of Individual Sperm and Expression in Somatic Cells. Fertil. Steril. 2012, 98, 423–431. [Google Scholar] [CrossRef]
- Escoffier, J.; Lee, H.C.; Yassine, S.; Zouari, R.; Martinez, G.; Karaouzène, T.; Coutton, C.; Kherraf, Z.E.; Halouani, L.; Triki, C.; et al. Homozygous Mutation of PLCZ1 Leads to Defective Human Oocyte Activation and Infertility That Is Not Rescued by the WW-Binding Protein PAWP. Hum. Mol. Genet. 2016, 25, 878–891. [Google Scholar] [CrossRef]
- Torra-Massana, M.; Rodríguez, A.; Vassena, R. Exonic Genetic Variants Associated with Unexpected Fertilization Failure and Zygotic Arrest after ICSI: A Systematic Review. Zygote 2023, 31, 316–341. [Google Scholar] [CrossRef]
- Torra-Massana, M.; Cornet-Bartolomé, D.; Barragán, M.; Durban, M.; Ferrer-Vaquer, A.; Zambelli, F.; Rodriguez, A.; Oliva, R.; Vassena, R. Novel Phospholipase C Zeta 1 Mutations Associated with Fertilization Failures after ICSI. Hum. Reprod. 2019, 34, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, H.; Azad, N.; Nazari, L.; Piryaei, A.; Heidari, M.H.; Masteri-Farahani, R.; Karimi, M.; Ghaffari-Novin, M. Effect of Artificial Oocyte Activation on Intra-Cytoplasmic Sperm Injection Outcomes in Patients with Lower Percentage of Sperm Containing Phospholipase Cζ: A Randomized Clinical Trial. J. Reprod. Infertil. 2019, 20, 3–9. [Google Scholar] [PubMed]
- Cheung, S.; Xie, P.; Parrella, A.; Keating, D.; Rosenwaks, Z.; Palermo, G.D. Identification and Treatment of Men with Phospholipase Cζ–Defective Spermatozoa. Fertil. Steril. 2020, 114, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Cardona Barberán, A.; Bonte, D.; Boel, A.; Thys, V.; Paredis, R.; Machtelinckx, F.; De Sutter, P.; De Croo, I.; Leybaert, L.; Stoop, D.; et al. Assisted Oocyte Activation Does Not Overcome Recurrent Embryo Developmental Problems. Hum. Reprod. 2023, 38, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Rahimizadeh, P.; Topraggaleh, T.R.; Nasr-Esfahani, M.H.; Ziarati, N.; Mirshahvaladi, S.; Esmaeili, V.; Seifi, S.; Eftekhari-Yazdi, P.; Shahverdi, A. The Alteration of PLCζ Protein Expression in Unexplained Infertile and Asthenoteratozoospermic Patients: A Potential Effect on Sperm Fertilization Ability. Mol. Reprod. Dev. 2020, 87, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tavalaee, M.; Nomikos, M.; Lai, F.A.; Nasr-Esfahani, M.H. Expression of Sperm PLCζ and Clinical Outcomes of ICSI-AOA in Men Affected by Globozoospermia Due to DPY19L2 Deletion. Reprod. Biomed. Online 2018, 36, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Zhang, Z.; Wu, L.; Fu, J.; Chen, B.; Yan, Z.; Li, B.; Zhou, Z.; Wang, W.; Zhao, L.; et al. The Identification of Novel Mutations in PLCZ1 Responsible for Human Fertilization Failure and a Therapeutic Intervention by Artificial Oocyte Activation. Mol. Hum. Reprod. 2020, 26, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huang, Y.; Li, B.; Zhang, T.; Niu, Y.; Hu, S.; Ding, Y.; Yao, G.; Wei, Z.; Yao, N.; et al. Novel Mutations in PLCZ1 Lead to Early Embryonic Arrest as a Male Factor. Front. Cell Dev. Biol. 2023, 11, 1193248. [Google Scholar] [CrossRef]
- Yuan, P.; Zheng, L.; Liang, H.; Lin, Q.; Ou, S.; Zhu, Y.; Lai, L.; Zhang, Q.; He, Z.; Wang, W. Novel Mutations in the PLCZ1 Gene Associated with Human Low or Failed Fertilization. Mol. Genet. Genom. Med. 2020, 8, e1470. [Google Scholar] [CrossRef]
- Dai, J.; Dai, C.; Guo, J.; Zheng, W.; Zhang, T.; Li, Y.; Lu, C.; Gong, F.; Lu, G.; Lin, G. Novel Homozygous Variations in PLCZ1 Lead to Poor or Failed Fertilization Characterized by Abnormal Localization Patterns of PLCζ in Sperm. Clin. Genet. 2020, 97, 347–351. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, Y.; Deng, K.; Shen, J.; Cui, Y.; Liu, J.; Yang, X.; Diao, F. Mutations in PLCZ1 Induce Male Infertility Associated with Polyspermy and Fertilization Failure. J. Assist. Reprod. Genet. 2023, 40, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, J.; Kong, S.; Li, C.; Zhang, Z.; He, X.; Wu, H.; Tang, D.; Zha, X.; Tan, Q.; et al. A Homozygous Nonsense Mutation of PLCZ1 Cause Male Infertility with Oocyte Activation Deficiency. J. Assist. Reprod. Genet. 2020, 37, 821–828. [Google Scholar] [CrossRef]
- Kashir, J.; Mistry, B.V.; BuSaleh, L.; Abu-Dawas, R.; Nomikos, M.; Ajlan, A.; Abu-Dawud, R.; AlYacoub, N.; AlHassan, S.; Lai, F.A.; et al. Phospholipase C Zeta Profiles Are Indicative of Optimal Sperm Parameters and Fertilisation Success in Patients Undergoing Fertility Treatment. Andrology 2020, 8, 1143–1159. [Google Scholar] [CrossRef]
- Tavalaee, M.; Kiani-Esfahani, A.; Nasr-Esfahani, M.H. Relationship between Potential Sperm Factors Involved in Oocyte Activation and Sperm DNA Fragmentation with Intra-Cytoplasmic Sperm Injection Clinical Outcomes. Cell J. 2016, 18, 588–596. [Google Scholar] [PubMed]
- Yelumalai, S.; Yeste, M.; Jones, C.; Amdani, S.N.; Kashir, J.; Mounce, G.; Da Silva, S.J.M.; Barratt, C.L.; McVeigh, E.; Coward, K. Total Levels, Localization Patterns, and Proportions of Sperm Exhibiting Phospholipase C Zeta Are Significantly Correlated with Fertilization Rates after Intracytoplasmic Sperm Injection. Fertil. Steril. 2015, 104, 561–568.e4. [Google Scholar] [CrossRef]
- Kamali-Dolat Abadi, M.; Tavalaee, M.; Shahverdi, A.; Nasr-Esfahani, M.H. Evaluation of PLCζ and PAWP Expression in Globozoospermic Individuals. Cell J. 2016, 18, 438–445. [Google Scholar] [PubMed]
- Suarez, S.S. Interactions of Gametes with the Female Reproductive Tract. Cell Tissue Res. 2016, 363, 185–194. [Google Scholar] [CrossRef]
- Suarez, S.S.; Pacey, A.A. Sperm Transport in the Female Reproductive Tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef]
- Hachem, A.; Godwin, J.; Ruas, M.; Lee, H.C.; Buitrago, M.F.; Ardestani, G.; Bassett, A.; Fox, S.; Navarrete, F.; De Sutter, P.; et al. Plcζ Is the Physiological Trigger of the Ca2+ Oscillations That Induce Embryogenesis in Mammals but Conception Can Occur in Its Absence. Development 2017, 144, 2914–2924. [Google Scholar] [CrossRef]
