Horses’ Tactile Reactivity Differs According to the Type of Work: The Example of Equine-Assisted Intervention
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Statement
2.2. Study 1: Horses’ Tactile Reactivity in EAI and Non EAI Horses
2.2.1. Methods
Study Sites
Horse Subjects
Tactile Reactivity Test
Data and Statistical Analyses
- (1)
- Total reactivity: sum of all responses, up to a maximum of 24 (4 filaments tested on both sides on 3 areas of the body).
- (2)
- The reactivity by filament size: the sum of the responses for each size of filaments tested. Since data were similar for thin (0.008 g and 0.002 g) or thick (1 g and 300 g) filaments, respectively, we used these categories for statistical analyses.
- (3)
- Reactivity per side of the body: the sum of the responses on the left and right sides of the body (areas and filaments pooled), respectively.
- (4)
- Reactivity by body area: the sum of the responses recorded for each body area tested (sides and filaments pooled).
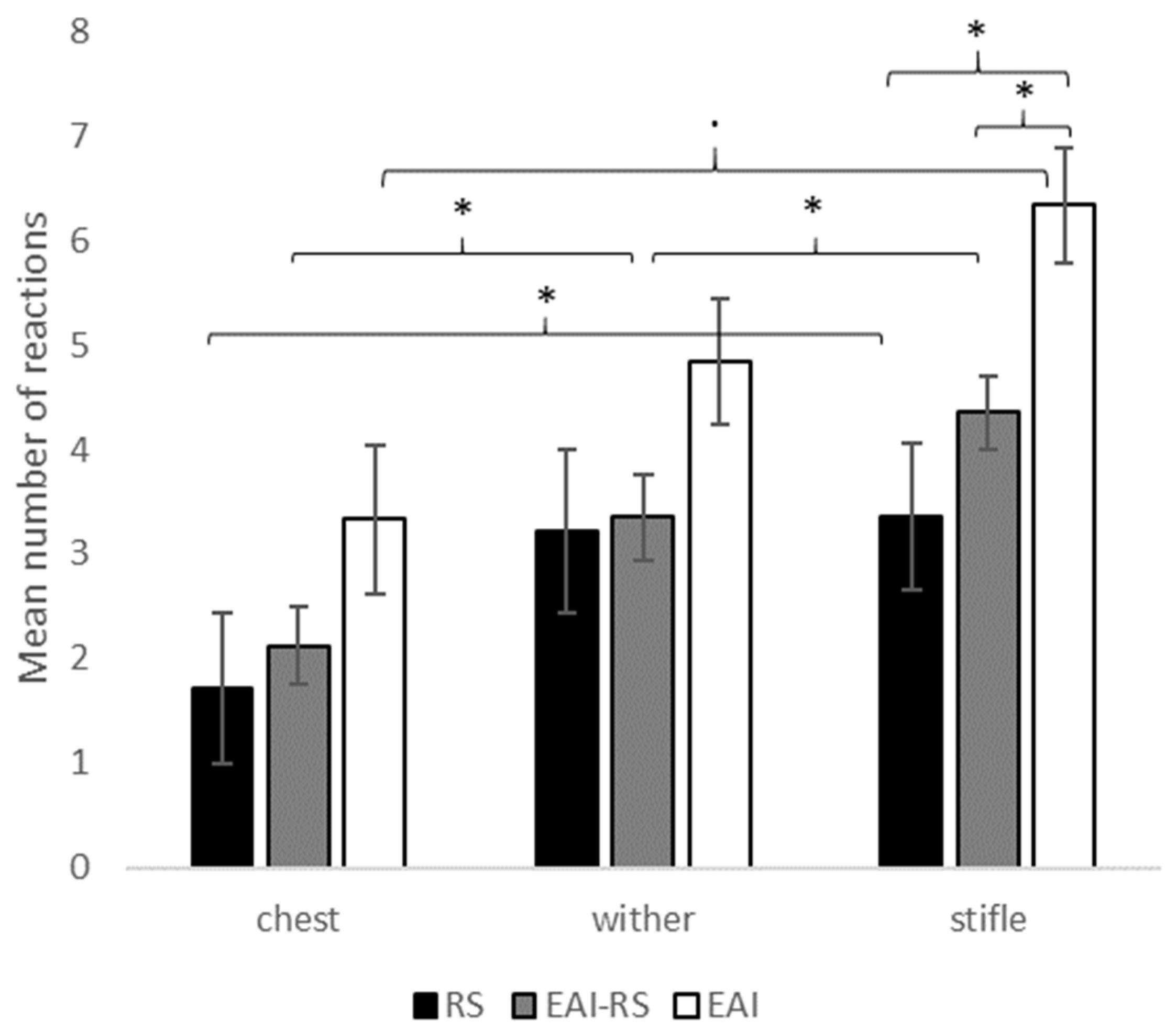
2.2.2. Results
2.2.3. Conclusion of Study 1
2.3. Study 2: Comparison of Grooming Procedure between Persons Diagnosed with or without Mental and/or Developmental Disorders
2.3.1. Methods
Subjects
- –
- Nine horses (6 mares, 3 geldings, age: ±SE = 13.4 ± 1.4 y.o) that had been involved only in EAI for at least one year. They lived in groups, in pasture with hay and water ad libitum.
