Chrysoeriol Improves In Vitro Porcine Embryo Development by Reducing Oxidative Stress and Autophagy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Chemicals
2.2. Oocyte Collection and In Vitro Maturation (IVM)
2.3. Parthenogenetic Activation and Embryo IVC
2.4. Total Cell Number Count and Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling (TUNEL) Assay
2.5. 5-Ethynyl-2′-Deoxyuridine (EdU) Assay
2.6. Intracellular ROS and GSH Level Assay
2.7. Mitochondrial Membrane Potential (MMP, ∆Ψ) Assay
2.8. Immunofluorescence Staining
2.9. Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.10. Statistical Analysis
3. Results
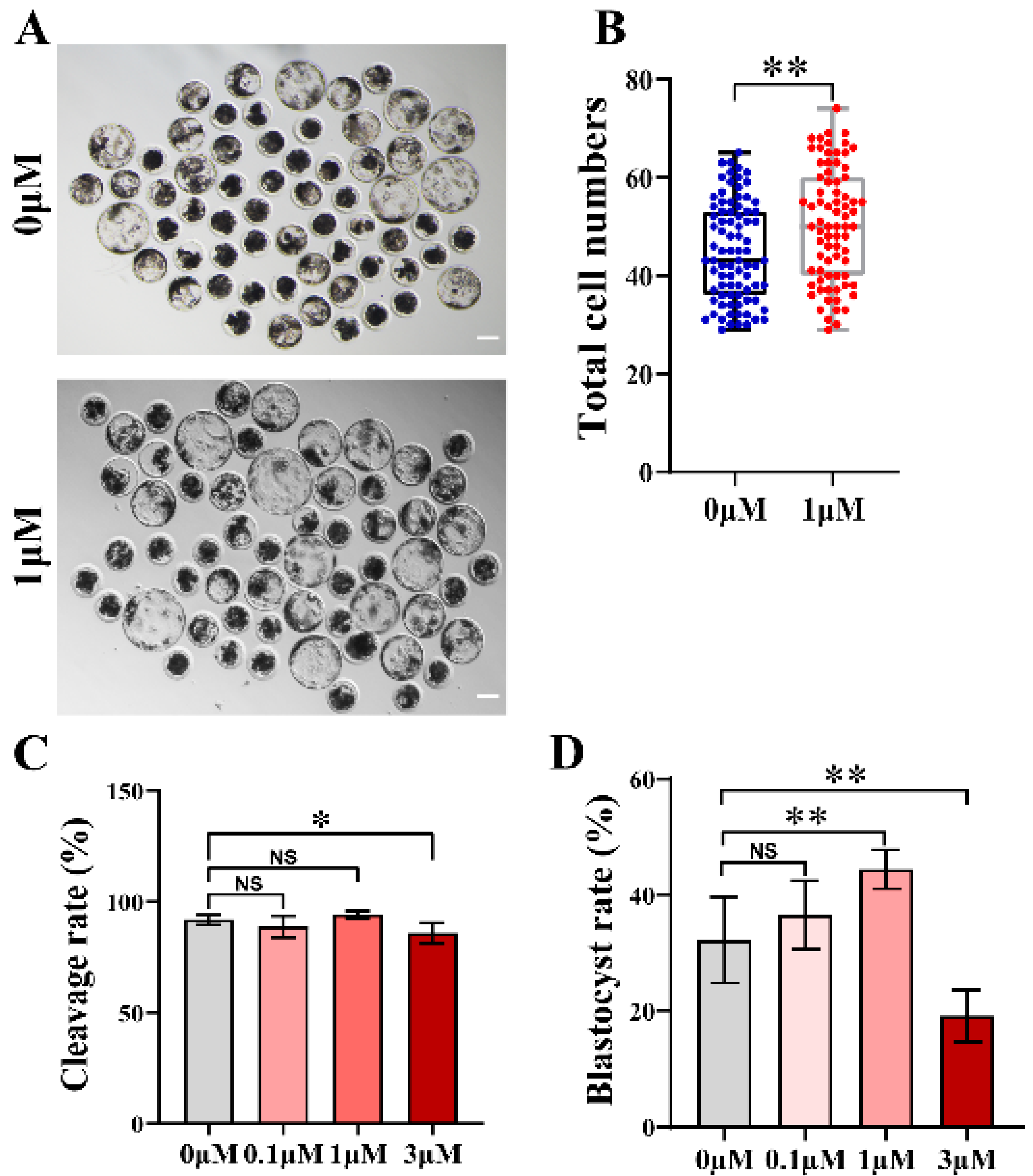
3.1. CHE Enhanced the Early Developmental Rates of the Porcine Embryos
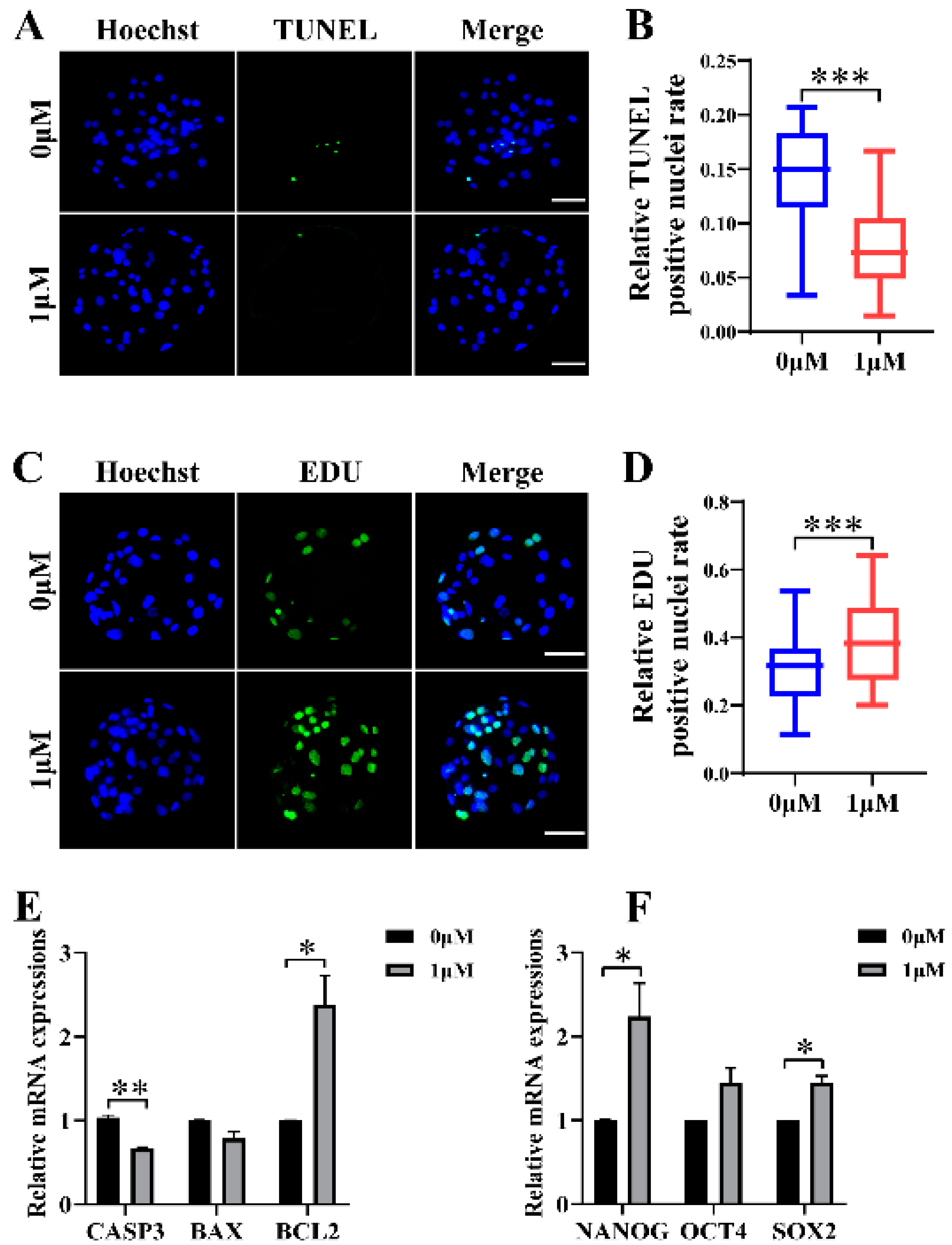
3.2. CHE Improved the Cell Proliferation Level and Reduced Apoptosis in Blastocysts
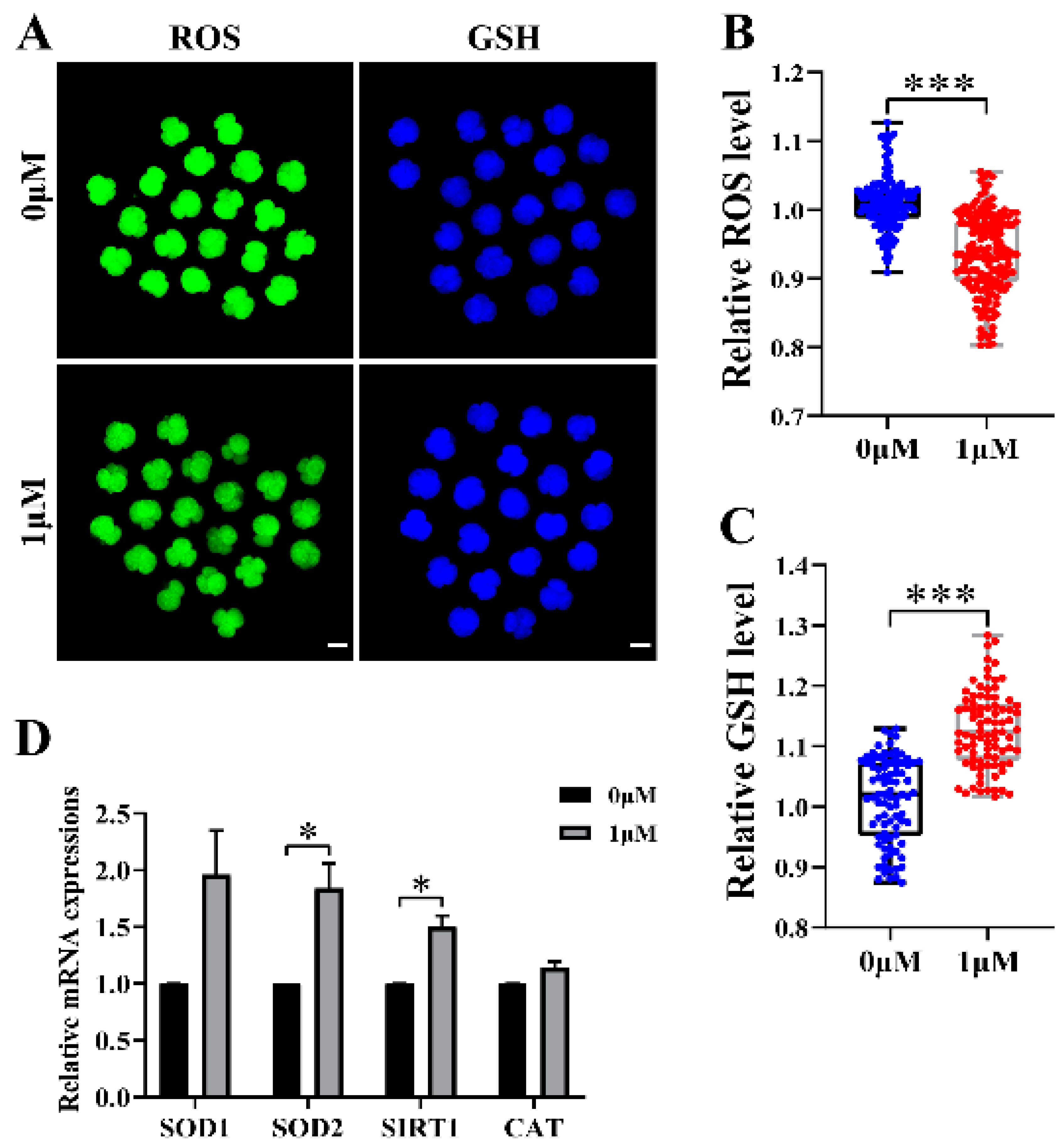
3.3. CHE Improved the Antioxidant Capacity of Early-Stage Embryos
3.4. CHE Reduces Autophagy Levels during Porcine Embryo Development
3.5. CHE Improved the Mitochondrial Function of Early-Stage Porcine Embryos
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Hajj, N.; Haaf, T. Epigenetic disturbances in in vitro cultured gametes and embryos: Implications for human assisted reproduction. Fertil. Steril. 2013, 99, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Hammadeh, M.E.; Fischer-Hammadeh, C.; Ali, K.R. Assisted hatching in assisted reproduction: A state of the art. J. Assist. Reprod. Genet. 2011, 28, 119–128. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Lin, E.; He, P.; Ru, G. Oxidative damage-induced hyperactive ribosome biogenesis participates in tumorigenesis of offspring by cross-interacting with the Wnt and TGF-β1 pathways in IVF embryos. Exp. Mol. Med. 2021, 53, 1792–1806. [Google Scholar] [CrossRef]
