Voluntary Biosurveillance of Streptococcus equi Subsp. equi in Nasal Secretions of 9409 Equids with Upper Airway Infection in the USA
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Sample Collection and Analysis
2.4. Statistical Analysis
3. Results
3.1. Prevalence of S. equi and Coinfections
3.2. Demographics
3.3. Vaccination Status and Clinical Signs
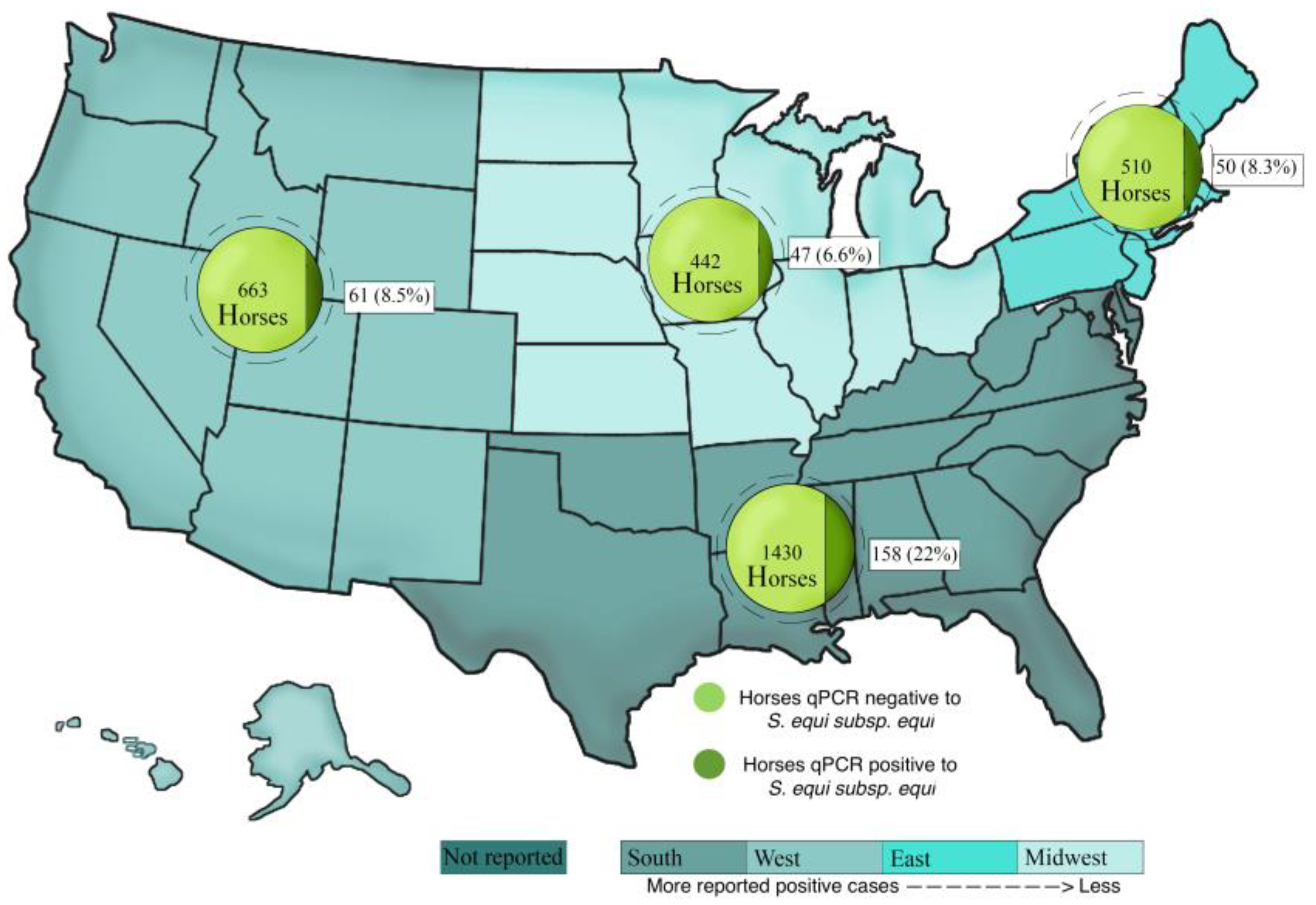
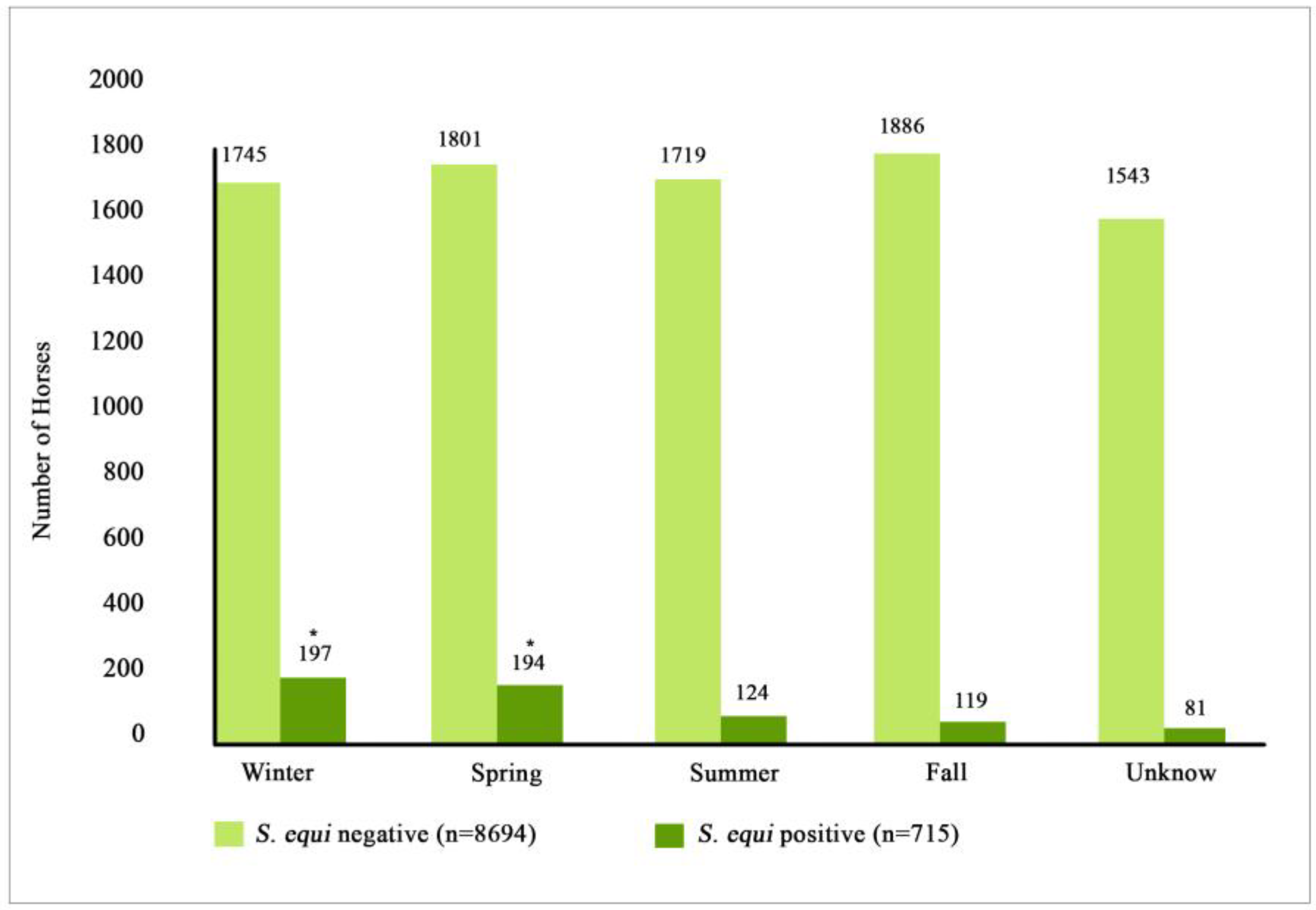
3.4. Location and Seasonality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paillot, R.; Lopez-Alvarez, M.R.; Newton, J.R.; Waller, A.S. Strangles: A modern clinical view from the 17th century. Equine Vet. J. 2017, 49, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Waller, A.S. Strangles: A pathogenic legacy of the war horse. Vet. Rec. 2016, 178, 91–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pusterla, N.; Kass, P.H.; Mapes, S.; Johnson, C.; Barnett, D.C.; Vaala, W.; Gutierrez, C.; McDaniel, R.; Whitehead, B.; Manning, J. Surveillance programme for important equine infectious respiratory pathogens in the USA. Vet. Rec. 2011, 169, 12. [Google Scholar] [CrossRef]
- Sweeney, C.R.; Timoney, J.F.; Newton, J.R.; Hines, M.T. Streptococcus equi infections in horses: Guidelines for treatment, control, and prevention of strangles. J. Vet. Intern. Med. 2005, 19, 123–134. [Google Scholar] [CrossRef]
- Boyle, A.G.; Timoney, J.F.; Newton, J.R.; Hines, M.T.; Waller, A.S.; Buchanan, B.R. Streptococcus equi infections in horses: Guidelines for treatment, control, and prevention of strangles—Revised consensus statement. J. Vet. Intern. Med. 2018, 32, 633–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, E.R.A.; Hillhouse, A.E.; Konganti, K.; Wu, J.; Lawhon, S.D.; Bordin, A.I.; Cohen, N.D. Comparison of whole genome sequences of Streptococcus equi subsp equi from an outbreak in Texas with isolates from within the region, Kentucky, USA, and other countries. Vet. Microbiol. 2020, 243, 108638. [Google Scholar]
- Kemp-Symonds, J.; Kemble, T.; Waller, A. Modified live Streptococcus equi (‘strangles’) vaccination followed by clinically adverse reactions associated with bacterial replication. Equine Vet. J. 2007, 39, 284–286. [Google Scholar] [CrossRef] [PubMed]
- McGlennon, A.; Waller, A.; Verheyen, K.; Slater, J.; Grewar, J.; Aanensen, D.; Newton, R. Surveillance of strangles in UK horses between 2015 and 2019 based on laboratory detection of Streptococcus equi. Vet. Rec. 2021, 189, e948. [Google Scholar] [CrossRef] [PubMed]
- Libardoni, F.; Machado, G.; Gressler, L.T.; Kowalski, A.P.; Diehl, G.N.; dos Santos, L.C.; Corbellini, L.G.; Castagna de Vargas, A. Prevalence of Streptococcus equi subsp equi in horses and associated risk factors in the State of Rio Grande do Sul, Brazil. Res. Vet. Sci. 2016, 104, 53–57. [Google Scholar]
- Jaramillo-Morales, C.; Gomez, D.E.; Renaud, D.; Arroyo, L.G. Streptococcus equi culture prevalence, associated risk factors and antimicrobial susceptibility in a horse population from Colombia. J. Equine Vet. Sci. 2022, 111, 103890. [Google Scholar] [CrossRef]
- Clark, C.; Greenwood, S.; Boison, J.O.; Chirino-Trejo, M.; Dowling, P.M. Bacterial isolates from equine infections in western Canada (1998–2003). Can. Vet. J. 2008, 49, 153–160. [Google Scholar] [PubMed]
- Ling, A.S.G.; Upjohn, M.M.; Webb, K.; Waller, A.S.; Verheyen, K.L.P. Seroprevalence of Streptococcus equi in working horses in Lesotho. Vet. Rec. 2011, 169, 72. [Google Scholar] [CrossRef] [PubMed]
- Tirosh-Levy, S.; Blum, S.E.; Steward, K.F.; Waller, A.S.; Steinman, A. Streptococcus equi subspecies equi in horses in Israel: Seroprevalence and strain types. Vet. Rec. Open 2016, 3, e000187. [Google Scholar] [CrossRef] [Green Version]
- Laing, G.; Christley, R.; Stringer, A.; Aklilu, N.; Ashine, T.; Newton, R.; Radford, A.; Pinchbeck, G. Respiratory disease and sero-epidemiology of respiratory pathogens in the working horses of Ethiopia. Equine Vet. J. 2018, 50, 793–799. [Google Scholar] [CrossRef] [Green Version]