- –
- Nine horses (7 mares, 2 geldings, age: ±SE = 14.4 ± 1.3 y.o) that had worked in conventional riding centre lessons for at least one year and lived in straw bedded, 3 × 3 m2 individual stalls with water provided ad libitum and hay and commercial pellets provided twice a day.
- –
- Eleven leisure horses (6 mares, 2 geldings, 3 stallions age: ±SE = 11.4 ± 2.2 y.o) that were occasionally ridden outdoors and lived in groups in pastures with water ad libitum and hay during winter.
Grooming Session
Data Recording and Analyses
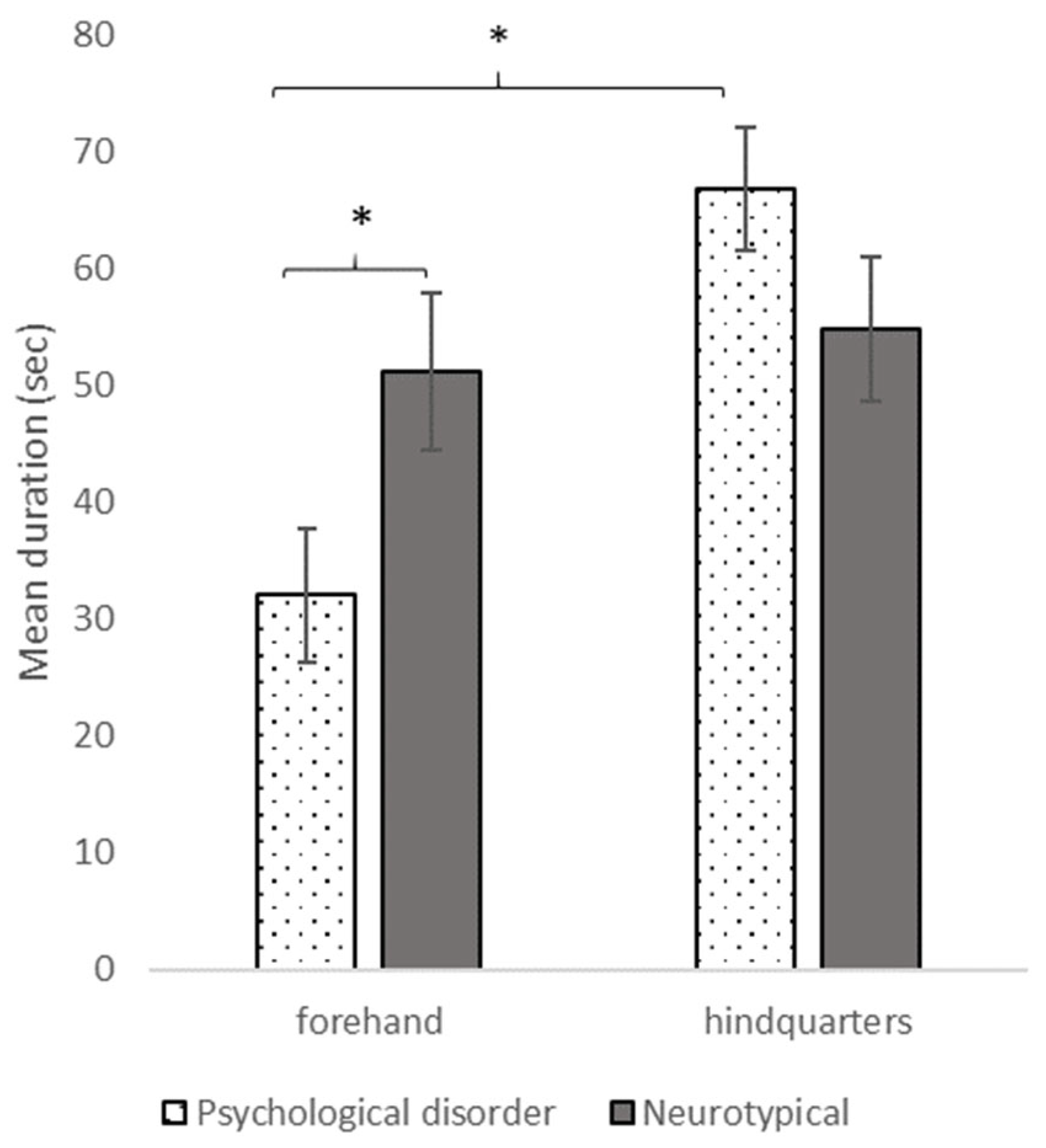
2.3.2. Results
2.3.3. Conclusion of Study 2
3. General Discussion
3.1. Higher Tactile Reactivity in EAI Horses
3.2. Differences in Tactile Reactivity between Body Areas and According to the Working Activity
3.3. Differences in Brushing Location and Modalities between People with or without Mental and/or Developmental Disorders in Grooming EAI Sessions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cauchoix, M.; Chaine, A.S. How Can We Study the Evolution of Animal Minds? Front. Psychol. 2016, 7, 358. [Google Scholar] [CrossRef] [PubMed]
- Bremner, A.J.; Spence, C. The Development of Tactile Perception. Adv. Child. Dev. Behav. 2017, 52, 227–268. [Google Scholar] [CrossRef]
- Bowden, J.L.; McNulty, P.A. Age-Related Changes in Cutaneous Sensation in the Healthy Human Hand. Age 2013, 35, 1077–1089. [Google Scholar] [CrossRef]
- Plomin, R. Developmental Behavioral Genetics. Child Dev. 1983, 54, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, S.; Stevens, B.; Beyene, J.; Chan, P.C.; Bagg, M.; Asztalos, E. Pain Behaviours in Extremely Low Gestational Age Infants. Early Hum. Dev. 2008, 84, 451–458. [Google Scholar] [CrossRef]
- André, V.; Durier, V.; Beuchée, A.; Roué, J.M.; Lemasson, A.; Hausberger, M.; Sizun, J.; Henry, S. Higher Tactile Sensitivity in Preterm Infants at Term-Equivalent Age: A Pilot Study. PLoS ONE 2020, 15, e0229270. [Google Scholar] [CrossRef]
- Ragert, P.; Schmidt, A.; Altenmüller, E.; Dinse, H.R. Superior Tactile Performance and Learning in Professional Pianists: Evidence for Meta-Plasticity in Musicians. Eur. J. Neurosci. 2004, 19, 473–478. [Google Scholar] [CrossRef]
- Reuter, E.M.; Voelcker-Rehage, C.; Vieluf, S.; Godde, B. Touch Perception throughout Working Life: Effects of Age and Expertise. Exp. Brain Res. 2012, 216, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, D.M.; Leknes, S.; Løseth, G.; Wessberg, J.; Olausson, H. The Neurobiology Shaping Affective Touch: Expectation, Motivation, and Meaning in the Multisensory Context. Front. Psychol. 2016, 6, 1986. [Google Scholar] [CrossRef]
- Sailer, U.; Ackerley, R. Exposure Shapes the Perception of Affective Touch. Dev. Cogn. Neurosci. 2019, 35, 109–114. [Google Scholar] [CrossRef]
