Anatomical and Three-Dimensional Study of the Female Feline Abdominal and Pelvic Vascular System Using Dissections, Computed Tomography Angiography and Magnetic Resonance Angiography
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anatomic Evaluation
2.3. Computed Tomography Angiography and 3D Printing
2.4. Magnetic Resonance Angiography
3. Results
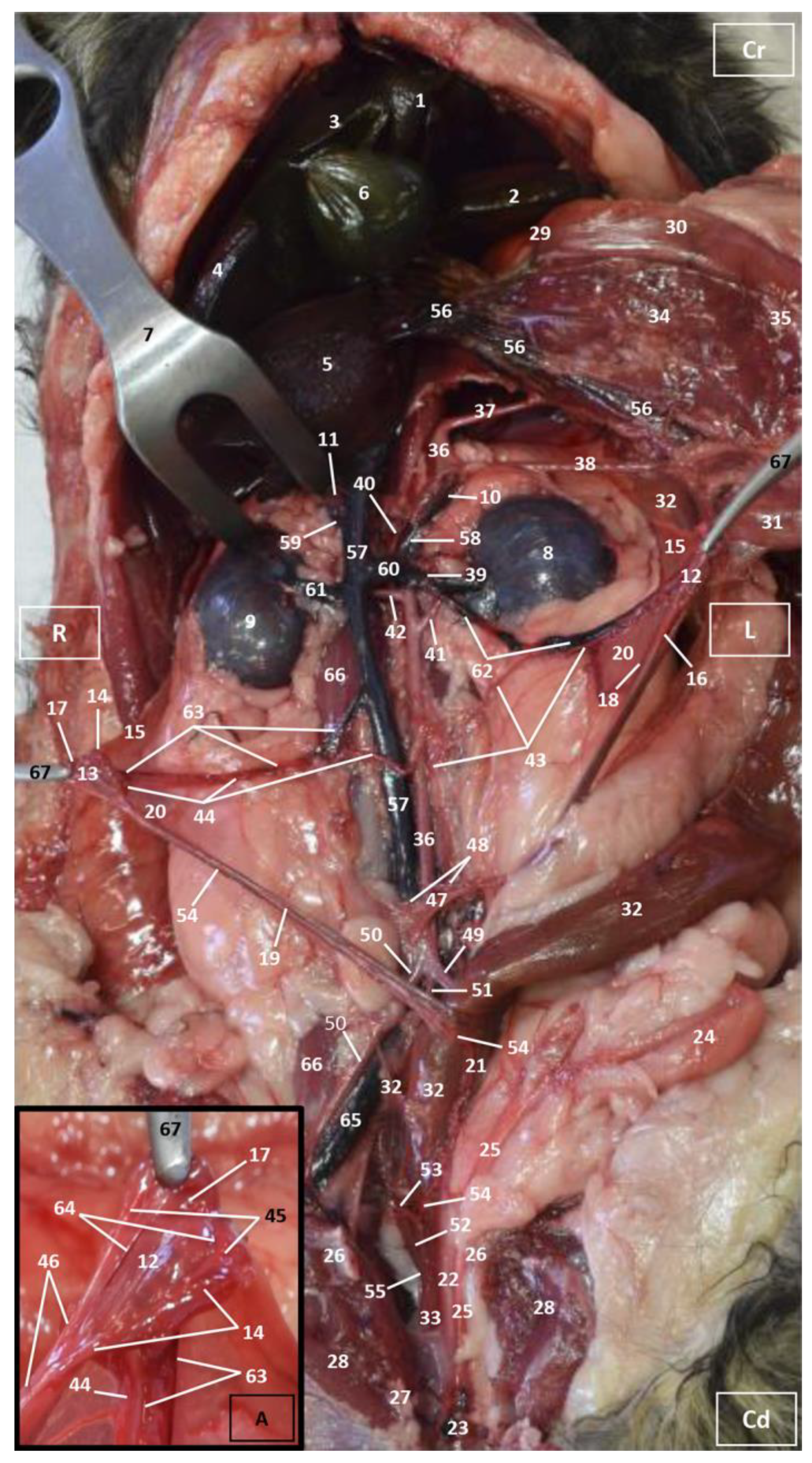
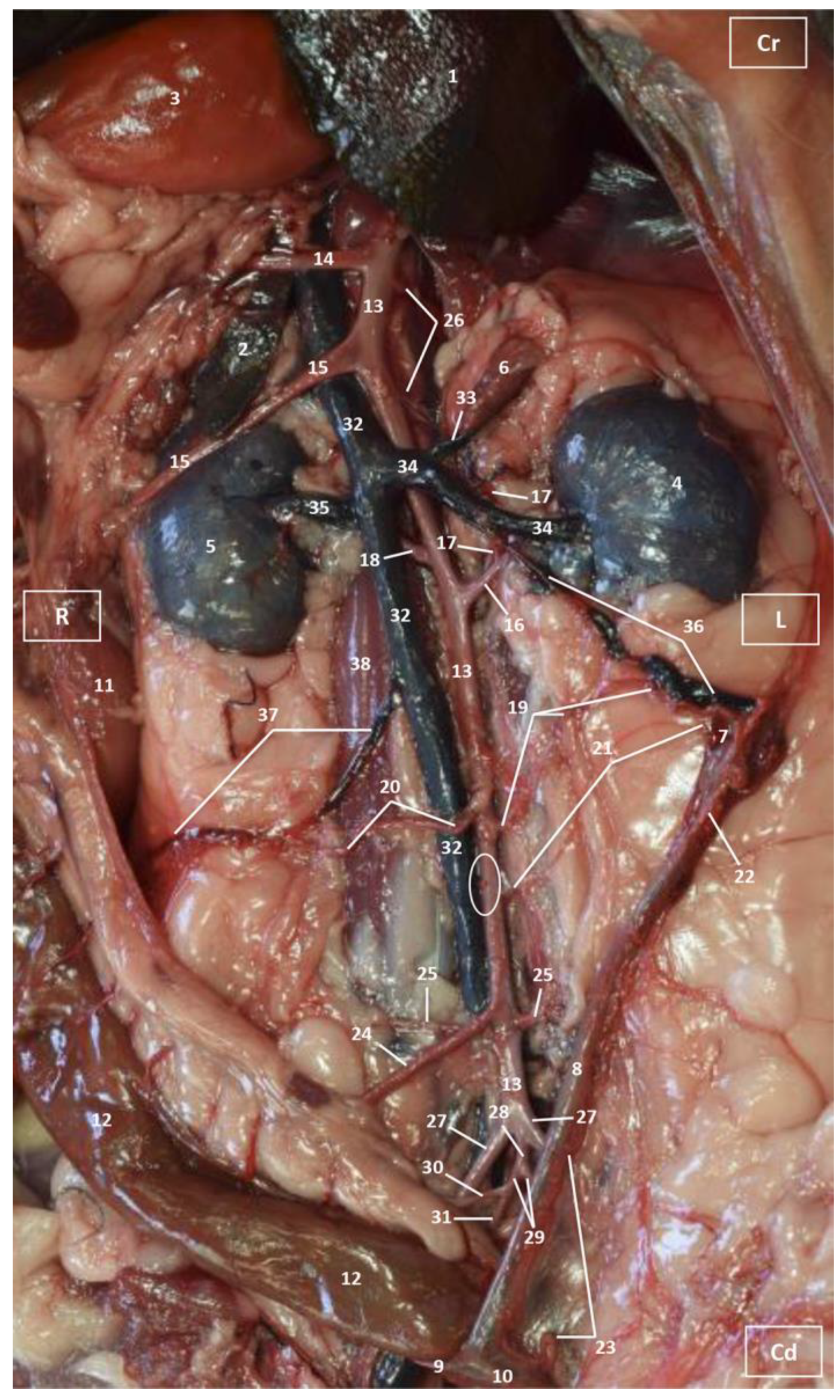
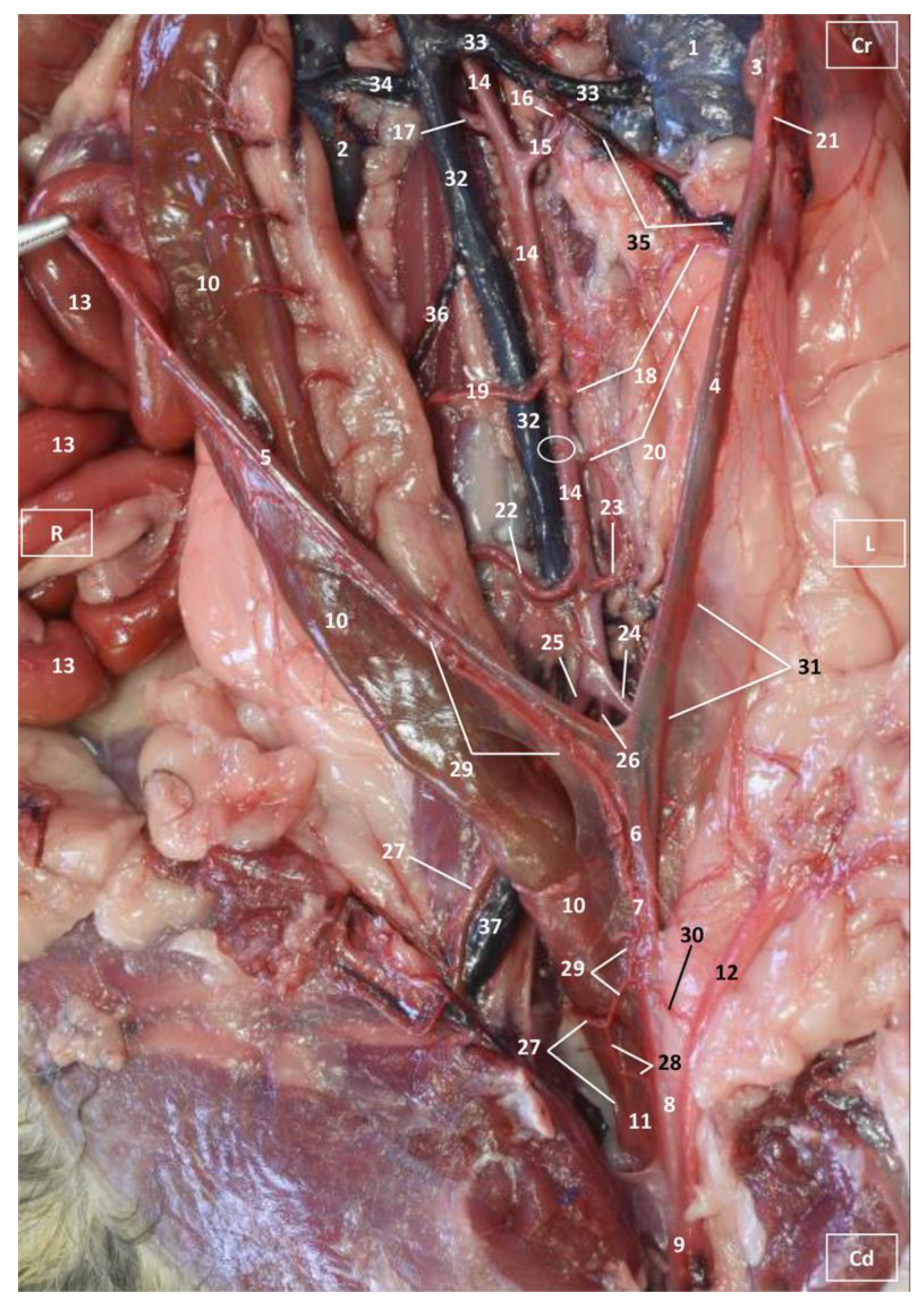
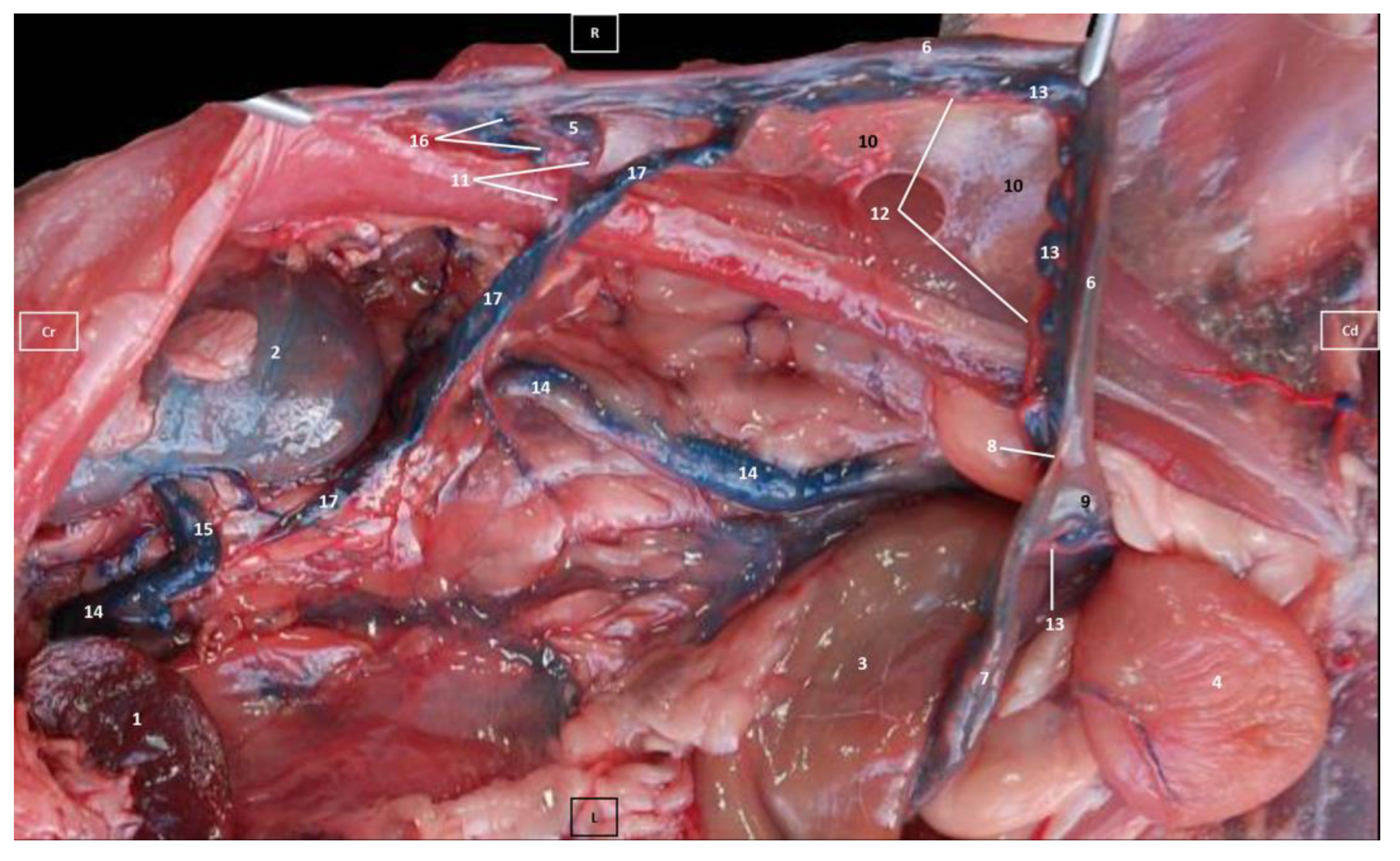
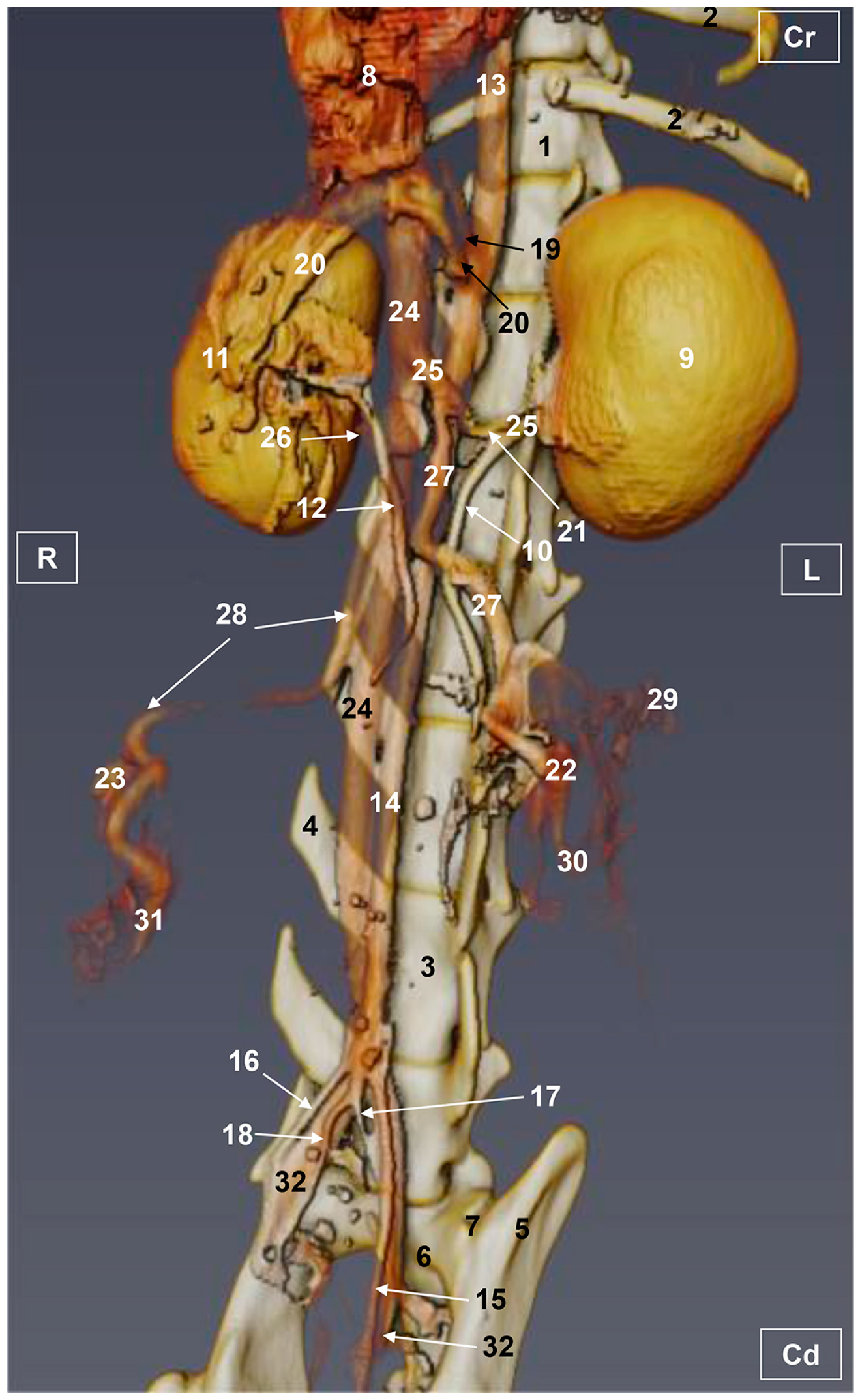
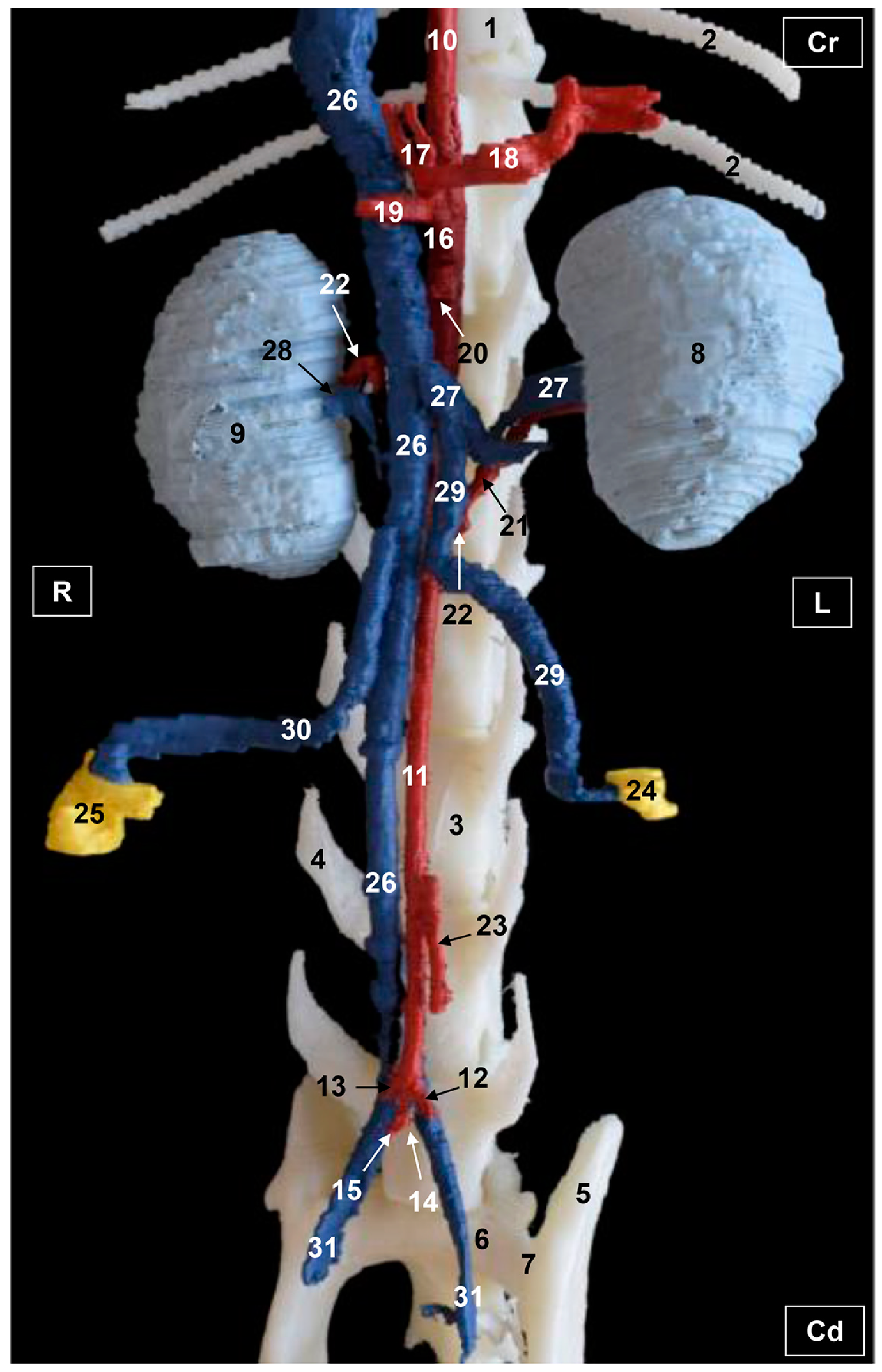
3.1. Gross Dissections
3.1.1. Arterial System
3.1.2. Venous System
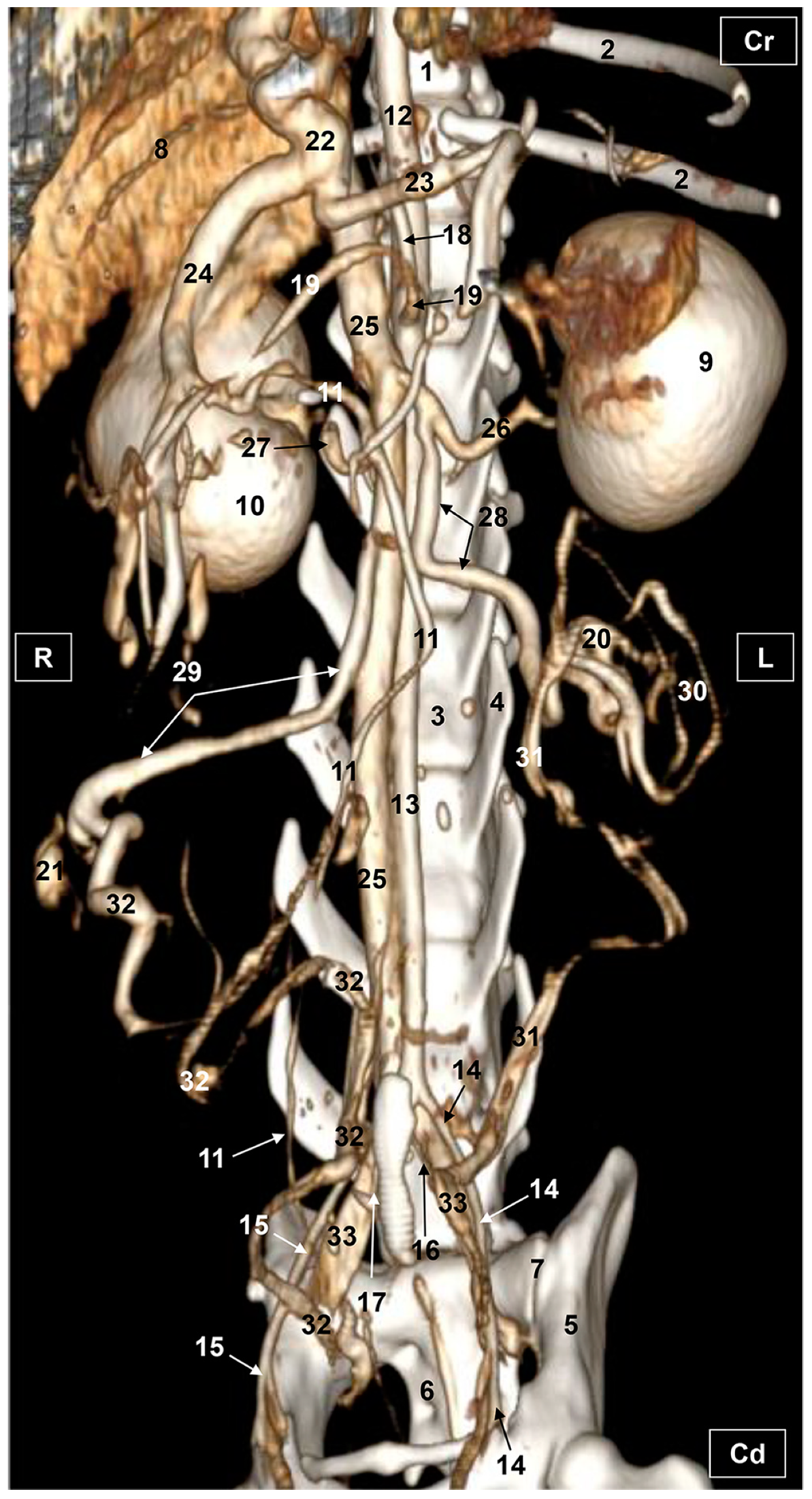
3.2. Computed Tomography Angiography and 3D Printing
3.2.1. Arterial System
3.2.2. Venous System
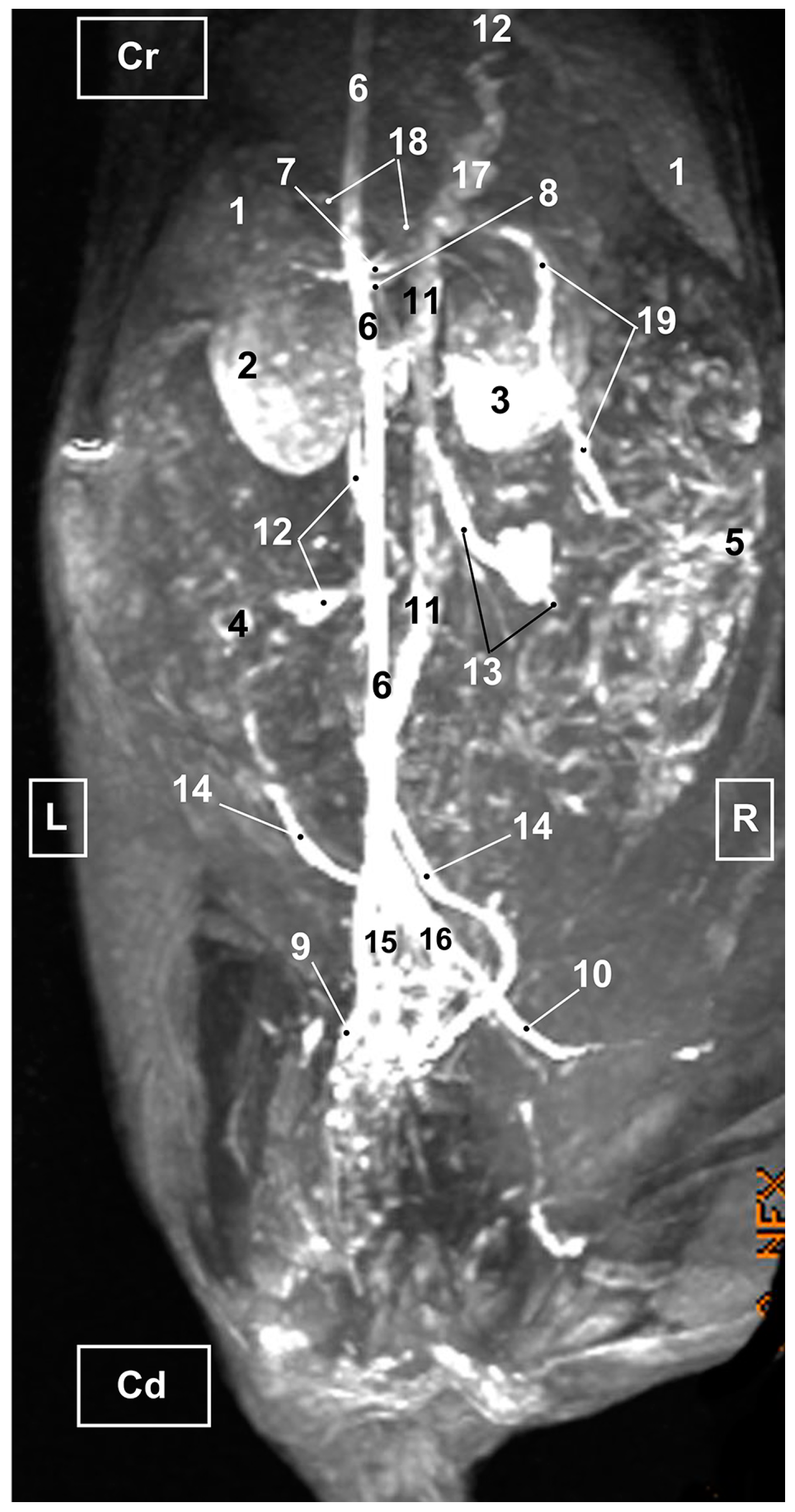
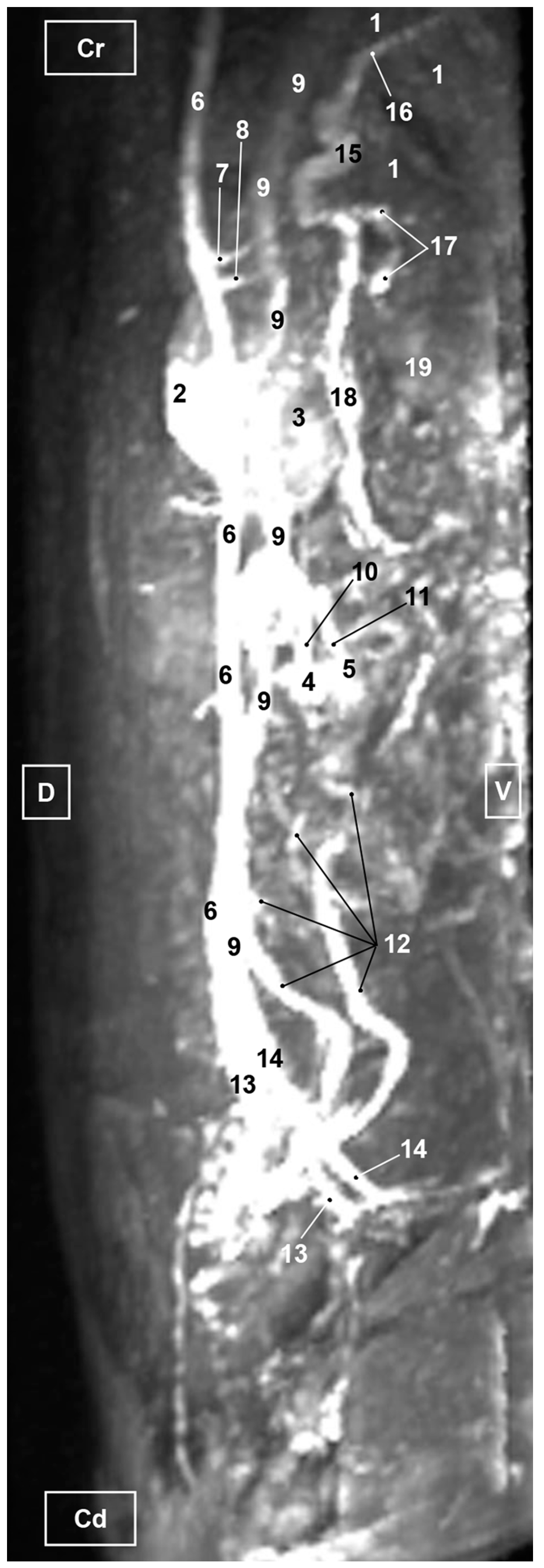
3.3. Magnetic Resonance Angiography
3.3.1. Arterial System
3.3.2. Venous System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weatherspoon, K.; Gilbertie, W.; Catanzano, T. Emergency computed tomography angiogram of the chest, abdomen, and pelvis. Semin. Ultrasound CT MRI 2017, 38, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Michaely, H.J.; Attenberger, U.I.; Kramer, H.; Nael, K.; Reiser, M.F.; Schoenberg, S.O. Abdominal and pelvic MR angiography. Magn. Reason. Imaging Clin. N. Am. 2007, 15, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.S.; Platt, J.L. CT angiography in the abdomen: A pictorial review and update. Abdom. Imaging 2014, 39, 196–214. [Google Scholar] [CrossRef] [PubMed]
- Halpern, E.J.; Rutter, C.M.; Gardiner, G.A., Jr.; Nazarian, L.N.; Wechsler, R.J.; Levin, D.B.; Kueny-Beck, M.; Moritz, M.J.; Carabasi, R.A.; Kahn, M.B.; et al. Comparison of Doppler US and CT angiography for evaluation of renal artery stenosis. Acad. Radiol. 1998, 5, 524–532. [Google Scholar] [CrossRef]
- Green, D.; Parker, D. CTA and MRA: Visualization without catheterization. Semin. Ultrasound CT MRI 2003, 24, 185–191. [Google Scholar] [CrossRef]
- Arfi, A.; Arfi-Rouche, J.; Barrau, V.; Nyangoh Timoh, K.; Touboul, C. Three-dimensional computed tomography angiography reconstruction of the origin of the uterine artery and its clinical significance. Surg. Radiol. Anat. 2018, 40, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Francois, C.J. Abdominal magnetic resonance angiography. Magn. Reson. Imaging Clin. N. Am. 2020, 28, 395–405. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Wang, X.M.; Hao, G.Y.; Bao, J.; Hu, S.; Hu, C.H. Enhanced radiation damage caused by iodinated contrast agents during CT examination. Eur. J. Radiol. 2017, 92, 72–77. [Google Scholar] [CrossRef]
- Edelman, R.R.; Koktzoglou, I. Noncontrast MR angiography: An update. J. Magn. Reson. Imaging 2019, 49, 355–373. [Google Scholar] [CrossRef]
- Korosec, F.R.; Mistretta, C.A. MR angiography: Basic principles and theory. Magn. Reson. Imaging Clin. N. Am. 1998, 6, 223–256. [Google Scholar] [CrossRef]
- Lutterbey, G.; Gieseke, J.; Sommer, T.; Keller, E.; Kuhl, C.; Schild, H.H. A rational study of the great abdominal veins using 2D-TOF- and turbo-spin-echo sequences. Rofo 1998, 169, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Roditi, G.; Wieben, O.; Prince, M.R.; Hecht, E.M. MR angiography series: Abdominal and pelvic MR angiography. Radiographics 2022, 42, E94–E95. [Google Scholar] [CrossRef] [PubMed]
- Ayache, J.B.; Collins, J.D. MR angiography of the abdomen and pelvis. Radiol. Clin. N. Am. 2014, 52, 839–859. [Google Scholar] [CrossRef] [PubMed]
- Lewin, J.S.; Laub, G.; Hausmann, R. Three-dimensional time-of-flight MR angiography: Applications in the abdomen and thorax. Radiology 1991, 179, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.T.; Heath, D.G.; Bliss, D.F.; Cabral, B.; Fishman, E.K. Three-dimensional CT: Real-time interactive volume rendering. AJR Am. J. Roentgenol. 1996, 167, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Perandini, S.; Faccioli, N.; Zaccarella, A.; Re, T.; Mucelli, R.P. The diagnostic contribution of CT volumetric rendering techniques in routine practice. Indian J. Radiol. Imaging. 2010, 20, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.D.; Walker, P.J.; Dake, M.D.; Napel, S.; Jeffrey, R.B.; McDonnell, C.H.; Mitchell, R.S.; Miller, D.C. Three-dimensional spiral computed tomographic angiography: An alternative imaging modality for the abdominal aorta and its branches. J. Vasc. Surg. 1993, 18, 656–664. [Google Scholar] [CrossRef]
- Pereles, F.S.; Baskaran, V. Abdominal magnetic resonance angiography: Principles and practical applications. Top. Magn. Reson. Imaging 2001, 12, 317–326. [Google Scholar] [CrossRef]
- Won, W.W.; Sharma, A.; Wu, W. Retrospective comparison of abdominal ultrasonography and radiography in the investigation of feline abdominal disease. Can. Vet. J. 2015, 56, 1065–1068. [Google Scholar]
- Griffin, S. Feline abdominal ultrasonography: What’s normal? what’s abnormal? Hepatic vascular anomalies. J. Feline Med. Surg. 2019, 21, 645–654. [Google Scholar] [CrossRef]
- Scrivani, P.V.; Martin-Flores, M.; van Hatten, R.; Bezuidenhout, A.J. Structural and functional changes relevant to maxillary arterial flow observed during computed tomography and nonselective digital subtraction angiography in cats with the mouth closed and opened. Vet. Radiol. Ultrasound 2014, 55, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.T.; O’Brien, M.A.; Hartman, S.K.; Mulherin, A.C.; McReynolds, C.J.; McMichael, M.; Rapoport, G.; O’Brien, R.T. Microdose computed tomographic cardiac angiography in normal cats. J. Vet. Cardiol. 2014, 16, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, D.H.; Kim, K.; Oh, D.; Yoon, J. Non-electrocardiography- and electrocardiography-gated computed tomography angiography for the evaluation of feline coronary arteries. Front. Vet. Sci. 2022, 9, 952412. [Google Scholar] [CrossRef] [PubMed]
- Gavrias, E.; Miró, F.; Blanco, B.; Lucena, R.; Ginel, P.J.; Novales, M. Helical computed tomography—Anatomy of the cat abdomen. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2016, 73, 149–152. [Google Scholar] [CrossRef]
- Rojo, D.; Vázquez, J.M.; Sánchez, C.; Arencibia, A.; García, M.I.; Soler, M.; Kilroy, D.; Ramírez, G. Sectional anatomic and tomographic study of the feline abdominal cavity for obtaining a three-dimensional vascular model. Iran. J. Vet. Res. 2020, 21, 279–286. [Google Scholar] [PubMed]
- Makara, M.; Chau, J.; Hall, E.; Kloeppel, H.; Podadera, J.; Barrs, V. Effects of two contrast injection protocols on feline aortic and hepatic enhancement using dynamic computed tomography. Vet. Radiol. Ultrasound 2015, 56, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.D.; Van der Vekens, E.; Rieger, J.; Forterre, F.; Vincenti, S. Preliminary studies on the intrahepatic anatomy of the venous vasculature in cats. Vet. Sci. 2022, 9, 607. [Google Scholar] [CrossRef] [PubMed]
- Rojo Ríos, D.; Ramírez Zarzosa, G.; Soler Laguía, M.; Kilroy, D.; Martínez Gomariz, F.; Sánchez Collado, C.; Gil Cano, F.; García García, M.I.; Jáber, J.R.; Arencibia Espinosa, A. Creation of three-dimensional anatomical vascular and biliary models for the study of the feline liver (Felis silvestris catus L.): A comparative CT, volume rendering (Vr), cast and 3D printing study. Animals 2023, 13, 1573. [Google Scholar] [CrossRef]
- Head, L.L.; Daniel, G.B.; Tobias, K.; Morandi, F.; DeNovo, R.C.; Donnell, R. Evaluation of the feline pancreas using computed tomography and radiolabeled leukocytes. Vet. Radiol. Ultrasound 2003, 44, 420–428. [Google Scholar] [CrossRef]
- Bouma, J.L.; Aronson, L.R.; Keith, D.G.; Saunders, H.M. Use of computed tomography renal angiography for screening feline renal transplant donors. Vet. Radiol. Ultrasound 2003, 44, 636–641. [Google Scholar] [CrossRef]
- Caceres, A.; Zwingenberger, A.; Aronson, L.; Mai, W. Characterization of normal feline renal vascular anatomy with dual-Phase CT angiography. Vet. Radiol. Ultrasound 2008, 49, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Suran, J.N.; Cáceres, A.V.; Reetz, J.A. Comparison between bolus tracking and timing-bolus techniques for renal computed tomographic angiography in normal cats. Vet. Radiol. Ultrasound 2013, 54, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Arencibia, A.; Corbera, J.A.; Ramírez, G.; Contreras, S.; Morales, M.; Jaber, J.R.; Orós, J.; Vázquez, J.M. Three-dimensional time of flight magnetic resonance angiography of the heart and associated vessels in a cat. J. Vet. Cardiol. 2016, 18, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Arencibia, A.; Corbera, J.A.; Gil, F.; Ramírez, G.; Jaber, J.R.; Morales, M.; Vázquez, J.M. Anatomical assessment of intrathoracic cardiovascular structures using fast spin-echo double inversion recovery and steady-state free precession magnetic resonance imaging in a normal cat. J. Vet. Cardiol. 2019, 24, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Ko, M.; Ahn, J.; Ahn, J.; Yu, J.; Chang, J.; Oh, S.; Chang, D. Evaluation of the abdominal aorta and external iliac arteries using three-dimensional time-of-flight, three dimensional electrocardiograph-gated fast spin-echo, and contrast-enhanced magnetic resonance angiography in clinically healthy cats. Front. Vet. Sci. 2002, 9, 819627. [Google Scholar] [CrossRef]
- Lauridsen, H.; Hansen, K.; Norgård, M.; Wang, T.; Pedersen, M. From tissue to silicon to plastic: Three-dimensional printing in comparative anatomy and physiology. R. Soc. Open Sci. 2016, 3, 150643. [Google Scholar] [CrossRef]
- Wilhite, R.; Wölfel, I. 3D Printing for veterinary anatomy: An overview. Anat. Histol. Embryol. 2019, 48, 609–620. [Google Scholar] [CrossRef]
- Schaller, O. Illustrated Veterinary Anatomical Nomenclature; Ferdinand Enke Verlag: Stuttgart, Germany, 1992; pp. 306–307, 310–313, 320–321, 372–383, 388–389. [Google Scholar]
- Nickel, R.; Schumer, A.; Seiferle, E. The Anatomy of the Domestic Animals. In The Circulatory System the Skin and the Cutaneus Organs of the Domestic Mammals; Verlag Paul Parey: Berlin/Hamburg, Germany, 1981; Volume 3, pp. 116, 159–183. [Google Scholar]
- Gil Cano, F.; Latorre Reviriego, R.; Ramírez Zarzosa, G.; López Albors, O.; Ayala Florenciano, M.D.; Martínez Gomariz, F.; Sánchez Collado, C.; Vázquez Autón, J.M. Atlas de Anatomía del Perro; Multimédica: San Cugat del Vallés, Spain, 2021; pp. 143–155, 183–187, 196. [Google Scholar]
- Gil Cano, F.; Latorre Reviriego, R.; Ramírez Zarzosa, G.; López Albors, O.; Ayala Florenciano, M.D.; Martínez Gomariz, F.; Sánchez Collado, C.; Vázquez Autón, J.M. Atlas de Anatomía del Gato; Multimédica: San Cugat del Vallés, Spain, 2022; pp. 1–240, 116–117, 121, 124, 128, 150. [Google Scholar]
- Brühschwein, A.; Klever, J.; Hoffmann, A.S.; Huber, D.; Kaufmann, E.; Reese, S.; Meyer-Lindenberg, A. Free DICOM-viewers for veterinary medicine: Survey and comparison of functionality and user-friendliness of medical imaging PACS-DICOM-viewer freeware for specific use in veterinary medicine practices. J. Digit. Imaging 2020, 33, 54–63. [Google Scholar] [CrossRef]
- OsiriX Lite DICOM-Viewer. Available online: http://www.osirix-viewer.com (accessed on 30 May 2022).
