The Serological Response in Cattle following Administration of a Heterologous Sheep Pox Virus Strain Vaccine for Protection from Lumpy Skin Disease; Current Situation in Armenia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
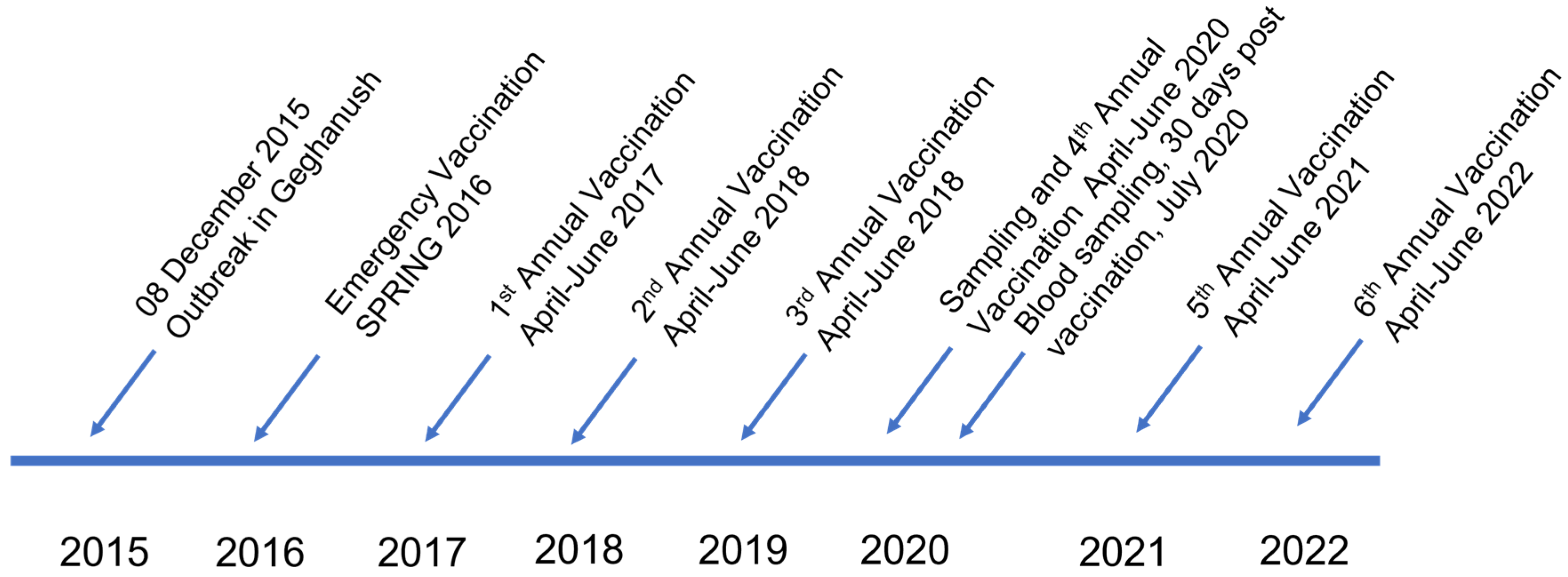
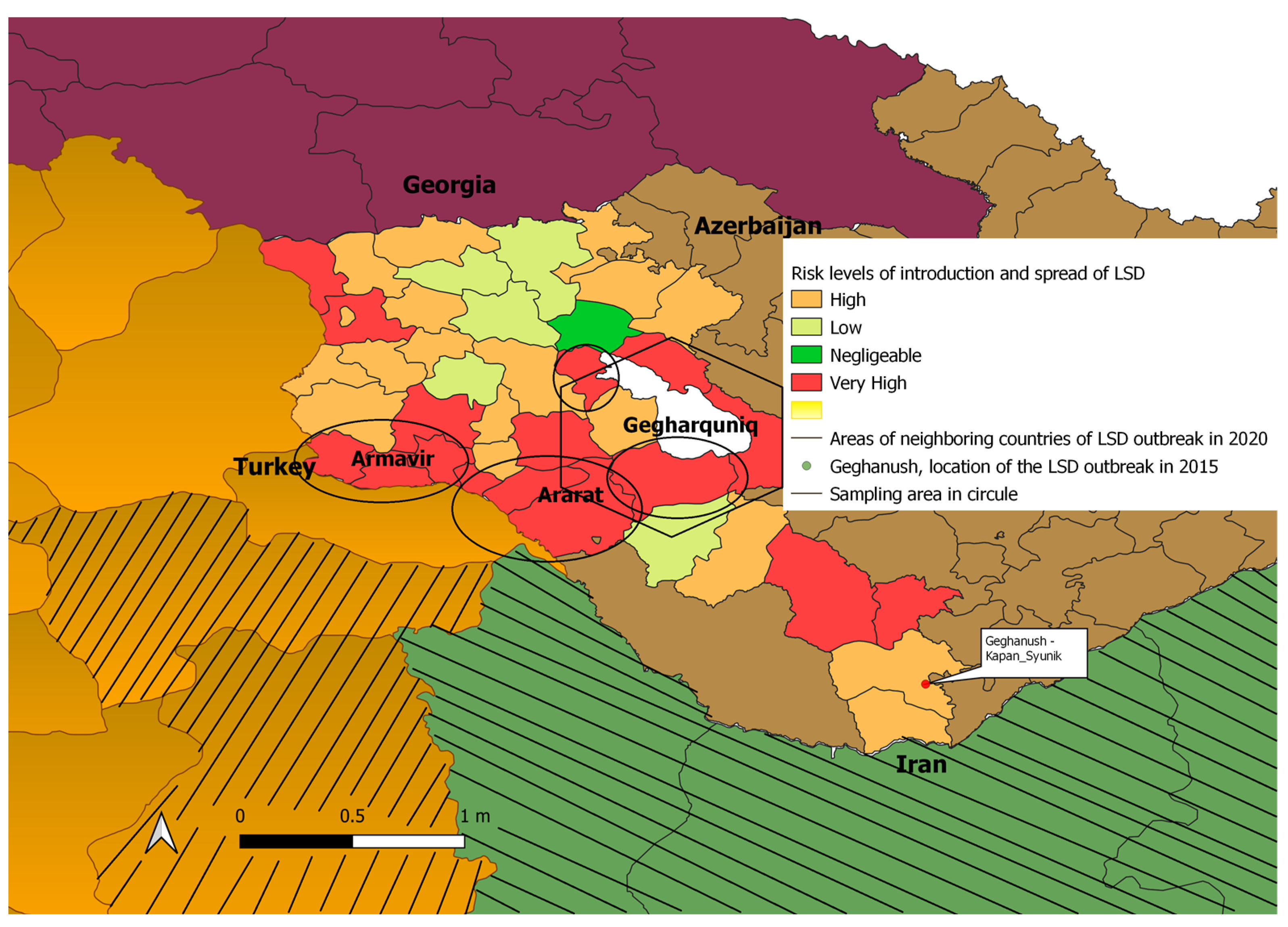
2.1. Study Area
2.2. Sample Size
2.3. Sample Collection
2.4. Vaccination
2.5. Detection of Antibodies in Cattle
2.6. Detection of LSDV in Ticks
3. Results
3.1. Serological Results
3.2. Vaccination Safety
3.3. Tick Species and LSDV Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIE. Ch. 1. Criteria for Inclusion of Diseases, Infections and Infestations in the OIE List. In OIE Terrestrial Animal Health Code, 23rd ed.; Office International des Epizooties: Paris, France, 2014; Volume 1. [Google Scholar]
- Sameea Yousefi, P.; Mardani, K.; Dalir-Naghadeh, B.; Jalilzadeh-Amin, G. Epidemiological Study of Lumpy Skin Disease Outbreaks in North-western Iran. Transbound. Emerg. Dis. 2017, 64, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, E.; Alexandrov, T.; Beltrán-Alcrudo, D. Lumpy Skin Disease Field Manual. A manual for Veterinarians; FAO Animal Production and Health Manual 20; Food and Agriculture Organization: Rome, Italy, 2017. [Google Scholar]
- Tuppurainen, E.S.M.; Venter, E.H.; Shisler, J.L.; Gari, G.; Mekonnen, G.A.; Juleff, N.; Lyons, N.A.; De Clercq, K.; Upton, C.; Bowden, T.R.; et al. Review: Capripoxvirus Diseases: Current Status and Opportunities for Control. Transbound. Emerg. Dis. 2017, 64, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Kosareva, O.; Kukushkina, M.; Konstantinov, A.; Diev, V.; Starov, S.; Yasneva, E.; Basova, D. Lumpy dermatitis (tubercle), clinical signs in experimental infection of cattle. Proc. Fed. Cent. Anim. Health 2010, 8, 73. [Google Scholar]
- Chihota, C.M.; Rennie, L.F.; Kitching, R.P.; Mellor, P.S. Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae). Epidemiol. Infect. 2001, 126, 317–321. [Google Scholar] [CrossRef]
- Makarov, V.; Sukharev, O.; Vasilevich, F.; Gulyukin, M. Transmission of Viral Infections by Insect Vectors. Issues Leg. Regul. Vet. Med. 2014, 2, 44–50. [Google Scholar]
- Paslaru, A.I.; Verhulst, N.O.; Maurer, L.M.; Brendle, A.; Pauli, N.; Vogtlin, A.; Renzullo, S.; Ruedin, Y.; Hoffmann, B.; Torgerson, P.R.; et al. Potential mechanical transmission of Lumpy skin disease virus (LSDV) by the stable fly (Stomoxys calcitrans) through regurgitation and defecation. Curr. Res. Insect Sci. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Sprygin, A.; Fedorova, O.; Nesterov, A.; Shumilova, I.; Byadovskaya, O. The Stable Fly Stomoxys Calcitrans L as a Potential Vector in the Spread of Lumpy Skin Disease Virus in Russia: Short Review. In Proceedings of the International Scientific and Practical Conference “Development of the Agro-lndustrial Complex in the Context of Robotization and Digitalization of Production in Russia and Abroad” (DAIC 2020), Yekaterinburg, Russia, 15–16 October 2020; p. 06026. [Google Scholar] [CrossRef]
- Sprygin, A.; Pestova, Y.; Wallace, D.B.; Tuppurainen, E.; Kononov, A.V. Transmission of lumpy skin disease virus: A short review. Virus Res. 2019, 269, 197637. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, E.S.; Lubinga, J.C.; Stoltsz, W.H.; Troskie, M.; Carpenter, S.T.; Coetzer, J.A.; Venter, E.H.; Oura, C.A. Evidence of vertical transmission of lumpy skin disease virus in Rhipicephalus decoloratus ticks. Ticks Tick Borne Dis. 2013, 4, 329–333. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.; Stoltsz, W.H.; Troskie, M.; Wallace, D.B.; Oura, C.A.; Mellor, P.S.; Coetzer, J.A.; Venter, E.H. A potential role for ixodid (hard) tick vectors in the transmission of lumpy skin disease virus in cattle. Transbound. Emerg. Dis. 2011, 58, 93–104. [Google Scholar] [CrossRef]
- Tuppurainen, E.; Dietze, K.; Wolff, J.; Bergmann, H.; Beltran-Alcrudo, D.; Fahrion, A.; Lamien, C.E.; Busch, F.; Sauter-Louis, C.; Conraths, F.J.; et al. Review: Vaccines and Vaccination against Lumpy Skin Disease. Vaccines 2021, 9, 1136. [Google Scholar] [CrossRef]
- Gari, G.; Abie, G.; Gizaw, D.; Wubete, A.; Kidane, M.; Asgedom, H.; Bayissa, B.; Ayelet, G.; Oura, C.A.; Roger, F.; et al. Evaluation of the safety, immunogenicity and efficacy of three capripoxvirus vaccine strains against lumpy skin disease virus. Vaccine 2015, 33, 3256–3261. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, E.S.; Pearson, C.R.; Bachanek-Bankowska, K.; Knowles, N.J.; Amareen, S.; Frost, L.; Henstock, M.R.; Lamien, C.E.; Diallo, A.; Mertens, P.P. Characterization of sheep pox virus vaccine for cattle against lumpy skin disease virus. Antivir. Res. 2014, 109, 1–6. [Google Scholar] [CrossRef] [PubMed]
- WOAH. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2022; World Organization for Animal Health: Paris, France, 2022. [Google Scholar]
- Saltykov, Y.; Kolosova, A.; Fedorova, V. Vaccines against infectious nodular dermatitis in cattle. Veterinary 2021, 10, 3–8. [Google Scholar]
- Kononov, A. Features of the diagnosis and prevention of infectious nodular dermatitis (lodular dermatitis) of cattle. In Proceedings of the All-Russian Research Institute of Experimental Veterinary Medicine named after JR Kovalenko Materials of the IX International Veterinary Congress “One World—One Health”, Svetlogorsk, Russia, 17–19 April 2019; Volume 81, pp. 74–75. [Google Scholar]
- Ospanov, E.; Kaimoldina, S.; Kirpichenko, V.; Kenesbek, M. Immunoprophylaxis of Nodular Dermatitis. Izdenister Natigeler 2021, 4, 12–21. [Google Scholar] [CrossRef]
- Abutarbush, S.M.; Tuppurainen, E.S.M. Serological and clinical evaluation of the Yugoslavian RM65 sheep pox strain vaccine use in cattle against lumpy skin disease. Transbound. Emerg. Dis. 2018, 65, 1657–1663. [Google Scholar] [CrossRef]
- Ben-Gera, J.; Klement, E.; Khinich, E.; Stram, Y.; Shpigel, N.Y. Comparison of the efficacy of Neethling lumpy skin disease virus and x10RM65 sheep-pox live attenuated vaccines for the prevention of lumpy skin disease—The results of a randomized controlled field study. Vaccine 2015, 33, 4837–4842. [Google Scholar] [CrossRef]
- Hamdi, J.; Bamouh, Z.; Jazouli, M.; Boumart, Z.; Tadlaoui, K.O.; Fihri, O.F.; El Harrak, M. Experimental evaluation of the cross-protection between Sheeppox and bovine Lumpy skin vaccines. Sci. Rep. 2020, 10, 8888. [Google Scholar] [CrossRef]
- Rouby, S.R.; Safwat, N.M.; Hussein, K.H.; Abdel-Ra’ouf, A.M.; Madkour, B.S.; Abdel-Moneim, A.S.; Hosein, H.I. Lumpy skin disease outbreaks in Egypt during 2017–2018 among sheeppox vaccinated cattle: Epidemiological, pathological, and molecular findings. PLoS ONE 2021, 16, e0258755. [Google Scholar] [CrossRef]
- Varshovi, H.R.; Norian, R.; Azadmehr, A.; Afzal Ahangaran, N. Immune response characteristics of Capri pox virus vaccines following emergency vaccination of cattle against lumpy skin disease virus. Iran. J. Vet. Sci. Technol. 2017, 9, 33–40. [Google Scholar] [CrossRef]
- Zhugunissov, K.; Bulatov, Y.; Orynbayev, M.; Kutumbetov, L.; Abduraimov, Y.; Shayakhmetov, Y.; Taranov, D.; Amanova, Z.; Mambetaliyev, M.; Absatova, Z.; et al. Goatpox virus (G20-LKV) vaccine strain elicits a protective response in cattle against lumpy skin disease at challenge with lumpy skin disease virulent field strain in a comparative study. Vet. Microbiol. 2020, 245, 108695. [Google Scholar] [CrossRef]
- Abdallah, F.M.; El Damaty, H.M.; Kotb, G.F. Sporadic cases of lumpy skin disease among cattle in Sharkia province, Egypt: Genetic characterization of lumpy skin disease virus isolates and pathological findings. Vet. World 2018, 11, 1150–1158. [Google Scholar] [CrossRef]
- Milovanovic, M.; Dietze, K.; Milicevic, V.; Radojicic, S.; Valcic, M.; Moritz, T.; Hoffmann, B. Humoral immune response to repeated lumpy skin disease virus vaccination and performance of serological tests. BMC Vet. Res. 2019, 15, 80. [Google Scholar] [CrossRef]
- Diagnostics, I. ID Screen® Capripox Double Antigen Multi-Species. Available online: https://www.innovative-diagnostics.com/produit/id-screen-capripox-double-antigen-multi-species/ (accessed on 11 January 2023).
- Markosyan, T.; Sargsyan, K.; Kharatyan, S.; Elbakyan, H.; Hakobyan, V.; Mkrtchyan, H. Lumpy Skin Disease in Armenia. J. Vet. Sci. Technol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Sargsyan, H.V.; Markosyan, T.A.; Akopyan, V.L.; Elbakyan, A.L.; Kharatyan, S.A. Studying the Virulent Properties of the Field Isolate of Bovine Nodular Dermatitis “GEGANUSH-2016”. Vet. Pathol. 2020, 1, 5–9. [Google Scholar] [CrossRef]
- Authority, E.F.S. Lumpy skin disease II. Data collection and analysis. Efsa J. 2018, 16, e05176. [Google Scholar]
- Rozstalnyy, A.; Aguanno, R.; Beltran-Alcrudo, D. Lumpy skin disease: Country situation and capacity reports. EMPRES-Anim. Health 2017, 360, 47. [Google Scholar]
- Katsoulos, P.-D.; Chaintoutis, S.C.; Dovas, C.I.; Polizopoulou, Z.S.; Brellou, G.D.; Agianniotaki, E.I.; Tasioudi, K.E.; Chondrokouki, E.; Papadopoulos, O.; Karatzias, H.; et al. Investigation on the incidence of adverse reactions, viraemia and haematological changes following field immunization of cattle using a live attenuated vaccine against lumpy skin disease. Transbound. Emerg. Dis. 2018, 65, 174–185. [Google Scholar] [CrossRef]
- Authority, E.F.S.; Calistri, P.; De Clercq, K.; Gubbins, S.; Klement, E.; Stegeman, A.; Cortiñas Abrahantes, J.; Marojevic, D.; Antoniou, S.-E.; Broglia, A. Lumpy skin disease epidemiological report IV: Data collection and analysis. EFSA J. 2020, 18, e06010. [Google Scholar] [CrossRef]
- Burkov, P.; Scherbakov, P.; Slobodyansky, S. Vaccinal Prevention of Nodular Dermatitis in Hereford Cattle (Clinical and Immunological Implications). In Proceedings of the E3S Web of Conferences, International Scientific and Practical Conference “Development of the Agro-lndustrial Complex in the Context of Robotization and Digitalization of Production in Russia and Abroad” (DAIC 2020), Yekaterinburg, Russia, 15–16 October 2020; p. 02038. [Google Scholar]
- Burkov, P.V.; Shcherbakov, P.N.; Slobodyanskiy, S.R.; Scherbakova, T.B.; Stepanova, K.V.; Epanchintseva, O.V.; Abdyramanova, T.D. Vaccine prophylaxis of lumpy skin disease. IOP Conf. Ser. Earth Environ. Sci. 2021, 677, 042017. [Google Scholar] [CrossRef]
- Diev, V.I.; Starov, S.K.; Basova, D.K.; Kulakov, V.Y.; Konstantinov, A.V. Testing of Sheep Pox Virus Vaccine in Sheep Under Experimental and Epizootic Conditions. Vet. Sci. Today 2017, 2, 62–66. [Google Scholar]
- Ragimkhanovich, B.N.; Gajdarovich, G.M.; Sharapovich, A.S.; Shagabudinovich, S.M.; Salikhovich, S.Y.; Albertovna, K.K.; Akaevich, O.R. Method of Preventing Nodular Dermatitis in Bovine Animals; Federal Service for Intellectual Property: Moscow, Russia, 2017. [Google Scholar]