- Nozawa, K.; Satouh, Y.; Fujimoto, T.; Oji, A.; Ikawa, M. Sperm-Borne Phospholipase C Zeta-1 Ensures Monospermic Fertilization in Mice. Sci. Rep. 2018, 8, 1315. [Google Scholar] [CrossRef]
- Vanden Meerschaut, F.; Leybaert, L.; Nikiforaki, D.; Qian, C.; Heindryckx, B.; De Sutter, P. Diagnostic and Prognostic Value of Calcium Oscillatory Pattern Analysis for Patients with ICSI Fertilization Failure. Hum. Reprod. 2013, 28, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Souza Setti, A.; Ferreira, R.C.; Paes De Almeida Ferreira Braga, D.; De Cássia Sávio Figueira, R.; Iaconelli, A.; Borges, E. Intracytoplasmic Sperm Injection Outcome versus Intracytoplasmic Morphologically Selected Sperm Injection Outcome: A Meta-Analysis. Reprod. Biomed. Online 2010, 21, 450–455. [Google Scholar] [CrossRef][Green Version]
- Ruggeri, E.; Deluca, K.F.; Galli, C.; Lazzari, G.; Deluca, J.G.; Carnevale, E.M. Cytoskeletal Alterations Associated with Donor Age and Culture Interval for Equine Oocytes and Potential Zygotes That Failed to Cleave after Intracytoplasmic Sperm Injection. Reprod. Fertil. Dev. 2015, 27, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Colleoni, S.; Duchi, R.; Lazzari, G. Male Factors Affecting the Success of Equine In Vitro Embryo Production by Ovum Pickup-Intracytoplasmic Sperm Injection in a Clinical Setting. J. Equine Vet. Sci. 2016, 43, S6–S10. [Google Scholar] [CrossRef]
- Gonzalez-Castro, R.A.; Amoroso-Sanches, F.; Stokes, J.E.; Graham, J.K.; Carnevale, E.M. Localisation of Phospholipase Cζ1 (PLCZ1) and Postacrosomal WW-Binding Protein (WBP2 N-Terminal like) on Equine Spermatozoa and Flow Cytometry Quantification of PLCZ1 and Association with Cleavage in Vitro. Reprod. Fertil. Dev. 2019, 31, 1778–1792. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, S.; Lazzari, G.; Duchi, R.; Baca Castex, C.; Mari, G.; Lagutina, I.; Galli, C. Fertilization and Development of Oocytes after ICSI with Semen of Stallions with Different in Vivo Fertility. J. Equine Vet. Sci. 2012, 32, 408–409. [Google Scholar] [CrossRef]
- Gonzalez-Castro, R.; Carnevale, E. Association between Equine Sperm Parameters and ICSI Outcome. J. Equine Vet. Sci. 2016, 41, 62. [Google Scholar] [CrossRef]
- Yoneda, A.; Kashima, M.; Yoshida, S.; Terada, K.; Nakagawa, S.; Sakamoto, A.; Hayakawa, K.; Suzuki, K.; Ueda, J.; Watanabe, T. Molecular Cloning, Testicular Postnatal Expression, and Oocyte-Activating Potential of Porcine Phospholipase Cζ. Reproduction 2006, 132, 393–401. [Google Scholar] [CrossRef]
- Kurokawa, M.; Sato, K.I.; Wu, H.; He, C.; Malcuit, C.; Black, S.J.; Fukami, K.; Fissore, R.A. Functional, Biochemical, and Chromatographic Characterization of the Complete [Ca2+]i Oscillation-Inducing Activity of Porcine Sperm. Dev. Biol. 2005, 285, 376–392. [Google Scholar] [CrossRef]
- Atabay, E.P.; Tadeo, R.D.; Atabay, E.C.; Venturina, E.V.; Fissore, R.A.; Mingala, C.N. Molecular Characterization and Comparison of Phospholipase C Zeta (PLCZ1) Gene Between Swamp (Bubalus Carabanensis) and Riverine (Bubalus Bubalis) Buffaloes: Its Implications and Future Perspectives. Anim. Biotechnol. 2018, 29, 190–198. [Google Scholar] [CrossRef]
- Kasimanickam, V.; Kasimanickam, R.; Arangasamy, A.; Saberivand, A.; Stevenson, J.S.; Kastelic, J.P. Association between MRNA Abundance of Functional Sperm Function Proteins and Fertility of Holstein Bulls. Theriogenology 2012, 78, 2007–2019.e2. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.A.; Malcuit, C.; Cheon, B.; Holland, M.K.; Fissore, R.A.; D’Cruz, N.T. Species-Specific Differences in the Activity and Nuclear Localization of Murine and Bovine Phospholipase C Zeta 1. Biol. Reprod. 2010, 83, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, V.; Kastelic, J.P.; Thundathil, J.C. Ouabain-Induced Activation of Phospholipase C Zeta and Its Contributions to Bovine Sperm Capacitation. Cell Tissue Res. 2021, 385, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Flores, I.; Chiquete-Félix, N.; Palma-Lara, I.; Uribe-Carvajal, S.; De Lourdes Juárez-Mosqueda, M. During Capacitation in Bull Spermatozoa, Actin and PLC-ζ Undergo Dynamic Interactions. Zygote 2017, 25, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, A.I.S.B.; Fioratti, E.G.; Fissore, R.A.; He, C.; Lee, H.C.; Souza, F.F.; Landim-Alvarenga, F.C.; Lopes, M.D. Identification of Phospholipase C Zeta in Normospermic and Teratospermic Domestic Cat Sperm. Theriogenology 2013, 80, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Yoda, A.; Oda, S.; Shikano, T.; Kouchi, Z.; Awaji, T.; Shirakawa, H.; Kinoshita, K.; Miyazaki, S. Ca2+ Oscillation-Inducing Phospholipase C Zeta Expressed in Mouse Eggs Is Accumulated to the Pronucleus during Egg Activation. Dev. Biol. 2004, 268, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Grasa, P.; Coward, K.; Davis, L.C.; Parrington, J. Phospholipase C Zeta Undergoes Dynamic Changes in Its Pattern of Localization in Sperm during Capacitation and the Acrosome Reaction. Fertil. Steril. 2009, 91, 2230–2242. [Google Scholar] [CrossRef] [PubMed]
- Bedford-Guaus, S.J.; McPartlin, L.A.; Xie, J.; Westmiller, S.L.; Buffone, M.G.; Roberson, M.S. Molecular Cloning and Characterization of Phospholipase C Zeta in Equine Sperm and Testis Reveals Species-Specific Differences in Expression of Catalytically Active Protein. Biol. Reprod. 2011, 85, 78–88. [Google Scholar] [CrossRef]
- Sato, K.; Wakai, T.; Seita, Y.; Takizawa, A.; Fissore, R.A.; Ito, J.; Kashiwazaki, N. Molecular Characteristics of Horse Phospholipase C Zeta (PLCζ). Anim. Sci. J. 2013, 84, 359–368. [Google Scholar] [CrossRef]
- Fujimoto, S.; Yoshida, N.; Fukui, T.; Amanai, M.; Isobe, T.; Itagaki, C.; Izumi, T.; Perry, A.C.F. Mammalian Phospholipase Cζ Induces Oocyte Activation from the Sperm Perinuclear Matrix. Dev. Biol. 2004, 274, 370–383. [Google Scholar] [CrossRef]
- Ito, M.; Shikano, T.; Oda, S.; Horiguchi, T.; Tanimoto, S.; Awaji, T.; Mitani, H.; Miyazaki, S. Difference in Ca2+ Oscillation-Inducing Activity and Nuclear Translocation Ability of PLCZ1, an Egg-Activating Sperm Factor Candidate, Between Mouse, Rat, Human, and Medaka Fish1. Biol. Reprod. 2008, 78, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M.; Mulgrew-Nesbitt, A.; Pallavi, P.; Mihalyne, G.; Zaitseva, I.; Swann, K.; Lai, F.A.; Murray, D.; McLaughlin, S. Binding of Phosphoinositide-Specific Phospholipase C-ζ (PLC-ζ) to Phospholipid Membranes: Potential Role of an Unstructured Cluster of Basic Residues. J. Biol. Chem. 2007, 282, 16644–16653. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M.; Swann, K.; Lai, F.A. Starting a New Life: Sperm PLC-Zeta Mobilizes the Ca2+ Signal That Induces Egg Activation and Embryo Development: An Essential Phospholipase C with Implications for Male Infertility. BioEssays 2012, 34, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M.; Kashir, J.; Swann, K.; Lai, F.A. Sperm PLCζ: From Structure to Ca2+ Oscillations, Egg Activation and Therapeutic Potential. FEBS Lett. 2013, 587, 3609–3616. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Ito, M.; Shikano, T.; Awaji, T.; Yoda, A.; Takeuchi, H.; Kinoshita, K.; Miyazaki, S. The Role of X/Y Linker Region and N-Terminal EF-Hand Domain in Nuclear Translocation and Ca2+ Oscillation-Inducing Activities of Phospholipase Cζ, a Mammalian Egg-Activating Factor. J. Biol. Chem. 2006, 281, 27794–27805. [Google Scholar] [CrossRef] [PubMed]
- Thanassoulas, A.; Swann, K.; Lai, F.A.; Nomikos, M. The Structure and Function Relationship of Sperm PLCZ1. Reproduction 2022, 164, F1–F8. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.R.; Ashley, B.; Moon, A.; Woolley, T.E.; Swann, K. PLCζ Induced Ca2+ Oscillations in Mouse Eggs Involve a Positive Feedback Cycle of Ca2+ Induced InsP3 Formation from Cytoplasmic PIP2. Front. Cell Dev. Biol. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Theodoridou, M.; Nomikos, M.; Parthimos, D.; Gonzalez-Garcia, J.R.; Elgmati, K.; Calver, B.L.; Sideratou, Z.; Nounesis, G.; Swann, K.; Lai, F.A. Chimeras of Sperm PLCζ Reveal Disparate Protein Domain Functions in the Generation of Intracellular Ca2+ Oscillations in Mammalian Eggs at Fertilization. Mol. Hum. Reprod. 2013, 19, 852–864. [Google Scholar] [CrossRef]
- Kurokawa, M.; Yoon, S.Y.; Alfandari, D.; Fukami, K.; Sato, K.-I.; Fissore, R.A. Proteolytic Processing of Phospholipase Cζ and [Ca2+]i Oscillations during Mammalian Fertilization. Dev. Biol. 2007, 312, 407–418. [Google Scholar] [CrossRef]
- Stein, P.; Savy, V.; Williams, A.M.; Williams, C.J. Modulators of Calcium Signalling at Fertilization. Open Biol. 2020, 10, 200118. [Google Scholar] [CrossRef]
- Jones, K.; Soeller, C.; Cannell, M. The Passage of Ca2+ and Fluorescent Markers between the Sperm and the Egg after Fusion in the Mouse. Development 1998, 125, 4627–4635. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, Y.; Whitaker, M.; Swann, K. Sperm-Egg Fusion Is the Prelude to the Initial Ca2+ Increase at Fertilization in the Mouse. Development 1997, 124, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Nomikos, M.; Lai, F.A.; Swann, K. Sperm-Induced Ca2+ Release during Egg Activation in Mammals. Biochem. Biophys. Res. Commun. 2014, 450, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M.; Kashir, J.; Lai, F.A. The Role and Mechanism of Action of Sperm PLC-Zeta in Mammalian Fertilisation. Biochem. J. 2017, 474, 3659–3673. [Google Scholar] [CrossRef] [PubMed]
- Coward, K.; Ponting, C.P.; Chang, H.Y.; Hibbitt, O.; Savolainen, P.; Jones, K.T.; Parrington, J. Phospholipase Cζ, the Trigger Egg Activation in Mammals, Is Present in a Non-Mammalian Species. Reproduction 2005, 130, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.J.; Beyhan, Z.; Iager, A.E.; Yoon, S.Y.; Malcuit, C.; Schellander, K.; Fissore, R.A.; Cibelli, J.B. Parthenogenetic Activation of Bovine Oocytes Using Bovine and Murine Phospholipase C Zeta. BMC Dev. Biol. 2008, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Swann, K.; Lai, F.A. PLCζ and the Initiation of Ca2+ Oscillations in Fertilizing Mammalian Eggs. Cell Calcium 2013, 53, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Amdani, S.N.; Yeste, M.; Jones, C.; Coward, K. Phospholipase C Zeta (PLCζ) and Male Infertility: Clinical Update and Topical Developments. Adv. Biol. Regul. 2016, 61, 58–67. [Google Scholar] [CrossRef]
- Abdulsamad, H.M.R.; Murtaza, Z.F.; AlMuhairi, H.M.; Bafleh, W.S.; AlMansoori, S.A.; AlQubaisi, S.A.; Hamdan, H.; Kashir, J. The Therapeutic and Diagnostic Potential of Phospholipase C Zeta, Oocyte Activation, and Calcium in Treating Human Infertility. Pharmaceuticals 2023, 16, 441. [Google Scholar] [CrossRef]
- Ozil, J.P.; Huneau, D. Activation of Rabbit Oocytes: The Impact of the Ca2+ Signal Regime on Development. Development 2001, 128, 917–928. [Google Scholar] [CrossRef]
- Bedford-Guaus, S.; Yoon, S.Y.; Fissore, R.A.; Choi, Y.O.; Hinrichs, K. Microinjection of Mouse Phospholipase Cζ Complementary RNA into Mare Oocytes Induces Long-Lasting Intracellular Calcium Oscillations and Embryonic Development. Reprod. Fertil. Dev. 2008, 20, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.M.; Bernhardt, M.L.; Kong, B.Y.; Ahn, R.W.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc Sparks Are Triggered by Fertilization and Facilitate Cell Cycle Resumption in Mammalian Eggs. ACS Chem. Biol. 2011, 6, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Que, E.L.; Bleher, R.; Duncan, F.E.; Kong, B.Y.; Gleber, S.C.; Vogt, S.; Chen, S.; Garwin, S.A.; Bayer, A.R.; Dravid, V.P.; et al. Quantitative Mapping of Zinc Fluxes in the Mammalian Egg Reveals the Origin of Fertilization-Induced Zinc Sparks. Nat. Chem. 2015, 7, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshida, N.; Suzuki, E.; Okuda, E.; Perry, A.C.F. Full-Term Mouse Development by Abolishing Zn2+-Dependent Metaphase II Arrest without Ca2+ Release. Development 2010, 137, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, M.L.; Kim, A.M.; O’Halloran, T.V.; Woodruff, T.K. Zinc Requirement during Meiosis I-Meiosis II Transition in Mouse Oocytes Is Independent of the MOS-MAPK Pathway. Biol. Reprod. 