- Smith, G.D.; Takayama, S. Application of microfluidic technologies to human assisted reproduction. Mol. Hum. Reprod. 2017, 23, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.R.; Redel, B.K.; Kerns, K.C.; Spate, L.D.; Prather, R.S. Challenges and Considerations during In Vitro Production of Porcine Embryos. Cells 2021, 10, 2770. [Google Scholar] [CrossRef]
- de Souza-Fabjan, J.M.; Panneau, B.; Duffard, N.; Locatelli, Y.; de Figueiredo, J.R.; Freitas, V.J.; Mermillod, P. In vitro production of small ruminant embryos: Late improvements and further research. Theriogenology 2014, 81, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Funahashi, H.; Yoshioka, K.; Kikuchi, K. Up date of in vitro production of porcine embryos. Front. Biosci. 2006, 11, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Sun, W.; Liu, H.; Zhu, H.; Gao, M.; Xu, S. Eucalyptol antagonized the apoptosis and immune dysfunction of grass carp hepatocytes induced by tetrabromobisphenol A by regulating ROS/ASK1/JNK pathway. Environ. Toxicol. 2023, 1–13. [Google Scholar] [CrossRef]
- Sudharshan, S.J.; Ananth, K.N.; Jemima, P.; Dyavaiah, M.; Nagegowda, D.A. Betulinic acid mitigates oxidative stress-mediated apoptosis and enhances longevity in the yeast Saccharomyces cerevisiae model. Free Radic. Res. 2023, 1–14. [Google Scholar] [CrossRef]
- Ding, Z.M.; Jiao, X.F.; Wu, D.; Zhang, J.Y.; Chen, F.; Wang, Y.S.; Huang, C.J.; Zhang, S.X.; Li, X.; Huo, L.J. Bisphenol AF negatively affects oocyte maturation of mouse in vitro through increasing oxidative stress and DNA damage. Chem. Biol. Interact. 2017, 278, 222–229. [Google Scholar] [CrossRef]
- Khazaei, M.; Aghaz, F. Reactive Oxygen Species Generation and Use of Antioxidants during In Vitro Maturation of Oocytes. Int. J. Fertil. Steril. 2017, 11, 63–70. [Google Scholar] [CrossRef]