- Kitching, R.P.; Hammond, J.M.; Black, D.N. Studies on the Major Common Precipitating Antigen of Capripoxvirus. J. Gen. Virol. 1986, 67, 139–148. [Google Scholar] [CrossRef] [PubMed]
- FAO; EuFMD. European Neighborhood EuFMD Pillar II; Report on Significant FAST Disease Events and Information; FAO: Rome, Italy, 2022; p. 21. [Google Scholar]
| Region | Area | Number of Cattle | Plan Vaccination | Vaccinated Animals | Vaccination Coverage |
|---|---|---|---|---|---|
| Ararat | Ararat | 15,464 | 13,918 | 13,996 | 90.5 |
| Artashat | 13,444 | 12,100 | 12,211 | 90.8 | |
| Masis | 11,272 | 10,145 | 10,211 | 90.6 | |
| Armavir | Ejmiatsin | 13,314 | 12,427 | 12,457 | 93.6 |
| Armavir | 29,543 | 26,589 | 26,621 | 90.1 | |
| Baghramyan | 11,217 | 10,095 | 10,096 | 90.0 | |
| Gegharquniq | Sevan | 12,613 | 12,613 | 12,612 | 99.99 |
| Martuni | 29,585 | 29,585 | 29,617 | 100.1 | |
| Vardenis | 23,114 | 14,215 | 14,271 | 61.7 | |
| Gavar | 17,610 | 10,830 | 10,822 | 61.5 | |
| Chambarak | 12,002 | 7381 | 7388 | 61.6 |
| Region | Cattle | Before Vaccination S/P% | 30 Days after Vaccination S/P% |
|---|---|---|---|
| Ararat | Cattle 1/1 | 0.7 | 49.9 |
| Cattle 1/2 | 17.5 | 85.2 | |
| Cattle 1/3 | 3.7 | 56.7 | |
| Cattle 1/4 | 0.1 | 60.4 | |
| Cattle 1/5 | 6.7 | 82.0 | |
| Cattle 1/6 | 1.9 | 45.9 | |
| Cattle 1/7 | 18.2 | 91.4 | |
| Cattle 1/8 | 19.6 | 89.4 | |
| Cattle 1/9 | 0.4 | 45.9 | |
| Cattle 1/10 | 6.7 | 48.3 | |
| Cattle 1/11 | 1.9 | 59.5 | |
| Cattle 1/12 | 2.5 | 36.1 | |
| Cattle 1/13 | 5.5 | 52.0 | |
| Cattle 1/14 | −1.4 | 52.4 | |
| Cattle 1/15 | −0.2 | 89.4 | |
| Cattle 1/16 | 3.9 | 82.7 | |
| Cattle 1/17 | −2.4 | 45.1 | |
| Cattle 1/18 | 0.4 | 49.9 | |
| Cattle 1/19 | 0.9 | 82.5 | |
| Cattle 1/20 | 9.9 | 91.4 | |
| Armavir | Cattle 2/1 | −1.7 | 52.0 |
| Cattle 2/2 | 9.8 | 59.2 | |
| Cattle 2/3 | −0.1 | 45.9 | |
| Cattle 2/4 | 8.0 | 61.2 | |
| Cattle 2/5 | 0.4 | 56.5 | |
| Cattle 2/6 | −2.1 | 44.1 | |
| Cattle 2/7 | −1.6 | 38.6 | |
| Cattle 2/8 | 8.0 | 63.6 | |
| Cattle 2/9 | 9.3 | 51.3 | |
| Cattle 2/10 | 6.3 | 62.2 | |
| Cattle 2/11 | 11.3 | 57.2 | |
| Cattle 2/12 | 1.7 | 35.1 | |
| Cattle 2/13 | 14.6 | 78.8 | |
| Cattle 2/14 | 11.4 | 76.1 | |
| Cattle 2/15 | 1.9 | 41.6 | |
| Cattle 2/16 | 2.0 | 46.9 | |
| Cattle 2/17 | −2.4 | 35.1 | |
| Cattle 2/18 | −0.5 | 49.8 | |
| Cattle 2/19 | 3.9 | 68.7 | |
| Cattle 2/20 | 5.5 | 74.9 | |
| Gegharqunik | Cattle 3/1 | 11.1 | 65.4 |
| Cattle 3/2 | 9.4 | 66.3 | |