- Pringle, J.; Venner, M.; Tscheschlok, L.; Waller, A.S.; Riihimäki, M. Markers of long term silent carriers of Streptococcus equi ssp. equi in horses. J. Vet. Intern. Med. 2020, 34, 2751–2757. [Google Scholar] [CrossRef]
- Ferraro, S.; Fecteau, G.; Dubuc, J.; Francoz, D.; Rousseau, M.; Roy, J.P.; Buczinski, S. Scoping review on clinical definition of bovine respiratory disease complex and related clinical signs in dairy cows. J. Dairy Sci. 2021, 104, 7095–7108. [Google Scholar] [CrossRef] [PubMed]
- Duffee, L.R.; Stefanovski, D.; Boston, R.C.; Boyle, A.G. Predictor variables for and complications associated with Streptococcus equi subsp equi infection in horses. Am. Vet. Med. Assoc. 2015, 247, 1161–1168. [Google Scholar] [CrossRef]
- Waller, A.S. New perspectives for the diagnosis, control, treatment, and prevention of strangles in horses. Vet. Clin. N. Am.-Equine Pract. 2014, 30, 591–607. [Google Scholar] [CrossRef]
- Tscheschlok, L.; Venner, M.; Steward, K.; Böse, R.; Riihimäki, M.; Pringle, J. Decreased clinical severity of strangles in weanlings associated with restricted seroconversion to optimized Streptococcus equi ssp equi assays. J. Vet. Intern. Med. 2018, 32, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.; Staempfli, H.; Prescott, J.; Viel, L. Field evaluation of a commercial M-protein vaccine against Streptococcus equi infection in foals. Am. J. Vet. Res. 1991, 52, 589–592. [Google Scholar]
- Robinson, C.; Waller, A.S.; Frykberg, L.; Flock, M.; Zachrisson, O.; Guss, B.; Flock, J.I. Intramuscular vaccination with Strangvac is safe and induces protection against equine strangles caused by Streptococcus equi. Vaccine 2020, 38, 4861–4868. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.A.C.; Goovaerts, D.; Nuijten, P.J.M.; Theelen, R.P.H.; Hartford, O.M.; Foster, T.J. Investigations towards an efficacious and safe strangles vaccine: Submucosal vaccination with a live attenuated Streptococcus equi. Vet. Rec. 2000, 147, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Heather, Z.; Slater, J.; Potts, N.; Steward, K.F.; Maskell, D.J.; Fontain, M.C.; Lee, J.J.; Smith, K.; Waller, A.S. Vaccination with a live multi-gene deletion strain protects horses against virulent challenge with Streptococcus equi. Vaccine 2015, 33, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.G.; Mitchell, C.; Stefanovski, D.; Waller, A.S. Horses vaccinated with live attenuated intranasal strangles vaccine seroconvert to SEQ2190 and SeM. Equine Vet. J. 2022, 54, 299–305. [Google Scholar] [CrossRef]
- Boyle, A.G.; Stefanovski, D.; Rankin, S.C. Comparison of nasopharyngeal and guttural pouch specimens to determine the optimal sampling site to detect Streptococcus equi subsp equi carriers by DNA amplification. BMC Vet. Res. 2017, 13, 75. [Google Scholar] [CrossRef]
| PCR Results | S. equi qPCR-Negative Horses (8694) | S. equi qPCR-Positive Horses (715) | p-Value |
|---|---|---|---|
| Positive for EHV-4 (992) | 934 (10.7%) | 58 (8.1%) | 0.037 |
| Positive for EIV (910) | 884 (10.2%) | 26 (3.6%) | <0.001 |
| Positive for ERBV (311) | 261 (3.0%) | 50 (7.0%) | <0.001 |
| Positive for EHV-1 (154) | 148 (1.7%) | 6 (0.8%) | 0.087 |
| Positive for ERAV (12) | 12 (0.1%) | 0 (0.0%) | 0.30 |
| Infection/coinfection | <0.001 | ||
| Single pathogen (6123) | 5715 (65.7%) | 408 (57.1%) | |
| Multiple pathogens (2409) | 2183 (25.1%) | 226 (31.6%) | |
| Not reported coinfection status (877) | 796 (9.2%) | 81 (11.3%) |
| Prevalence Factors | S. equi qPCR-Negative Horses (8694) | S. equi qPCR-Positive Horses (715) | p-Value |
|---|---|---|---|
| Breed | <0.001 | ||
| Quarter Horse (3341) | 3018 (34.7%) | 323 (45.2%) | |
| Warmblood (1498) | 1436 (16.5%) | 62 (8.7%) | |
| Thoroughbred (1015) | 987 (11.4%) | 28 (3.9%) | |
| Arabian (645) | 588 (6.8%) | 57 (8.0%) | |
| Paint (427) | 391 (4.5%) | 36 (5.0%) | |
| Pony/Miniature (350) | 291 (3.3%) | 59 (8.3%) | |
| Draft Horse (238) | 219 (2.5%) | 19 (2.7%) | |
| Other Breed (1895) | 1764 (20.3%) | 131 (18.3%) | |
| Age (years) | 6 ± 2.1 | 8 ± 4.1 | |