- Salonen, H.M.; Åhlberg, T.M.; Laitinen-Vapaavuori, O.M.; Mölsä, S.H. CT measurement of prostate volume using OsiriX® viewer is reliable, repeatable, and not dependent on observer, CT protocol, or contrast enhancement in dogs. Vet. Radiol. Ultrasound 2022, 63, 729–738. [Google Scholar] [CrossRef]
- Haverkamp, K.; Harder, L.K.; Kuhnt, N.S.M.; Lüpke, M.; Nolte, I.; Wefstaedt, P. Validation of canine prostate volumetric measurements in computed tomography determined by the slice addition technique using the Amira program. BMC Vet. Res. 2019, 15, 49. [Google Scholar] [CrossRef]
- Dietrich, J.; Handschuh, S.; Steidl, R.; Böhler, A.; Forstenpointner, G.; Egerbacher, M.; Peham, C.; Schöpper, H. Muscle fibre architecture of thoracic and lumbar longissimus dorsi muscle in the horse. Animals 2021, 11, 915. [Google Scholar] [CrossRef]
- Czeibert, K.; Baksa, G.; Grimm, A.; Nagy, S.A.; Kubinyi, E.; Petneházy, Ö. MRI, CT and high resolution macro-anatomical images with cryosectioning of a Beagle brain: Creating the base of a multimodal imaging atlas. PLoS ONE 2019, 14, e0213458. [Google Scholar] [CrossRef] [PubMed]
- Büttelmann, G.; Harder, L.K.; Nolte, I.; Wefstaedt, P. Impact of body weight and sex in selected dog breeds on the canine adrenal gland dimensions measured by computed tomographic imaging. BMC Vet. Res. 2023, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- RadiAnt DICOM-Viewer. Available online: http://www.radiantviewer.com (accessed on 14 December 2022).
- Bertolini, G. Anomalies of the portal venous system in dogs and cats as seen on multidetector-row computed tomography: An overview and systematization proposal. Vet. Sci. 2019, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G. Acquired portal collateral circulation in the dog and cat. Vet. Radiol. Ultrasound 2010, 51, 25–33. [Google Scholar] [CrossRef]
- Konstantinidis, A.O.; Patsikas, M.N.; Papazoglou, L.G.; Adamama-Moraitou, K.K. Congenital portosystemic shunts in dogs and cats: Classification, pathophysiology, clinical presentation and diagnosis. Vet. Sci. 2023, 10, 160. [Google Scholar] [CrossRef]
- Pey, P.; Marcon, O.; Drigo, M.; Specchi, S.; Bertolini, G. Multidetector-row computed tomographic characteristics of presumed preureteral vena cava in cats. Vet. Radiol. Ultrasound 2015, 56, 359–366. [Google Scholar] [CrossRef]
- Jirasakul, J.; Thammasiri, N.; Darawiroj, D.; Choisunirachon, N.; Thanaboonnipat, C. Computed tomographic appearance of circumcaval and circumuterine ureter in a cat. Vet. Med. Sci. 2020, 6, 335–341. [Google Scholar] [CrossRef]
- Spediacci, C.; Longo, M.; Specchi, S.; Pey, P.; Rabba, S.; Mavraki, E.; Di Giancamillo, M.; Panopoulos, I. Computed tomographic appearance of transcaval ureter in two dogs and three cats: A novel CVC congenital malformation. Front. Vet. Sci. 2022, 9, 965185. [Google Scholar] [CrossRef]
- Nelson, N.C.; Nelson, L.L. Anatomy of extrahepatic portosystemic shunts in dogs as determined by computed tomography angiography. Vet. Radiol. Ultrasound 2011, 52, 498–506. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojo Ríos, D.; Ramírez Zarzosa, G.; Soler Laguía, M.; Kilroy, D.; Martínez Gomariz, F.; Sánchez Collado, C.; Gil Cano, F.; García García, M.I.; Ayala Florenciano, M.D.; Arencibia Espinosa, A. Anatomical and Three-Dimensional Study of the Female Feline Abdominal and Pelvic Vascular System Using Dissections, Computed Tomography Angiography and Magnetic Resonance Angiography. Vet. Sci. 2023, 10, 704. https://doi.org/10.3390/vetsci10120704
Rojo Ríos D, Ramírez Zarzosa G, Soler Laguía M, Kilroy D, Martínez Gomariz F, Sánchez Collado C, Gil Cano F, García García MI, Ayala Florenciano MD, Arencibia Espinosa A. Anatomical and Three-Dimensional Study of the Female Feline Abdominal and Pelvic Vascular System Using Dissections, Computed Tomography Angiography and Magnetic Resonance Angiography. Veterinary Sciences. 2023; 10(12):704. https://doi.org/10.3390/vetsci10120704
Chicago/Turabian StyleRojo Ríos, Daniel, Gregorio Ramírez Zarzosa, Marta Soler Laguía, David Kilroy, Francisco Martínez Gomariz, Cayetano Sánchez Collado, Francisco Gil Cano, María I. García García, María Dolores Ayala Florenciano, and Alberto Arencibia Espinosa. 2023. "Anatomical and Three-Dimensional Study of the Female Feline Abdominal and Pelvic Vascular System Using Dissections, Computed Tomography Angiography and Magnetic Resonance Angiography" Veterinary Sciences 10, no. 12: 704. https://doi.org/10.3390/vetsci10120704
APA StyleRojo Ríos, D., Ramírez Zarzosa, G., Soler Laguía, M., Kilroy, D., Martínez Gomariz, F., Sánchez Collado, C., Gil Cano, F., García García, M. I., Ayala Florenciano, M. D., & Arencibia Espinosa, A. (2023). Anatomical and Three-Dimensional Study of the Female Feline Abdominal and Pelvic Vascular System Using Dissections, Computed Tomography Angiography and Magnetic Resonance Angiography. Veterinary Sciences, 10(12), 704. https://doi.org/10.3390/vetsci10120704