2011, 84, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Duncan, F.E.; Que, E.L.; Zhang, N.; Feinberg, E.C.; O’Halloran, T.V.; Woodruff, T.K. The Zinc Spark Is an Inorganic Signature of Human Egg Activation. Sci. Rep. 2016, 6, 24737. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.M.; Vogt, S.; O’Halloran, T.V.; Woodruff, T.K. Zinc Availability Regulates Exit from Meiosis in Maturing Mammalian Oocytes. Nat. Chem. Biol. 2010, 6, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Anthony, K.; Neuberger, T.; Diaz, F.J. Preconception Zinc Deficiency Disrupts Postimplantation Fetal and Placental Development in Mice. Biol. Reprod. 2014, 90, 83. [Google Scholar] [CrossRef]
- Kong, B.Y.; Bernhardt, M.L.; Kim, A.M.; O’Halloran, T.V.; Woodruff, T.K. Zinc Maintains Prophase i Arrest in Mouse Oocytes through Regulation of the: Mos-Mapk Pathway. Biol. Reprod. 2012, 87, 11. [Google Scholar] [CrossRef]
- Kong, B.Y.; Duncan, F.E.; Que, E.L.; Kim, A.M.; O’Halloran, T.V.; Woodruff, T.K. Maternally-Derived Zinc Transporters ZIP6 and ZIP10 Drive the Mammalian Oocyte-to-Egg Transition. Mol. Hum. Reprod. 2014, 20, 1077–1089. [Google Scholar] [CrossRef]
- Kong, B.Y.; Duncan, F.E.; Que, E.L.; Xu, Y.; Vogt, S.; O’halloran, T.V.; Woodruff, T.K. The Inorganic Anatomy of the Mammalian Preimplantation Embryo and the Requirement of Zinc During the First Mitotic Divisions. Dev. Dyn. 2015, 244, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Duncan, F.E.; Que, E.L.; O’Halloran, T.V.; Woodruff, T.K. The Fertilization-Induced Zinc Spark Is a Novel Biomarker of Mouse Embryo Quality and Early Development. Sci. Rep. 2016, 6, 22772. [Google Scholar] [CrossRef]
- Bernhardt, M.L.; Kong, B.Y.; Kim, A.M.; O’Halloran, T.V.; Woodruff, T.K. A Zinc-Dependent Mechanism Regulates Meiotic Progression in Mammalian Oocytes. Biol. Reprod. 2012, 86, 114. [Google Scholar] [CrossRef] [PubMed]
- Que, E.L.; Duncan, F.E.; Lee, H.C.; Hornick, J.E.; Vogt, S.; Fissore, R.A.; O’Halloran, T.V.; Woodruff, T.K. Bovine Eggs Release Zinc in Response to Parthenogenetic and Sperm-Induced Egg Activation. Theriogenology 2019, 127, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Malcuit, C.; Knott, J.G.; He, C.; Wainwright, T.; Parys, J.B.; Robl, J.M.; Fissore, R.A. Fertilization and Inositol 1,4,5-Trisphosphate (IP3)-Induced Calcium Release in Type-1 Inositol 1,4,5-Trisphosphate Receptor down-Regulated Bovine Eggs. Biol. Reprod. 2005, 73, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Malcuit, C.; Maserati, M.; Takahashi, Y.; Page, R.; Fissore, R.A. Intracytoplasmic Sperm Injection in the Bovine Induces Abnormal [Ca 2+]i Responses and Oocyte Activation. Reprod. Fertil. Dev. 2006, 18, 39–51. [Google Scholar] [CrossRef]
- Larman, M.G.; Saunders, C.M.; Carroll, J.; Lai, F.A.; Swann, K. Cell Cycle-Dependent Ca2+ Oscillations in Mouse Embryos Are Regulated by Nuclear Targeting of PLCζ. J. Cell Sci. 2004, 117, 2513–2521. [Google Scholar] [CrossRef]
- Escoffier, J.; Yassine, S.; Lee, H.C.; Martinez, G.; Delaroche, J.; Coutton, C.; Karaouzéne, T.; Zouari, R.; Metzler-Guillemain, C.; Pernet-Gallay, K.; et al. Subcellular Localization of Phospholipase Cz in Human Sperm and Its Absence in DPY19L2-Deficient Sperm Are Consistent with Its Role in Oocyte Activation. Mol. Hum. Reprod. 2014, 21, 157–168. [Google Scholar] [CrossRef]
- Grasa, P.; Coward, K.; Young, C.; Parrington, J. The Pattern of Localization of the Putative Oocyte Activation Factor, Phospholipase Cζ, in Uncapacitated, Capacitated, and Ionophore-Treated Human Spermatozoa. Hum. Reprod. 2008, 23, 2513–2522. [Google Scholar] [CrossRef]
- Sutovsky, P.; Manandhar, G.; Wu, A.; Oko, R. Interactions of Sperm Perinuclear Theca with the Oocyte: Implications for Oocyte Activation, Anti-Polyspermy Defense, and Assisted Reproduction. Microsc. Res. Tech. 2003, 61, 362–378. [Google Scholar] [CrossRef]
- Oko, R.; Sutovsky, P. Biogenesis of Sperm Perinuclear Theca and Its Role in Sperm Functional Competence and Fertilization. J. Reprod. Immunol. 2009, 83, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Castro, R.; Graham, J.; Carnevale, E. Flow Cytometric Expression Levels of Phospholipase C Zeta in Capacitated and Acrosome Reacted Stallion Sperm. Anim. Reprod. Sci. 2018, 194, e2. [Google Scholar] [CrossRef]
- Wu, H.; He, C.-L.; Jehn, B.; Black, S.J.; Fissore, R.A. Partial Characterization of the Calcium-Releasing Activity of Porcine Sperm Cytosolic Extracts. Dev. Biol. 1998, 203, 369–381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, H.; Smyth, J.; Luzzi, V.; Fukami, K.; Takenawa, T.; Black, S.L.; Allbritton, N.L.; Fissore, R.A. Sperm Factor Induces Intracellular Calcium Oscillations by Stimulating the Phosphoinositide Pathway. Biol. Reprod. 2001, 64, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, M.; Fissore, R.A. ICSI-generated Mouse Zygotes Exhibit Altered Calcium Oscillations, Inositol 1,4,5-trisphosphate Receptor-1 Down-regulation, and Embryo Development. MHR Basic. Sci. Reprod. Med. 2003, 9, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Yanagimachi, R.; Kuretake, S.; Bortkiewicz, H.; Perry, A.C.F.; Yanagimachi, H. Analysis of Mouse Oocyte Activation Suggests the Involvement of Sperm Perinuclear Material’. Biol. Reprod. 1998, 58, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Shirakawa, H.; Nakada, K.; Honda, Y. Essential Role of the Inositol 1,4,5-Trisphosphate Receptor/Ca2+ Release Channel in Ca2+ Waves and Ca2+ Oscillations at Fertilization of Mammalian Eggs. Dev. Biol. 1993, 158, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Igusa, Y.; Miyazaki, S.-I.; Yamashita, N. Periodic Hyperpolarizing Responses in Hamster and Mouse Eggs Fertilized with Mouse Sperm. J. Physiol. 1983, 340, 633–647. [Google Scholar] [CrossRef]