| Less than 1 (1516) | 1448 (16.7%) | 68 (9.5%) | <0.001 |
| 1–5 (2345) | 2159 (24.8%) | 186 (26.0%) | |
| 6–10 (2005) | 1807 (20.8%) | 198 (27.7%) | |
| 11–15 (1413) | 1308 (15.0%) | 105 (14.7%) | |
| 16–20 (859) | 789 (9.1%) | 70 (9.8%) | |
| Over 20 (573) | 540 (6.2%) | 33 (4.6%) | |
| No reported (698) | 643 (7.4%) | 55 (7.7%) | |
| Sex | 0.24 | ||
| Female (3305) | 3056 (35.2%) | 249 (34.8%) | |
| Male 1 (4597) | 4231 (48.7%) | 366 (51.2%) | |
| No reported (1507) | 1407 (16.2%) | 100 (14.0%) | |
| Use | 0.006 | ||
| Competition (3831) | 3581 (41.2%) | 250 (35.0%) | |
| Ranch/Farm horse (3458) | 3157 (36.3%) | 301 (42.1%) | |
| Breeding (442) | 408 (4.7%) | 34 (4.8%) | |
| Other Use (842) | 779 (9.0%) | 63 (8.8%) | |
| No reported (836) | 769 (8.8%) | 67 (9.4%) | |
| Transportation (2465) | 2264 (26.0%) | 201 (28.1%) | 0.14 |
| PCR Results | S. equi qPCR-Negative Horses (8694) | S. equi qPCR-Positive Horses (715) | p-Value |
|---|---|---|---|
| Vaccine History | |||
| S. equi (913) | 824 (9.5%) | 89 (12.4%) | <0.001 |
| EHV 1 and 4 (3054) | 2839 (32.7%) | 215 (30.1%) | 0.11 |
| EIV (3032) | 2817 (32.4%) | 215 (30.1%) | 0.061 |
| Clinical Signs | |||
| Fever presence (6954) | 6364 (73.2%) | 590 (82.5%) | <0.001 |
| Lethargy (6246) | 5707 (65.6%) | 539 (75.4%) | <0.001 |
| Anorexia (5144) | 4696 (54.0%) | 448 (62.7%) | <0.001 |
| Nasal discharge (6510) | 5871 (67.5%) | 639 (89.4%) | <0.001 |
| Cough (4175) | 3807 (43.9%) | 368 (51.5%) | <0.001 |
| Ocular discharge (2107) | 1902 (21.9%) | 205 (28.7%) | <0.001 |
| Distal limb edema | 806 (9.3%) | 62 (8.7%) | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo-Morales, C.; James, K.; Barnum, S.; Vaala, W.; Chappell, D.E.; Schneider, C.; Craig, B.; Bain, F.; Barnett, D.C.; Gaughan, E.; et al. Voluntary Biosurveillance of Streptococcus equi Subsp. equi in Nasal Secretions of 9409 Equids with Upper Airway Infection in the USA. Vet. Sci. 2023, 10, 78. https://doi.org/10.3390/vetsci10020078
Jaramillo-Morales C, James K, Barnum S, