Semen Quality Traits of Two Thai Native Chickens Producing a High and a Low of Semen Volumes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Semen Collection and Evaluation
2.3. Semen Color, Volume, and pH
2.4. Sperm Motility
2.5. Sperm Viability
2.6. Sperm Concentration
2.7. Statistical Analysis
- = observation values from treatment combination at breed i (i = 1 to 2) and volume j (j = 1 to 2), and replication k (k = 1 to 12)
- = overall mean
- = the main factor of rooster breeds i (i = 1 to 2)
- = the main factor of semen volume j (j = 1 to 2)
- = the interaction between rooster breeds and semen volume ij (i = 1 to 2 and j = 1 to 2)
- = experimental error
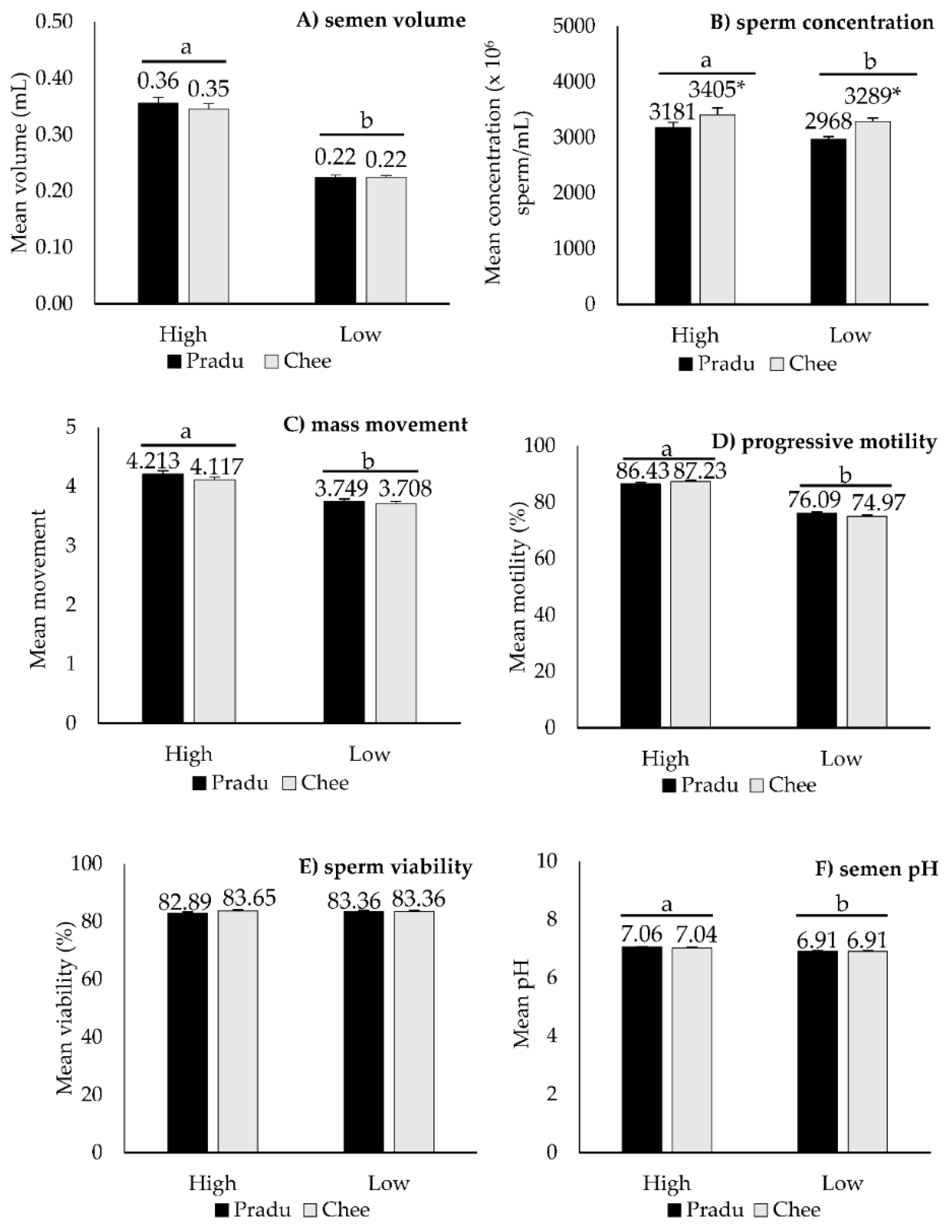
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chuaychu-noo, N.; Thananurak, P.; Chankitisakul, V.; Vongpralub, T. Supplementing rooster sperm with cholesterol-loaded-cyclodextrin improves fertility after cryopreservation. Cryobiology 2017, 74, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Thananurak, P.; Chuaychu-noo, N.; Thelie, A.; Phasuk, Y.; Vongpralub, T.; Blesbois, E. Different concentrations of cysteamine, ergothioneine, and serine modulate quality and fertilizing ability of cryopreserved chicken sperm. Poult. Sci. 2020, 99, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Feyisa, S.G.; Park, Y.H.; Kim, Y.M.; Lee, B.R.; Jung, K.M.; Choi, S.B.; Han, J.Y. Morphological defects of sperm and their association with motility, fertility, and hatchability in four Korean native chicken breeds. Asian-Australas. J. Anim. Sci. 2018, 31, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Haron, A.W.; Yusoff, R.; Nesa, M.; Bukar, M.; Kasim, A. Evaluation of the ejaculate quality of the red jungle fowl, domestic chicken, and bantam chicken in Malaysia. Turk. J. Vet. Anim. Sci. 2013, 37, 564–568. [Google Scholar] [CrossRef]
- State, K.; State, O. Evaluation of semen quality of five different cockerel breed used in poultry industry in Nigeria. J. Environ. Issues Agric. Dev. Ctries. 2014, 6, 30–36. [Google Scholar]
- Ajayi, F.O.; Agaviezor, B.O.; Ajuogu, P.K.; Harcourt, P.; Harcourt, P. Semen characteristics of three strains of local cocks in the humid tropical environment of Nigeria. J. Anim. Vet. Adv. 2011, 3, 125–127. [Google Scholar]
- Donoghue, A.M. Prospective approaches to avoid flock fertility problems: Predictive assessment of sperm function traits in poultry. Poult. Sci. 1999, 78, 437–443. [Google Scholar] [CrossRef]
- Peters, S.O.; Shoyebo, O.D.; Ilori, B.M.; Ozoje, M.O.; Ikeobi, C.O.N.; Adebambo, O.A. Semen quality traits of seven strain of chickens raised in the humid tropics. Int. J. Poult. Sci. 2008, 7, 949–953. [Google Scholar] [CrossRef]
- Denisa, V.F.; Radekand, M.; Adislav, L. Assessment of ejaculate quality in roosters of three laying lines. Mendel Net 2015, 11–12, 174–177. [Google Scholar]
- Tarif, A.; Bhuiyan, M.; Ferdousy, R. Evaluation of semen quality among four chicken lines. J. Agric. Vet. Sci. 2013, 6, 7–13. [Google Scholar] [CrossRef]
- Farahi, M.; Masoudi, A.A.; Ehsani, A. Does the change in sperm motility during the production period differ between high and low motility groups? Livest. Sci. 2018, 216, 1–5. [Google Scholar] [CrossRef]