- Dominguez, E.M.; Moreno-Irusta, A.; Rodriguez, M.B.; Salamone, D.F.; de Arruda, R.P.; Losinno, L.; Giojalas, L.C. Chemotactic Selection of Frozen-Thawed Stallion Sperm Improves Sperm Quality and Heterologous Binding to Oocytes. Anim. Reprod. Sci. 2020, 221, 106582. [Google Scholar] [CrossRef]
- Ito, J.; Kashiwazaki, N. Molecular Mechanism of Fertilization in the Pig. Anim. Sci. J. 2012, 83, 669–682. [Google Scholar] [CrossRef]
- Gonzalez-Castro, R.A.; Whitcomb, L.A.; Pinsinski, E.C.; Carnevale, E.M. Cryopreservation of Equine Spermatozoa Reduces Plasma Membrane Integrity and Phospholipase C Zeta 1 Content as Associated with Oocyte Activation. Andrology 2023, 1–14, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bedford-Guaus, S.J.; McPartlin, L.A.; Varner, D.D. Characterization of Equine Phospholipase C Zeta: A Review and Preliminary Results on Expression Defects in Subfertile Stallions. J. Equine Vet. Sci. 2012, 32, 445–450. [Google Scholar] [CrossRef]
- Carnevale, E.M. Clinical Considerations Regarding Assisted Reproductive Procedures in Horses. J. Equine Vet. Sci. 2008, 28, 686–690. [Google Scholar] [CrossRef]
- Gonzalez-Castro, R.A.; Trentin, J.M.; Carnevale, E.M.; Graham, J.K. Effects of Extender, Cryoprotectants and Thawing Protocol on Motility of Frozen-Thawed Stallion Sperm That Were Refrozen for Intracytoplasmic Sperm Injection Doses. Theriogenology 2019, 136, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Sieme, H.; Oldenhof, H.; Wolkers, W.F. Sperm Membrane Behaviour during Cooling and Cryopreservation. Reprod. Domest. Anim. 2015, 50, 20–26. [Google Scholar] [CrossRef]
- Bogle, O.A.; Kumar, K.; Attardo-Parrinello, C.; Lewis, S.E.M.; Estanyol, J.M.; Ballescà, J.L.; Oliva, R. Identification of Protein Changes in Human Spermatozoa throughout the Cryopreservation Process. Andrology 2017, 5, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Castro, R.A.; Carnevale, E.M. Semiquantitative and Quantitative Assessments of Phospholipase C Zeta 1 in Stallion Sperm. Reprod. Fertil. Dev. 2023, 35, 150. [Google Scholar] [CrossRef]
- Gonzalez-Castro, R.A.; Carnevale, E.M. Phospholipase C Zeta Quantification by Flow Cytometry in Fresh, Frozen and Refrozen Stallion Sperm. J. Equine Vet. Sci. 2020, 89, 103033. [Google Scholar] [CrossRef]
- Kashir, J.; Jones, C.; Mounce, G.; Ramadan, W.M.; Lemmon, B.; Heindryckx, B.; De Sutter, P.; Parrington, J.; Turner, K.; Child, T.; et al. Variance in Total Levels of Phospholipase C Zeta (PLC-ζ) in Human Sperm May Limit the Applicability of Quantitative Immunofluorescent Analysis as a Diagnostic Indicator of Oocyte Activation Capability. Fertil. Steril. 2013, 99, 107–117.e3. [Google Scholar] [CrossRef]
- Moreau, J.; Fargeon, S.; Gatimel, N.; Parinaud, J.; Léandri, R.D. Expression of Phospholipase PLC Zeta in Human Spermatozoa: Impact of Cryopreservation. Andrology 2019, 7, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Heynen, A.; Jones, C.; Durrans, C.; Craig, J.; Gadea, J.; Turner, K.; Parrington, J.; Coward, K. Effects of Cryopreservation and Density-Gradient Washing on Phospholipase C Zeta Concentrations in Human Spermatozoa. Reprod. Biomed. Online 2011, 23, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Hirose, N.; Kamimura, S.; Wakayama, S.; Ito, J.; Ooga, M.; Wakayama, T. Production of Mouse Offspring from Inactivated Spermatozoa Using Horse PLCζ MRNA. J. Reprod. Dev. 2020, 66, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Homa, S.T.; Swann, K. Fertilization and Early Embryology: A Cytosolic Sperm Factor Triggers Calcium Oscillations and Membrane Hyperpolarizations in Human Oocytes. Hum. Reprod. 1994, 9, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Swann, K. A Cytosolic Sperm Factor Stimulates Repetitive Calcium Increases and Mimics Fertilization in Hamster Eggs. Development 1990, 110, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.T.; Hobson, E.; Pickering, S.; Lai, F.A.; Braude, P.; Swann, K. Phospholipase Cζ Causes Ca2+ Oscillations and Parthenogenetic Activation of Human Oocytes. Reproduction 2004, 128, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Gradil, C.; Yoon, S.; Brown, J.; He, C.; Visconti, P.; Fissore, R. PLC Zeta: A Marker of Fertility for Stallions? Anim. Reprod. Sci. 2006, 94, 23–25. [Google Scholar] [CrossRef]
- Sessions-Bresnahan, D.R.; Graham, J.K.; Carnevale, E.M. Validation of a Heterologous Fertilization Assay and Comparison of Fertilization Rates of Equine Oocytes Using Invitro Fertilization, Perivitelline, and Intracytoplasmic Sperm Injections. Theriogenology 2014, 82, 274–282. [Google Scholar] [CrossRef]
- Amoroso-Sanches, F.; Gonzalez-Castro, R.A.; Stokes, J.E.; Carnevale, E.M. Stallion Sperm Phospholipase C Zeta Affects Cleavage Rates after Intracytoplasmic Injection in Bovine Oocytes. Reprod. Fertil. Dev. 2018, 31, 214–251. [Google Scholar] [CrossRef]
- Ferrer-Vaquer, A.; Barragan, M.; Freour, T.; Vernaeve, V.; Vassena, R. PLCζ Sequence, Protein Levels, and Distribution in Human Sperm Do Not Correlate with Semen Characteristics and Fertilization Rates after ICSI. J. Assist. Reprod. Genet. 2016, 33, 747–756. [Google Scholar] [CrossRef]
- Giesecke, K.; Hamann, H.; Sieme, H.; Distl, O. Evaluation of Prolactin Receptor (PRLR) as Candidate Gene for Male Fertility in Hanoverian Warmblood Horses. Reprod. Domest. Anim. 2010, 45, e124–e130. [Google Scholar] [CrossRef]
- Giesecke, K.; Hamann, H.; Sieme, H.; Distl, O. INHBA-Associated Markers as Candidates for Stallion Fertility. Reprod. Domest. Anim. 2010, 45, 342–347. [Google Scholar] [CrossRef]
- Giesecke, K.; Hamann, H.; Stock, K.F.; Klewitz, J.; Martinsson, G.; Distl, O.; Sieme, H. Evaluation of ACE, SP17, and FSHB as Candidates for Stallion Fertility in Hanoverian Warmblood Horses. Anim. Reprod. Sci. 2011, 126, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Giesecke, K.; Hamann, H.; Stock, K.F.; Woehlke, A.; Sieme, H.; Distl, O. Evaluation of SPATA1-Associated Markers for Stallion Fertility. Anim. Genet. 2009, 40, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hamann, H.; Jude, R.; Sieme, H.; Mertens, U.; Töpfer-Petersen, E.; Distl, O.; Leeb, T. A Polymorphism within the Equine CRISP3 Gene Is Associated with Stallion Fertility in Hanoverian Warmblood Horses. Anim. Genet. 2007, 38, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Raudsepp, T.; McCue, M.E.; Das, P.J.; Dobson, L.; Vishnoi, M.; Fritz, K.L.; Schaefer, R.; Rendahl, A.K.; Derr, J.N.; Love, C.C.; et al. Genome-Wide Association Study Implicates Testis-Sperm Specific FKBP6 as a Susceptibility Locus for Impaired Acrosome Reaction in Stallions. PLoS Genet. 2012, 8, e1003139. [Google Scholar] [CrossRef]
- Schrimpf, R.; Dierks, C.; Martinsson, G.; Sieme, H.; Distl, O. Genome-Wide Association Study Identifies Phospholipase C Zeta 1 (PLCz1) as a Stallion Fertility Locus in Hanoverian Warmblood Horses. PLoS ONE 2014, 9, e109675. [Google Scholar] [CrossRef]
- Ju, Z.H.; Pan, Q.; Zhang, Y.; Huang, J.M.; Qi, C.; Wang, X.G.; Li, Q.L.; Zhong, J.F.; Liu, M.; Wang, C.F. Identification and Characterization of a Novel Splice Variant of the PLCζ1 Gene in Bull Testis Tissues. Genet. Mol. Res. 2014, 13, 9899–9909. [Google Scholar] [CrossRef]
- Yuan, Z.; Cai, T.; Tian, J.; Ivanov, A.V.; Giovannucci, D.R.; Xie, Z. Na/K-ATPase Tethers Phospholipase C and IP3 Receptor into a Calcium-Regulatory Complex. Mol. Biol. Cell 2005, 16, 4034–4045. [Google Scholar] [CrossRef]
- Thundathil, J.C.; Anzar, M.; Buhr, M.M. Na+/K+ ATPase as a Signaling Molecule during Bovine Sperm Capacitation. Biol. Reprod. 2006, 75, 308–317. [Google Scholar] [CrossRef]
- Newton, L.D.; Krishnakumar, S.; Menon, A.G.; Kastelic, J.P.; Van Der Hoorn, F.A.; Thundathil, J.C. Na+/K+ ATPase Regulates Sperm Capacitation through a Mechanism Involving Kinases and Redistribution of Its Testis-Specific Isoform. Mol. Reprod. Dev. 2010, 77, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, T.; Sánchez, G.; Wertheimer, E.; Blanco, G. Activity of the Na,K-ATPase A4 Isoform Is Important for Membrane Potential, Intracellular Ca2+, and PH to Maintain Motility in Rat Spermatozoa. Reproduction 2010, 139, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, T.; Sánchez, G.; Blanco, G. Activity of the Na,K-ATPase A4 Isoform Is Regulated during Sperm Capacitation to Support Sperm Motility. J. Androl. 2012, 33, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Thundathil, J.C.; Rajamanickam, G.D.; Kastelic, J.P. Na/K-ATPase and Regulation of Sperm Function. Anim. Reprod. 2018, 15, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, V.; Kastelic, J.; Thundathil, J. Intracytoplasmic Sperm Injection in Cattle. Genes 2021, 12, 198. [Google Scholar] [CrossRef] [PubMed]
- Águila, L.; Felmer, R.; Arias, M.E.; Navarrete, F.; Martin-Hidalgo, D.; Lee, H.C.; Visconti, P.; Fissore, R. Defective Sperm Head Decondensation Undermines the Success of ICSI in the Bovine. Reproduction 2017, 154, 307–318. [Google Scholar] [CrossRef]
- Hara, H.; Abdalla, H.; Morita, H.; Kuwayama, M.; Hirabayashi, M.; Hochi, S. Procedure for Bovine ICSI, nor Sperm Freezing-Drying, Impairs the Function of the Microtubule-Organizing Center. J. Reprod. Dev. 2011, 57, 428–432. [Google Scholar] [CrossRef]
- Ferré, L.B.; Kjelland, M.E.; Taiyeb, A.M.; Campos-Chillon, F.; Ross, P.J. Recent Progress in Bovine in Vitro-Derived Embryo Cryotolerance: Impact of in Vitro Culture Systems, Advances in Cryopreservation and Future Considerations. Reprod. Domest. Anim. 2020, 55, 659–676. [Google Scholar] [CrossRef]
- Gupta, N.; Akizawa, H.; Lee, H.C.; Fissore, R.A. ICSI and the Discovery of the Sperm Factor and PLCZ1. Reproduction 2022, 164, F9–F20. [Google Scholar] [CrossRef]
- Sutovsky, P.; Oko, R.; Hewitson, L.; Schatten, G. The Removal of the Sperm Perinuclear Theca and Its Association with the Bovine Oocyte Surface during Fertilization. Dev. Biol. 1997, 188, 75–84. [Google Scholar] [CrossRef]
- Jager, S.; Wijchman, J.; Kremer, J. Studies on the Decondensation of Human, Mouse, and Bull Sperm Nuclei by Heparin and Other Polyanions. J. Exp. Zool. 1990, 256, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Perreault, S.D.; Barbee, R.R.; Elstein, K.H.; Zucker, R.M.; Keefer, C.L. Interspecies Differences in the Stability of Mammalian Sperm Nuclei Assessed in Vivo by Sperm Microinjection and in Vitro by Flow Cytometry. Biol. Reprod. 1988, 39, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, F.; Aguila, L.; Arias, M.E.; Sánchez, R.; Felmer, R. Improved Preimplantation Development of Bovine ICSI Embryos Generated with Spermatozoa Pretreated with Membrane-Destabilizing Agents Lysolecithin and Triton X-100. Theriogenology 2016, 86, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Vassiliev, I.; Lagutina, I.; Galli, A.; Lazzari, G. Bovine Embryo Development Following ICSI: Effect of Activation, Sperm Capacitation and Pre-Treatment with Dithiothreitol. Theriogenology 2003, 60, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Morozumi, K.; Yanagimachi, R. Incorporation of the Acrosome into the Oocyte during Intracytoplasmic Sperm Injection Could Be Potentially Hazardous to Embryo Development. Proc. Natl. Acad. Sci. USA 2005, 102, 14209–14214. [Google Scholar] [CrossRef] [PubMed]
- Seita, Y.; Ito, J.; Kashiwazaki, N. Removal of Acrosomal Membrane from Sperm Head Improves Development of Rat Zygotes Derived from Intracytoplasmic Sperm Injection. J. Reprod. Dev. 2009, 55, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Daghigh-Kia, H. Identification and SNP Detection for Preimplantation Active Genes and Their Association with Embryo Development and Male Fertility in Cattle. Ph.D. Thesis, University of Bonn, Boon, Germany, 2007. [Google Scholar]
- Pan, Q.; Ju, Z.; Huang, J.; Zhang, Y.; Qi, C.; Gao, Q.; Zhou, L.; Li, Q.; Wang, L.; Zhong, J.; et al. PLCz Functional Haplotypes Modulating Promoter Transcriptional Activity Are Associated with Semen Quality Traits in Chinese Holstein Bulls. PLoS ONE 2013, 8, e58795. [Google Scholar] [CrossRef]
- Steger, K. Perspectives in the Diagnosis of Testicular Biopsies Using Molecular Biological Techniques. Andrologia 2003, 35, 183. [Google Scholar] [CrossRef]
- Ostermeier, G.C.; Dix, D.J.; Miller, D.; Khatri, P.; Krawetz, S.A. Spermatozoal RNA Profiles of Normal Fertile Men. Lancet 2002, 360, 772–777. [Google Scholar] [CrossRef]
- Dadoune, J.-P. Expression of Mammalian Spermatozoal Nucleoproteins. Microsc. Res. Tech. 2003, 61, 56–75. [Google Scholar] [CrossRef]
- Lambard, S.; Galeraud-Denis, I.; Martin, G.; Levy, R.; Chocat, A.; Carreau, S. Analysis and Significance of MRNA in Human Ejaculated Sperm from Normozoospermic Donors: Relationship to Sperm Motility and Capacitation. Mol. Hum. Reprod. 2004, 10, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Melo, P.; Jones, C.; Ross, C.; Mounce, G.; Turner, K.; Child, T.; Coward, K. Use of Phospholipase C Zeta Analysis to Identify Candidates for Artificial Oocyte Activation: A Case Series of Clinical Pregnancies and a Proposed Algorithm for Patient Management. Fertil. Steril. 2020, 114, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Aghajanpour, S.; Ghaedi, K.; Salamian, A.; Deemeh, M.R.; Tavalaee, M.; Moshtaghian, J.; Parrington, J.; Nasr-Esfahani, M.H. Quantitative Expression of Phospholipase C Zeta, as an Index to Assess Fertilization Potential of a Semen Sample. Hum. Reprod. 2011, 26, 2950–2956. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Fan, Y.; Wang, F.; Yan, Z.; Li, M.; Ouyang, J.; Wu, L.; Yin, M.; Zhao, J.; Kuang, Y.; et al. Novel Mutations in PLCZ1 Cause Male Infertility Due to Fertilization Failure or Poor Fertilization. Hum. Reprod. 2020, 35, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Tavalaee, M.; Nasr-Esfahani, M.H. Expression Profile of PLCζ, PAWP, and TR-KIT in Association with Fertilization Potential, Embryo Development, and Pregnancy Outcomes in Globozoospermic Candidates for Intra-Cytoplasmic Sperm Injection and Artificial Oocyte Activation. Andrology 2016, 4, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Ostermeier, G.C.; Miller, D.; Huntriss, J.D.; Diamond, M.P.; Krawetz, S.A. Delivering Spermatozoan RNA to the Oocyte. Nature 2004, 429, 154. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Ostermeier, G.C.; Krawetz, S.A. The Controversy, Potential and Roles of Spermatozoal RNA. Trends Mol. Med. 2005, 11, 156–163. [Google Scholar] [CrossRef]
- Tavalaee, M.; Kiani-Esfahani, A.; Nasr-Esfahani, M.H. Relationship between Phospholipase C-Zeta, Semen Parameters, and Chromatin Status. Syst. Biol. Reprod. Med. 2017, 63, 259–268. [Google Scholar] [CrossRef]
- Aras-Tosun, D.; Cakar, Z.; Can, A.; Ozkavukcu, S.; Kaplanoglu, I.; Cinar, O. Phospholipase C-Zeta Levels Are Not Correlated with Fertilisation Rates in Infertile Couples. Andrologia 2022, 54, e14269. [Google Scholar] [CrossRef]
- Alsaed, O.S.; Alamlih, L.I.; Al-Radideh, O.; Chandra, P.; Alemadi, S.; Al-Allaf, A.-W. Clinical Utility of ANA-ELISA vs. ANA-Immunofluorescence in Connective Tissue Diseases. Sci. Rep. 2021, 11, 8229. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Jellerette, T.; Salicioni, A.M.; Lee, H.C.; Yoo, M.; Coward, K.; Parrington, J.; Grow, D.; Cibelli, J.; Visconti, P.; et al. Human Sperm Devoid of PLC, Zeta 1 Fail to Induce Ca2+ Release and Are Unable to Initiate the First Step of Embryo Development. J. Clin. Investig. 2008, 118, 3671–3681. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Buntwal, L.; Nomikos, M.; Calver, B.L.; Stamatiadis, P.; Ashley, P.; Vassilakopoulou, V.; Sanders, D.; Knaggs, P.; Livaniou, E.; et al. Antigen Unmasking Enhances Visualization Efficacy of the Oocyte Activation Factor, Phospholipase C Zeta, in Mammalian Sperm. Mol. Hum. Reprod. 2017, 23, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Mistry, B.V.; BuSaleh, L.; Nomikos, M.; Almuqayyil, S.; Abu-Dawud, R.; AlYacoub, N.; Hamdan, H.; AlHassan, S.; Lai, F.A.; et al. Antigen Unmasking Is Required to Clinically Assess Levels and Localisation Patterns of Phospholipase C Zeta in Human Sperm. Pharmaceuticals 2023, 16, 198. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Jones, C.; Melo, P.; Ross, C.; Mounce, G.; Child, T.; Coward, K. Antigen Unmasking Does Not Improve the Visualization of Phospholipase C Zeta in Human Spermatozoa. Asian J. Androl. 2022, 24, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Heindryckx, B.; Van der Elst, J.; De Sutter, P.; Dhont, M. Treatment Option for Sperm- or Oocyte-Related Fertilization Failure: Assisted Oocyte Activation Following Diagnostic Heterologous ICSI. Hum. Reprod. 2005, 20, 2237–2241. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Buitrago, M.; Dhaenens, L.; Lu, Y.; Bonte, D.; Vanden Meerschaut, F.; De Sutter, P.; Leybaert, L.; Heindryckx, B. Human Oocyte Calcium Analysis Predicts the Response to Assisted Oocyte Activation in Patients Experiencing Fertilization Failure after ICSI. Hum. Reprod. 2018, 33, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Bonte, D.; Thys, V.; De Sutter, P.; Boel, A.; Leybaert, L.; Heindryckx, B. Vitrification Negatively Affects the Ca2+-Releasing and Activation Potential of Mouse Oocytes, but Vitrified Oocytes Are Potentially Useful for Diagnostic Purposes. Reprod. Biomed. Online 2020, 40, 13–25. [Google Scholar] [CrossRef]
- Bonte, D.; Ferrer-Buitrago, M.; Dhaenens, L.; Popovic, M.; Thys, V.; De Croo, I.; De Gheselle, S.; Steyaert, N.; Boel, A.; Vanden Meerschaut, F.; et al. Assisted Oocyte Activation Significantly Increases Fertilization and Pregnancy Outcome in Patients with Low and Total Failed Fertilization after Intracytoplasmic Sperm Injection: A 17-Year Retrospective Study. Fertil. Steril. 2019, 112, 266–274. [Google Scholar] [CrossRef]