| Cattle 3/3 | 7.9 | 58.4 | |
| Cattle 3/4 | 3.4 | 46.9 | |
| Cattle 3/5 | 3.2 | 41.6 | |
| Cattle 3/6 | 0.6 | 37.5 | |
| Cattle 3/7 | 2.1 | 35.1 | |
| Cattle 3/8 | 2.4 | 52.2 | |
| Cattle 3/9 | 0.9 | 42.1 | |
| Cattle 3/10 | 0.4 | 43.1 | |
| Cattle 3/11 | 5.6 | 76.0 | |
| Cattle 3/12 | 0.6 | 37.1 | |
| Cattle 3/13 | 3.6 | 52.1 | |
| Cattle 3/14 | 12.1 | 59.6 | |
| Cattle 3/15 | 18.9 | 93.7 | |
| Cattle 3/16 | 3.4 | 59.1 | |
| Cattle 3/17 | 1.6 | 41.6 | |
| Cattle 3/18 | 6.2 | 73.9 | |
| Cattle 3/19 | 8.9 | 74.9 | |
| Cattle 3/20 | 11.8 | 85.3 |
| Regions | Number of Examined Samples | 30 Days after Vaccination | |||
|---|---|---|---|---|---|
| Seronegative | Seropositive | ||||
| n | % | n | % | ||
| Gegharkunik | 266 | 43 | 16.17 | 223 | 83.83 |
| Armavir | 266 | 36 | 13.53 | 230 | 86.47 |
| Ararat | 266 | 32 | 12.03 | 234 | 87.97 |
| TOTAL | 798 | 111 | 13.91 * | 687 | 86.09 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakobyan, V.; Sargsyan, K.; Kharatyan, S.; Elbakyan, H.; Sargsyan, V.; Markosyan, T.; Vardanyan, T.; Badalyan, M.; Achenbach, J.E. The Serological Response in Cattle following Administration of a Heterologous Sheep Pox Virus Strain Vaccine for Protection from Lumpy Skin Disease; Current Situation in Armenia. Vet. Sci. 2023, 10, 102. https://doi.org/10.3390/vetsci10020102
Hakobyan V, Sargsyan K, Kharatyan S, Elbakyan H, Sargsyan V, Markosyan T, Vardanyan T, Badalyan M, Achenbach JE. The Serological Response in Cattle following Administration of a Heterologous Sheep Pox Virus Strain Vaccine for Protection from Lumpy Skin Disease; Current Situation in Armenia. Veterinary Sciences. 2023; 10(2):102. https://doi.org/10.3390/vetsci10020102
Chicago/Turabian StyleHakobyan, Varduhi, Khachik Sargsyan, Satenik Kharatyan, Hasmik Elbakyan, Vazgen Sargsyan, Tigran Markosyan, Tigranuhi Vardanyan, Manvel Badalyan, and Jenna E. Achenbach. 2023. "The Serological Response in Cattle following Administration of a Heterologous Sheep Pox Virus Strain Vaccine for Protection from Lumpy Skin Disease; Current Situation in Armenia" Veterinary Sciences 10, no. 2: 102. https://doi.org/10.3390/vetsci10020102
APA StyleHakobyan, V., Sargsyan, K., Kharatyan, S., Elbakyan, H., Sargsyan, V., Markosyan, T., Vardanyan, T., Badalyan, M., & Achenbach, J. E. (2023). The Serological Response in Cattle following Administration of a Heterologous Sheep Pox Virus Strain Vaccine for Protection from Lumpy Skin Disease; Current Situation in Armenia. Veterinary Sciences, 10(2), 102. https://doi.org/10.3390/vetsci10020102