- Khongsen, M.; Wanichapichart, W.; Khunchamnan, S.; Niyomdecha, A. The methods of semen collection and artificial insemination in Thai native chicken. Prince Naradhiwas Univ. J. 2013, 5, 132–143. [Google Scholar]
- Sonseeda, P.; Vongpralub, T.; Laopaiboon, B. Effects of environmental factors, ages and breeds on semen characteristics in Thai indigenous chickens: A one-year study. Thai. J. Vet. Med. 2013, 43, 347–352. [Google Scholar]
- Dorji, S.; Dorji, N. Semen characteristics of three strains of Bhutanese indigenous chickens. Bhutan. J. Anim. Sci. 2018, 2, 19–25. [Google Scholar]
- Machebe, N.S.; Ezekwe, A.G. Ejaculate characteristics of three genotypes of local cocks in the humid tropics. Agro. Sci. 2006, 3, 33–36. [Google Scholar] [CrossRef]
- Chuaychu-noo, N.; Thananurak, P.; Boonkum, W.; Vongpralub, T.; Chankitisakul, V. Effect of organic selenium dietary supplementation on quality and fertility of cryopreserved chicken sperm. Cryobiology 2021, 98, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Mussa, N.J.; Ratchamak, R.; Ratsiri, T.; Chumchai, R.; Vongpralub, T.; Boonkum, W.; Semaming, Y.; Chantikisakul, V. Lipid peroxidation and antioxidant enzyme activity in fresh rooster semen with high and low sperm motility. Vet. Integr. Sci. 2020, 18, 183–192. [Google Scholar]
- Lukaszewicz, E. Effects of semen filtration and dilution rate on morphology and fertility of frozen gander spermatozoa. Theriogenology 2001, 55, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Haunshi, S.; Niranjan, M.; Shanmugam, M.; Padhi, M.K.; Reddy, M.R.; Sunitha, R.; Panda, A.K. Characterization of two Indian native chicken breeds for production, egg and semen quality, and welfare traits. Poult. Sci. 2011, 90, 314–320. [Google Scholar] [CrossRef]
- Shanmugam, M.; Rajkumar, U.; Reddy, M.R.; Rao, S.V.R. Effect of age on semen quality in naked neck and dwarf chicken under tropical climatic conditions. Anim. Prod. Sci. 2012, 52, 964–968. [Google Scholar] [CrossRef]
- Hambu, E.K.; Arifiantini, R.I.; Purwantara, B.; Darwati, S. Raw semen characteristics of three different Indonesian local Roosters. Anim. Prod. 2016, 18, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Usman, T.H.; Sir, S.M.; Sani, B. Comparison of semen characteristics between indigenous and Amo breeds cockerel of Gombe State, Nigeria. J. Fundam. Appl. Sci. 2019, 2018, 303–306. [Google Scholar] [CrossRef]
- Shahbazi, S.; Mirhosseini, S.Z.; Romanov, M.N. Genetic diversity in five Iranian native chicken populations estimated by microsatellite markers. Biochem. Genet. 2007, 45, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Akaboot, P.; Duangjinda, M.; Phasuk, Y.; Kaenchan, C.; Chinchiyanond, W. Genetic characterization of Red Junglefowl (Gallus gallus), Thai indigenous chicken (Gallus domesticus), two commercial lines using selective functional genes compared to microsatellite markers. Genet. Mol. Res. 2012, 11, 1881–1890. [Google Scholar] [CrossRef]
- Shanmugam, M.; Vinoth, A.; Rajaravindra, K.S.; Rajkumar, U. Evaluation of semen quality in roosters of different age during hot climatic condition. Anim. Reprod. Sci. 2014, 145, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, P.B.; Kinet, H.; Özdoğan, N.; Demİral, Ö.O. Evaluation of some spermatological characteristics in Denizli cocks. Erciyes Üniversitesi Veteriner Fakültesi Dergisi 2006, 3, 37–42. [Google Scholar]
- Churchil, R.R.; Praveena, P.E.; Sharma, D. Semen quality parameters, their inter-relationship and post-washing sperm attributes of Rhode Island Red roosters. Vet. World 2014, 7, 1117–1122. [Google Scholar] [CrossRef] [Green Version]
- Das, K.K.; Ahmed, K.; Kalita, N.; Deka, N. Semen characteristics of Vanaraja and indigenous chicken of Assam. Int. J. Appl. Pure Sci. Agric. 2016, 1, 108–110. [Google Scholar]
- Parker, J.E. Relation of time of day of artificial insemination to fertility and hatchability of hens’ eggs. Poult. Sci. 1945, 24, 314–317. [Google Scholar] [CrossRef]
- Jones, D.G.; Lamoreux, W.F. Semen production of White Leghorn males from strains selected for high and low fecundity. Poult. Sci. 1942, 21, 173–184. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.; Li, J.; Li, H.; Hong, Z.; Xie, M.; Chen, S.; Yao, B. The semen pH affects sperm motility and capacitation. PLoS ONE 2015, 10, e0132974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.H.; Dong, H.B.; Ma, D.L.; Li, Y.W.; Han, D.; Luo, M.J.; Tan, J.H. Effects of pH during liquid storage of goat semen on sperm viability and fertilizing potential. Anim. Reprod. Sci. 2016, 164, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Mothibedi, K.; Nsoso, S.J.; Waugh, E.E.; Kgwatalala, P.M. Semen characteristics of purebred naked neck Tswana and Black Australorp X naked neck Tswana crossbred chickens under an intensive management system in Botswana. Am. J. Res. Commun. 2016, 4, 38–47. [Google Scholar]
| Parameters | Semen Volume | Sperm Concentration | Mass Movement | Progressive Motility | Sperm Viability | Semen pH |
|---|---|---|---|---|---|---|
| Semen volume | - | 0.110 (0.031) | 0.303 (0.001) | 0.484 (0.001) | 0.124 (0.015) | 0.417 (0.001) |
| Sperm concentration | - | 0.189 (0.001) | 0.254 (0.001) | −0.078 (0.126) | −0.061 (0.228) | |
| Mass movement | - | 0.604 (0.001) | −0.003 (0.949) | 0.285 (0.001) | ||
| Progressive motility | - | 0.030 (0.558) | 0.407 (0.001) | |||
| Sperm viability | - | −0.043 (0.398) | ||||
| Semen pH | - |
| Parameters | Semen Volume | Sperm Concentration | Mass Movement | Progressive Motility | Sperm Viability | Semen pH |
|---|---|---|---|---|---|---|
| Breeds a | ns | ** | ns | ns | ns | ns |
| Volume b | ** | * | ** | ** | ns | ** |
| Interaction | ns | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussa, N.J.; Boonkum, W.; Chankitisakul, V. Semen Quality Traits of Two Thai Native Chickens Producing a High and a Low of Semen Volumes. Vet. Sci. 2023, 10, 73. https://doi.org/10.3390/vetsci10020073
Mussa NJ, Boonkum W, Chankitisakul V. Semen Quality Traits of Two Thai Native Chickens Producing a High and a Low of Semen Volumes. Veterinary Sciences. 2023; 10(2):73. https://doi.org/10.3390/vetsci10020073
Chicago/Turabian StyleMussa, Ngassa Julius, Wuttigrai Boonkum, and Vibuntita Chankitisakul. 2023. "Semen Quality Traits of Two Thai Native Chickens Producing a High and a Low of Semen Volumes" Veterinary Sciences 10, no. 2: 73. https://doi.org/10.3390/vetsci10020073