- Gazzola, V.; Spezio, M.L.; Etzel, J.A.; Castelli, F.; Adolphs, R.; Keysers, C. Primary Somatosensory Cortex Discriminates Affective Significance in Social Touch. Proc. Natl. Acad. Sci. USA 2012, 109, E1657–E1666. [Google Scholar] [CrossRef]
- Henry, S.; Richard-Yris, M.A.; Hausberger, M. Influence of Various Early Human-Foal Interferences on Subsequent Human-Foal Relationship. Dev. Psychobiol. 2006, 48, 712–718. [Google Scholar] [CrossRef]
- Des Roches, A.D.B.; Durier, V.; Richard-Yris, M.A.; Blois-Heulin, C.; Ezzaouïa, M.; Hausberger, M.; Henry, S. Differential Outcomes of Unilateral Interferences at Birth. Biol. Lett. 2011, 7, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Richard-Yris, M.A.; Tordjman, S.; Hausberger, M. Neonatal Handling Affects Durably Bonding and Social Development. PLoS ONE 2009, 4, e5216. [Google Scholar] [CrossRef] [PubMed]
- Tallet, C.; Sy, K.; Prunier, A.; Nowak, R.; Boissy, A.; Boivin, X. Behavioural and Physiological Reactions of Piglets to Gentle Tactile Interactions Vary According to Their Previous Experience with Humans. Livest. Sci. 2014, 167, 331–341. [Google Scholar] [CrossRef]
- Lange, A.; Franzmayr, S.; Wisenöcker, V.; Futschik, A.; Waiblinger, S.; Lürzel, S. Effects of Different Stroking Styles on Behaviour and Cardiac Parameters in Heifers. Animals 2020, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Fine, A.H.; Andersen, S.J. A Commentary on the Contemporary Issues Confronting Animal Assisted and Equine Assisted Interactions. J. Equine Vet. Sci. 2021, 100, 103436. [Google Scholar] [CrossRef]
- Jegatheesan, B.; Yamazaki, K. The IAHAIO Definitions for Animal Assisted Intervention and Guidelines for Wellness of Animals Involved in AAI. In Handbook on Animal-Assisted Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 499–504. [Google Scholar] [CrossRef]
- Contalbrigo, L.; Borgi, M.; de Santis, M.; Collacchi, B.; Tuozzi, A.; Toson, M.; Redaelli, V.; Odore, R.; Vercelli, C.; Stefani, A.; et al. Equine-Assisted Interventions (EAIs) for Children with Autism Spectrum Disorders (ASD): Behavioural and Physiological Indices of Stress in Domestic Horses (Equus caballus) during Riding Sessions. Animals 2021, 11, 1562. [Google Scholar] [CrossRef]
- Gonzalez-De Cara, C.A.; Perez-Ecija, A.; Aguilera-Aguilera, R.; Rodero-Serrano, E.; Mendoza, F.J. Temperament Test for Donkeys to Be Used in Assisted Therapy. Appl. Anim. Behav. Sci. 2017, 186, 64–71. [Google Scholar] [CrossRef]
- De Santis, M.; Contalbrigo, L.; Borgi, M.; Cirulli, F.; Luzi, F.; Redaelli, V.; Stefani, A.; Toson, M.; Odore, R.; Vercelli, C.; et al. Equine Assisted Interventions (EAIs): Methodological Considerations for Stress Assessment in Horses. Vet. Sci. 2017, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Glenk, L.M. Current Perspectives on Therapy Dog Welfare in Animal-Assisted Interventions. Animals 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Kuhne, F.; Hößler, J.; Struwe, R. Affective Behavioural Responses by Dogs to Tactile Human-Dog Interactions. Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 371–378. [Google Scholar]
- Gueguen, L.; Lerch, N.; Grandgeorge, M.; Hausberger, M. Testing Individual Variations of Horses’ Tactile Reactivity: When, Where, How? Sci. Nat. 2022, 109, 41. [Google Scholar] [CrossRef] [PubMed]
- Saslow, C.A. Understanding the Perceptual World of Horses. Appl. Anim. Behav. Sci. 2002, 78, 209–224. [Google Scholar] [CrossRef]
- Lansade, L.; Pichard, G.; Leconte, M. Sensory Sensitivities: Components of a Horse’s Temperament Dimension. Appl. Anim. Behav. Sci. 2008, 114, 534–553. [Google Scholar] [CrossRef]
- Boyd, L.E. Time Budgets of Adult Przewalski Horses: Effects of Sex, Reproductive Status and Enclosure. Appl. Anim. Behav. Sci. 1988, 21, 19–39. [Google Scholar] [CrossRef]
- Feh, C.; de Mazierès, J. Grooming at a Preferred Site Reduces Heart Rate in Horses. Anim. Behav. 1993, 46, 1191–1194. [Google Scholar] [CrossRef]
- Spier, S.J.; Berger Pusterla, J.; Villarroel, A.; Pusterla, N. Outcome of Tactile Conditioning of Neonates, or “Imprint Training” on Selected Handling Measures in Foals. Vet. J. 2004, 168, 252–258. [Google Scholar] [CrossRef]
- Van Iwaarden, A.; Stubbs, N.C.; Clayton, H.M. Topographical Anatomy of the Equine M. Cutaneus Trunci in Relation to the Position of the Saddle and Girth. J. Equine Vet. Sci. 2012, 32, 519–524. [Google Scholar] [CrossRef]
- Vidament, M.; Lansade, L.; Danvy, S.; Saint Priest, B.D.; Sabbagh, M.; Ricard, A. Personality in Young Horses and Ponies Evaluated during Breeding Shows: Phenotypic Link with Jumping Competition Results. J. Vet. Behav. 2021, 44, 1–11. [Google Scholar] [CrossRef]
- Vidament, M.; Yvon, J.; Bon, M.; Saint Priest, B.; Danvy, S.; Lansade, L. The Temperament of Horses Measured by Standardized Tests: Relationship with Age, Race and Rider Level. In Proceedings of the 41ème Journée de la Recherche Équine, Paris, France, 12 March 2015; Volume 1, pp. 15–24. [Google Scholar]
- Hausberger, M.; Bruderer, C.; le Scolan, N.; Pierre, J.-S. Interplay Between Environmental and Genetic Factors in Temperament/Personality Traits in Horses (Equus caballus). J. Comp. Psychol. 2004, 118, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Hausberger, M.; Gautier, E.; Biquand, V.; Lunel, C.; Jégo, P. Could Work Be a Source of Behavioural Disorders? A Study in Horses. PLoS ONE 2009, 4, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Hausberger, M.; Muller, C.; Lunel, C. Does Work Affect Personality? A Study in Horses. PLoS ONE 2011, 6, e14659. [Google Scholar] [CrossRef]