- Tesarik, J.; Rienzi, L.; Ubaldi, F.; Mendoza, C.; Greco, E. Use of a Modified Intracytoplasmic Sperm Injection Technique to Overcome Sperm-Borne and Oocyte-Borne Oocyte Activation Failures. Fertil. Steril. 2002, 78, 619–624. [Google Scholar] [CrossRef]
- Vanden Meerschaut, F.; Nikiforaki, D.; Heindryckx, B.; De Sutter, P. Assisted Oocyte Activation Following ICSI Fertilization Failure. Reprod. Biomed. Online 2014, 28, 560–571. [Google Scholar] [CrossRef]
- Taylor, S.L.; Yoon, S.Y.; Morshedi, M.S.; Lacey, D.R.; Jellerette, T.; Fissore, R.A.; Oehninger, S. Complete Globozoospermia Associated with PLCζ Deficiency Treated with Calcium Ionophore and ICSI Results in Pregnancy. Reprod. Biomed. Online 2010, 20, 559–564. [Google Scholar] [CrossRef]
- Ozil, J.P.; Banrezes, B.; Tóth, S.; Pan, H.; Schultz, R.M. Ca2+ oscillatory Pattern in Fertilized Mouse Eggs Affects Gene Expression and Development to Term. Dev. Biol. 2006, 300, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Bridges, P.J.; Jeoung, M.; Kim, H.; Kim, J.H.; Lee, D.R.; Ko, C.; Baker, D.J. Methodology Matters: IVF versus ICSI and Embryonic Gene Expression. Reprod. Biomed. Online 2011, 23, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Heindryckx, B.; De Gheselle, S.; Gerris, J.; Dhont, M.; De Sutter, P. Efficiency of Assisted Oocyte Activation as a Solution for Failed Intracytoplasmic Sperm Injection. Reprod. Biomed. Online 2008, 17, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Matoba, S.; Ogonuki, N.; Namiki, T.; Ito, J.; Kashiwazaki, N.; Ogura, A. Application of Auxin-Inducible Degron Technology to Mouse Oocyte Activation with PLCζ. J. Od Reprod. Dev. 2018, 64, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Bedford, S.J.; Kurokawa, M.; Hinrichs, K.; Fissore, R. a Patterns of Intracellular Calcium Oscillations in Horse Oocytes Fertilized by Intracytoplasmic Sperm Injection: Possible Explanations for the Low Success of This Assisted Reproduction Technique in the Horse. Biol. Reprod. 2004, 70, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-Y.; Eum, J.H.; Lee, J.E.; Lee, H.C.; Kim, Y.S.; Han, J.E.; Won, H.J.; Park, S.H.; Shim, S.H.; Lee, W.S.; et al. Recombinant Human Phospholipase C Zeta 1 Induces Intracellular Calcium Oscillations and Oocyte Activation in Mouse and Human Oocytes. Hum. Reprod. 2012, 27, 1768–1780. [Google Scholar] [CrossRef]
- Kashir, J.; Jones, C.; Lee, H.C.; Rietdorf, K.; Nikiforaki, D.; Durrans, C.; Ruas, M.; Tee, S.T.; Heindryckx, B.; Galione, A.; et al. Loss of Activity Mutations in Phospholipase C Zeta (PLCζ) Abolishes Calcium Oscillatory Ability of Human Recombinant Protein in Mouse Oocytes. Hum. Reprod. 2011, 26, 3372–3387. [Google Scholar] [CrossRef]
- Sanusi, R.; Yu, Y.; Nomikos, M.; Lai, F.A.; Swann, K. Rescue of Failed Oocyte Activation after ICSI in a Mouse Model of Male Factor Infertility by Recombinant Phospholipase Cζ. Mol. Hum. Reprod. 2015, 21, 783–791. [Google Scholar] [CrossRef]

| Assay | Features Analyzed | Advantages | Disadvantages |
|---|---|---|---|
| Genetic analysis | Genomic DNA detects PLCZ1 mutations. Mutations associate with fertilization failure. | Characterization of PCLZ1 mutation in individuals or families for eligibility for oocyte activation treatment. Used in clinical trials | No information on protein content, distribution, or function. |
| Quantitative polymerase chain reaction | PLCZ1 mRNA expression associates with fertilization rates. | Characterization of PCLZ1 expression in sperm. Used in clinical trials. | No direct information on PLCZ1 protein. Relatively high cost. |
| Immunofluorescence | Protein abundance and distribution associate with fertilization rates. | Relatively low cost and rapid to perform. Used in clinical trials. | Evaluation of hundreds of sperm. Relies on antibody specificity. Differences inter-laboratory. |
| Immunoblotting | Relative protein abundance associates with fertilization rates. | Relatively low cost and rapid to perform. Used in clinical trials. | Proportional evaluation of sperm proteins. Relies on antibody specificity. Differences inter-laboratory. |
| Flow cytometry | Relative protein abundance associates with fertilization rates. | Relatively low cost and fast to perform. Evaluation of thousands of sperm. Used in clinical trials. | Relies on antibody specificity. Differences intra- and inter-laboratory. Requires flow cytometer. |
| Enzyme-linked immunoassay (ELISA) | Assessment of protein quantity | Relatively low cost. Fast to conduct in batches to reduce variation. | Relies on antibody specificity. Has not been tested in clinical trials. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Castro, R.A.; Carnevale, E.M. Phospholipase C Zeta 1 (PLCZ1): The Function and Potential for Fertility Assessment and In Vitro Embryo Production in Cattle and Horses. Vet. Sci. 2023, 10, 698. https://doi.org/10.3390/vetsci10120698
Gonzalez-Castro RA, Carnevale EM. Phospholipase C Zeta 1 (PLCZ1): The Function and Potential for Fertility Assessment and In Vitro Embryo Production in Cattle and Horses. Veterinary Sciences. 2023; 10(12):698. https://doi.org/10.3390/vetsci10120698
Chicago/Turabian StyleGonzalez-Castro, Raul A., and Elaine M. Carnevale. 2023. "Phospholipase C Zeta 1 (PLCZ1): The Function and Potential for Fertility Assessment and In Vitro Embryo Production in Cattle and Horses" Veterinary Sciences 10, no. 12: 698. https://doi.org/10.3390/vetsci10120698
APA StyleGonzalez-Castro, R. A., & Carnevale, E. M. (2023). Phospholipase C Zeta 1 (PLCZ1): The Function and Potential for Fertility Assessment and In Vitro Embryo Production in Cattle and Horses. Veterinary Sciences, 10(12), 698. https://doi.org/10.3390/vetsci10120698