- McEvoy, T.G.; Coull, G.D.; Broadbent, P.J.; Hutchinson, J.S.; Speake, B.K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J. Reprod. Fertil. 2000, 118, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Ekwall, H.; Tienthai, P.; Kawai, Y.; Noguchi, J.; Kaneko, H.; Rodriguez-Martinez, H. Morphological features of lipid droplet transition during porcine oocyte fertilisation and early embryonic development to blastocyst in vivo and in vitro. Zygote 2002, 10, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Osorio, N.; Kim, I.J.; Wang, H.; Kaya, A.; Memili, E. Melatonin increases cleavage rate of porcine preimplantation embryos in vitro. J. Pineal Res. 2007, 43, 283–288. [Google Scholar] [CrossRef]
- Liang, S.; Jin, Y.X.; Yuan, B.; Zhang, J.B.; Kim, N.H. Melatonin enhances the developmental competence of porcine somatic cell nuclear transfer embryos by preventing DNA damage induced by oxidative stress. Sci. Rep. 2017, 7, 11114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liang, S.; Yao, X.R.; Jin, Y.X.; Shen, X.H.; Yuan, B.; Zhang, J.B.; Kim, N.H. Laminarin improves developmental competence of porcine early stage embryos by inhibiting oxidative stress. Theriogenology 2018, 115, 38–44. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, J.B.; Peng, Y.X.; Liu, J.B.; Han, D.X.; Wang, Y.; Zhang, Z.; Yuan, B.; Gao, Y.; Chen, C.Z.; et al. Imperatorin improves in vitro porcine embryo development by reducing oxidative stress and autophagy. Theriogenology 2020, 146, 145–151. [Google Scholar] [CrossRef]
- Liu, R.P.; Wang, X.Q.; Wang, J.; Dan, L.; Li, Y.H.; Jiang, H.; Xu, Y.N.; Kim, N.H. Oroxin A reduces oxidative stress, apoptosis, and autophagy and improves the developmental competence of porcine embryos in vitro. Reprod. Domest. Anim. 2022, 57, 1255–1266. [Google Scholar] [CrossRef]
- Wang, X.Q.; Liu, R.P.; Wang, J.; Luo, D.; Li, Y.H.; Jiang, H.; Xu, Y.N.; Kim, N.H. Wedelolactone facilitates the early development of parthenogenetically activated porcine embryos by reducing oxidative stress and inhibiting autophagy. PeerJ 2022, 10, e13766. [Google Scholar] [CrossRef]
- Alex, A.M.; Arehally Marappa, M.; Joghee, S.; Chidambaram, S.B. Therapeutic benefits of flavonoids against neuroinflammation: A systematic review. Inflammopharmacology 2022, 30, 111–136. [Google Scholar] [CrossRef]
- Kim, M.H.; Kwon, S.Y.; Woo, S.Y.; Seo, W.D.; Kim, D.Y. Antioxidative Effects of Chrysoeriol via Activation of the Nrf2 Signaling Pathway and Modulation of Mitochondrial Function. Molecules 2021, 26, 313. [Google Scholar] [CrossRef] [PubMed]
- Limboonreung, T.; Tuchinda, P.; Chongthammakun, S. Chrysoeriol mediates mitochondrial protection via PI3K/Akt pathway in MPP(+) treated SH-SY5Y cells. Neurosci. Lett. 2020, 714, 134545. [Google Scholar] [CrossRef] [PubMed]
- Nickavar, B.; Rezaee, J.; Nickavar, A. Effect-Directed Analysis for the Antioxidant Compound in Salvia verticillata. Iran. J. Pharm. Res. 2016, 15, 241–246. [Google Scholar] [PubMed]
- Choi, D.Y.; Lee, J.Y.; Kim, M.R.; Woo, E.R.; Kim, Y.G.; Kang, K.W. Chrysoeriol potently inhibits the induction of nitric oxide synthase by blocking AP-1 activation. J. Biomed. Sci. 2005, 12, 949–959. [Google Scholar] [CrossRef]
- Zhang, T.; Ikejima, T.; Li, L.; Wu, R.; Yuan, X.; Zhao, J.; Wang, Y.; Peng, S. Impairment of Mitochondrial Biogenesis and Dynamics Involved in Isoniazid-Induced Apoptosis of HepG2 Cells Was Alleviated by p38 MAPK Pathway. Front. Pharmacol. 2017, 8, 753. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Priyadarsini, K.I.; Kumar, M.S.; Unnikrishnan, M.K.; Mohan, H. Effect of O-glycosilation on the antioxidant activity and free radical reactions of a plant flavonoid, chrysoeriol. Bioorg. Med. Chem. 2003, 11, 2677–2685. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Min, Q.; Palida, A.; Zhang, Y.; Tang, R.; Chen, L.; Li, H. 8-Chrysoeriol, as a potential BCL-2 inhibitor triggers apoptosis of SW1990 pancreatic cancer cells. Bioorg. Chem. 2018, 77, 478–484. [Google Scholar] [CrossRef]
- Min, D.Y.; Jung, E.; Ahn, S.S.; Lee, Y.H.; Lim, Y.; Shin, S.Y. Chrysoeriol Prevents TNFα-Induced CYP19 Gene Expression via EGR-1 Downregulation in MCF7 Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7523. [Google Scholar] [CrossRef]
- Cha, B.Y.; Shi, W.L.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. An inhibitory effect of chrysoeriol on platelet-derived growth factor (PDGF)-induced proliferation and PDGF receptor signaling in human aortic smooth muscle cells. J. Pharmacol. Sci. 2009, 110, 105–110. [Google Scholar] [CrossRef]
- Liu, Z.; Song, X.D.; Xin, Y.; Wang, X.J.; Yu, H.; Bai, Y.Y.; Liu, J.H.; Zhang, C.N.; Hui, R.T. Protective effect of chrysoeriol against doxorubicin-induced cardiotoxicity in vitro. Chin. Med. J. 2009, 122, 2652–2656. [Google Scholar] [PubMed]
- Abo-Qotb, S.M.S.; Hassanein, A.M.M.; Desoukey, S.Y.; Wanas, A.S.; Tawfik, H.M.; Orabi, M.A.A. In vivo anti-inflammatory and hepatoprotective activities of Orobanche crenata (Forssk.) aerial parts in relation to its phytomolecules. Nat. Prod. Res. 2022, 36, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Sun, N.; Li, C.; Lei, Y.; Huang, Z.; Wu, J.; Si, C.; Dai, X.; Liu, C.; Wei, J.; et al. Dissecting primate early post-implantation development using long-term in vitro embryo culture. Science 2019, 366, eaaw5754. [Google Scholar] [CrossRef]
- Tam, P.P.L. Modeling the early development of a primate embryo. Science 2019, 366, 798–799. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Park, S.M.; Lee, E.; Kim, J.H.; Jeong, Y.I.; Lee, J.Y.; Park, S.W.; Kim, H.S.; Hossein, M.S.; Jeong, Y.W.; et al. Anti-apoptotic effect of melatonin on preimplantation development of porcine parthenogenetic embryos. Mol. Reprod. Dev. 2008, 75, 1127–1135. [Google Scholar] [CrossRef]
- Popovic, M.; Azpiroz, F.; Chuva de Sousa Lopes, S.M. Engineered models of the human embryo. Nat. Biotechnol. 2021, 39, 918–920. [Google Scholar] [CrossRef]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol. 2012, 86, 1649–1665. [Google Scholar] [CrossRef]
- Harvey, A.J. Mitochondria in early development: Linking the microenvironment, metabolism and the epigenome. Reproduction 2019, 157, R159–R179. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef]
- Kumariya, S.; Ubba, V.; Jha, R.K.; Gayen, J.R. Autophagy in ovary and polycystic ovary syndrome: Role, dispute and future perspective. Autophagy 2021, 17, 2706–2733. [Google Scholar] [CrossRef]
- Navarro-Yepes, J.; Burns, M.; Anandhan, A.; Khalimonchuk, O.; del Razo, L.M.; Quintanilla-Vega, B.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Oxidative stress, redox signaling, and autophagy: Cell death versus survival. Antioxid. Redox Signal. 2014, 21, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Murthi, P.; Fitzpatrick, E.; Borg, A.J.; Donath, S.; Brennecke, S.P.; Kalionis, B. GAPDH, 18S rRNA and YWHAZ are suitable endogenous reference genes for relative gene expression studies in placental tissues from human idiopathic fetal growth restriction. Placenta 2008, 29, 798–801. [Google Scholar] [CrossRef] [PubMed]


| Genes | Sequences 5′–3′ | Base |
|---|---|---|
| GAPDH | F: TTCCACGGCACAGTCAAG | 18 |
| R: ATACTCAGCACCAGCATCG | 19 | |
| SOD1 | F: CAAAGGATCAAGAGAGGCACG | 21 |
| R: CGAGAGGGCGATCACAGAAT | 20 | |
| SOD2 | F: TTCTGGACAAATCTGAGCCCTAACG | 25 |
| R: CGACGGATACAGCGGTCAACTTC | 23 | |
| SIRT1 | F: GAGAAGGAAACAATGGGCCG | 20 |
| R: ACCAAACAGAAGGTTATCTCGGT | 23 | |
| CAT | F: AACTGTCCCTTCCGTGCTA | 19 |
| R: CCTGGGTGACATTATCTTCG | 20 | |
| NANOG | F: TGTCTCTCCTCTTCCTTCCTCCATG | 25 |
| R: TCCTCCTTCTCTGTGCTCTTCTCTG | 25 | |
| OCT4 | F: CCTATGACTTCTGCGGAGGGA | 21 |
| R: TTTGATGTCCTGGGACTCCTCG | 22 | |
| SOX2 | F: GAACAGCCCAGACCGAGTTAAGC | 23 |
| R: CTGATCTCCGAGTTGTGCATCTTGG | 25 | |
| CASP3 | F: AGAATTGGACTGTGGGATTGAGACG | 25 |
| R: GCCAGGAATAGTAACCAGGTGCTG | 24 | |
| BAX | F: GGACTTCCTTCGAGATCGGC | 20 |
| R: GCGTCCCAAAGTAGGAGAGG | 20 | |
| BCL2 | F: GGATAACGGAGGCTGGGATG | 20 |
| R: TTATGGCCCAGATAGGCACC | 20 | |
| LC3B | F: TTCAAACAGCGCCGAACCTT | 20 |
| R: TTTGGTAGGATGCTGCTCTCG | 21 | |
| ATG5 | F: TTGCAGTGGCTGAGTGAACA | 20 |
| R: TCAATCTGTTGGTTGCGGGA | 20 | |
| BECLIN1 | F: GATGGTGGCTTTCCTGGACTGTG | 23 |
| R: ACTGCCTCCTGTGTCTTCAATCTTG | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-R.; Ji, H.-W.; He, S.-Y.; Liu, R.-P.; Wang, X.-Q.; Wang, J.; Huang, C.-M.; Xu, Y.-N.; Li, Y.-H.; Kim, N.-H. Chrysoeriol Improves In Vitro Porcine Embryo Development by Reducing Oxidative Stress and Autophagy. Vet. Sci. 2023, 10, 143. https://doi.org/10.3390/vetsci10020143
Wang C-R, Ji H-W, He S-Y, Liu R-P, Wang X-Q, Wang J, Huang C-M, Xu Y-N, Li Y-H, Kim N-H. Chrysoeriol Improves In Vitro Porcine Embryo Development by Reducing Oxidative Stress and Autophagy. Veterinary Sciences. 2023; 10(2):143. https://doi.org/10.3390/vetsci10020143
Chicago/Turabian StyleWang, Chao-Rui, He-Wei Ji, Sheng-Yan He, Rong-Ping Liu, Xin-Qin Wang, Jing Wang, Chu-Man Huang, Yong-Nan Xu, Ying-Hua Li, and Nam-Hyung Kim. 2023. "Chrysoeriol Improves In Vitro Porcine Embryo Development by Reducing Oxidative Stress and Autophagy" Veterinary Sciences 10, no. 2: 143. https://doi.org/10.3390/vetsci10020143
APA StyleWang, C.-R., Ji, H.-W., He, S.-Y., Liu, R.-P., Wang, X.-Q., Wang, J., Huang, C.-M., Xu, Y.-N., Li, Y.-H., & Kim, N.-H. (2023). Chrysoeriol Improves In Vitro Porcine Embryo Development by Reducing Oxidative Stress and Autophagy. Veterinary Sciences, 10(2), 143. https://doi.org/10.3390/vetsci10020143