- Hausberger, M.; Stomp, M.; Sankey, C.; Brajon, S.; Lunel, C.; Henry, S. Mutual Interactions between Cognition and Welfare: The Horse as an Animal Model. Neurosci. Biobehav. Rev. 2019, 107, 540–559. [Google Scholar] [CrossRef] [PubMed]
- Lesimple, C.; Fureix, C.; Menguy, H.; Hausberger, M. Human Direct Actions May Alter Animal Welfare, a Study on Horses (Equus caballus). PLoS ONE 2010, 5, e10257. [Google Scholar] [CrossRef]
- Rochais, C.; Henry, S.; Sankey, C.; Nassur, F.; Góracka-Bruzda, A.; Hausberger, M. Visual Attention, an Indicator of Human-Animal Relationships? A Study of Domestic Horses (Equus caballus). Front. Psychol. 2014, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Rochais, C.; Stomp, M.; Sébilleau, M.; Houdebine, M.; Henry, S.; Hausberger, M. Horses’ Attentional Characteristics Differ According to the Type of Work. PLoS ONE 2022, 17, e0269974. [Google Scholar] [CrossRef]
- Philippe-Peyroutet, C.; Grandgeorge, M. Animal-Assisted Interventions for Children With Autism Spectrum Disorders: A Survey of French Facilities. People Anim. Int. J. Res. Pract. 2018, 1, 8. [Google Scholar]
- Pluta, M.; Kȩdzierski, W. Emotional Responses of Horses to Patients Requiring Therapy. Soc. Anim. 2018, 26, 426–436. [Google Scholar] [CrossRef]
- Fazio, E.; Medica, P.; Cravana, C.; Ferlazzo, A. Hypothalamic-Pituitary-Adrenal Axis Responses of Horses to Therapeutic Riding Program: Effects of Different Riders. Physiol. Behav. 2013, 118, 138–143. [Google Scholar] [CrossRef]
- Lerch, N.; Cirulli, F.; Rochais, C.; Lesimple, C.; Guilbaud, E.; Contalbrigo, L.; Borgi, M.; Grandgeorge, M.; Hausberger, M. Interest in Humans: Comparisons between Riding School Lesson Equids and Assisted Intervention Equids. Animals 2021, 11, 2533. [Google Scholar] [CrossRef]
- Mendonça, T.; Bienboire-Frosini, C.; Menuge, F.; Leclercq, J.; Lafont-Lecuelle, C.; Arroub, S.; Pageat, P. The Impact of Equine-Assisted Therapy on Equine Behavioral and Physiological Responses. Animals 2019, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Fureix, C.; Jego, P.; Sankey, C.; Hausberger, M. How Horses (Equus caballus) See the World: Humans as Significant “Objects”. Anim. Cogn. 2009, 12, 643–654. [Google Scholar] [CrossRef]
- Hausberger, M.; Roche, H.; Henry, S.; Visser, E.K. A Review of the Human–Horse Relationship. Appl. Anim. Behav. Sci. 2008, 109, 1–24. [Google Scholar] [CrossRef]
- Lansade, L.; Bonneau, C.; Parias, C.; Biau, S. Horse’s Emotional State and Rider Safety during Grooming Practices, a Field Study. Appl. Anim. Behav. Sci. 2019, 217, 43–47. [Google Scholar] [CrossRef]
- Mills, D.; Nankervis, K. Equine Behaviour: Principles and Practice; Blackwell: Oxford, UK, 1999. [Google Scholar]
- R Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 January 2020).
- Siegel, S.; Castellan, N.J. Nonparametric Statistics for the Behavioral Sciences; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Burn, C.C.; Dennison, T.L.; Whay, H.R. Relationships between Behaviour and Health in Working Horses, Donkeys, and Mules in Developing Countries. Appl. Anim. Behav. Sci. 2010, 126, 109–118. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- World Health Organization. The Composite International Diagnostic Interview, Version 1.1; Researcher’s Manual; WHO: Geneva, Switzerland, 1994. [Google Scholar]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- British Horse Society and The Pony Club. The Manual of Horsemanship; William Clowes Ltd.: London, UK, 1985. [Google Scholar]
- Anderson, M.K.; Friend, T.H.; Evans, J.W.; Bushong, D.M. Behavioral Assessment of Horses in Therapeutic Riding Programs. Appl. Anim. Behav. Sci. 1999, 63, 11–24. [Google Scholar] [CrossRef]
- Minero, M.; Zucca, D.; Canali, E. A Note on Reaction to Novel Stimulus and Restraint by Therapeutic Riding Horses. Appl. Anim. Behav. Sci. 2006, 97, 335–342. [Google Scholar] [CrossRef]
- Rankins, E.M.; Wickens, C.L.; McKeever, K.H.; Malinowski, K. A Survey of Horse Selection, Longevity, and Retirement in Equine-Assisted Services in the United States. Animals 2021, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Fureix, C.; Jego, P.; Henry, S.; Lansade, L.; Hausberger, M. Towards an Ethological Animal Model of Depression? A Study on Horses. PLoS ONE 2012, 7, e39280. [Google Scholar] [CrossRef] [PubMed]
- Haussler, K.K.; Erb, H.N. Mechanical Nociceptive Thresholds in the Axial Skeleton of Horses. Equine Vet. J. 2006, 38, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Durier, V.; Henry, S.; Sankey, C.; Sizun, J.; Hausberger, M. Locomotor Inhibition in Adult Horses Faced to Stressors: A Single Postpartum Experience May Be Enough! Front. Psychol. 2012, 3, 442. [Google Scholar] [CrossRef]
- Brajon, S.; Laforest, J.P.; Bergeron, R.; Tallet, C.; Hötzel, M.J.; Devillers, N. Persistency of the Piglet’s Reactivity to the Handler Following a Previous Positive or Negative Experience. Appl. Anim. Behav. Sci. 2015, 162, 9–19. [Google Scholar] [CrossRef]
- Croy, I.; Sehlstedt, I.; Wasling, H.B.; Ackerley, R.; Olausson, H. Gentle Touch Perception: From Early Childhood to Adolescence. Dev. Cogn. Neurosci. 2019, 35, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, L.; Schroeder, K.; Sherwood, D.; Stroud, D.; Udell, M.A.R. Horse Behavior towards Familiar and Unfamiliar Humans: Implications for Equine-Assisted Services. Animals 2021, 11, 2369. [Google Scholar] [CrossRef]
- Mendonça, T.; Bienboire-Frosini, C.; Kowalczyk, I.; Leclercq, J.; Arroub, S.; Pageat, P. Equine Activities Influence Horses’ Responses to Different Stimuli: Could This Have an Impact on Equine Welfare? Animals 2019, 9, 290. [Google Scholar] [CrossRef]
- Lansade, L.; Nowak, R.; Lainé, A.L.; Leterrier, C.; Bonneau, C.; Parias, C.; Bertin, A. Facial Expression and Oxytocin as Possible Markers of Positive Emotions in Horses. Sci. Rep. 2018, 8, 14680. [Google Scholar] [CrossRef]
- Sankey, C.; Henry, S.; Górecka-Bruzda, A.; Richard-Yris, M.A.; Hausberger, M. The Way to a Man’s Heart Is through His Stomach: What about Horses? PLoS ONE 2010, 5, e15446. [Google Scholar] [CrossRef]
- Osthaus, B.; Proops, L.; Long, S.; Bell, N.; Hayday, K.; Burden, F. Hair Coat Properties of Donkeys, Mules and Horses in a Temperate Climate. Equine Vet. J. 2018, 50, 339–342. [Google Scholar] [CrossRef]
- Cymbaluk, N.F. Thermoregulation of Horses in Cold, Winter Weather: A Review. Livest. Prod. Sci. 1994, 40, 65–71. [Google Scholar] [CrossRef]
- Tyler, S.J. The Behaviour and Social Organization of the New Forest Ponies. Anim. Behav. Monogr. 1972, 5, 87–196. [Google Scholar] [CrossRef]
- Hausberger, M.; Lesimple, C.; Henry, S. Detecting Welfare in a Non-Verbal Species: Social/Cultural Biases and Difficulties in Horse Welfare Assessment. Animals 2021, 11, 2249. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.L.H.; Thompson, H.; Guijarro, C.; Zulch, H.E. The Influence of Body Region, Handler Familiarity and Order of Region Handled on the Domestic Cat’s Response to Being Stroked. Appl. Anim. Behav. Sci. 2015, 173, 60–67. [Google Scholar] [CrossRef]
- Sankey, C.; Henry, S.; Clouard, C.; Richard-Yris, M.A.; Hausberger, M. Asymmetry of Behavioral Responses to a Human Approach in Young Naive vs. Trained Horses. Physiol. Behav. 2011, 104, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Waring, G. Horse Behavior; Noyes Publications: Norwich, NY, USA, 2003. [Google Scholar]

| Centre 1 | Centre 2 | ||
|---|---|---|---|
| Equid type | Ponies | 12 | 11 |
| Horses | 15 | 22 | |
| Sex | Mares | 20 | 12 |
| Geldings | 7 | 21 | |
| Age (mean ± SE) Y.O | 13.2 ± 1.2 | 14.8 ± 0.8 | |
| Riding activity 1 | RS | 2 | 12 |
| EAI | 5 | 1 | |
| EAI-RS | 20 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rochais, C.; Lerch, N.; Gueguen, L.; Schmidlin, M.; Bonamy, O.; Grandgeorge, M.; Hausberger, M. Horses’ Tactile Reactivity Differs According to the Type of Work: The Example of Equine-Assisted Intervention. Vet. Sci. 2023, 10, 130. https://doi.org/10.3390/vetsci10020130
Rochais C, Lerch N, Gueguen L, Schmidlin M, Bonamy O, Grandgeorge M, Hausberger M. Horses’ Tactile Reactivity Differs According to the Type of Work: The Example of Equine-Assisted Intervention. Veterinary Sciences. 2023; 10(2):130. https://doi.org/10.3390/vetsci10020130
Chicago/Turabian StyleRochais, Céline, Noémie Lerch, Léa Gueguen, Margaux Schmidlin, Ombeline Bonamy, Marine Grandgeorge, and Martine Hausberger. 2023. "Horses’ Tactile Reactivity Differs According to the Type of Work: The Example of Equine-Assisted Intervention" Veterinary Sciences 10, no. 2: 130. https://doi.org/10.3390/vetsci10020130
APA StyleRochais, C., Lerch, N., Gueguen, L., Schmidlin, M., Bonamy, O., Grandgeorge, M., & Hausberger, M. (2023). Horses’ Tactile Reactivity Differs According to the Type of Work: The Example of Equine-Assisted Intervention. Veterinary Sciences, 10(2), 130. https://doi.org/10.3390/vetsci10020130







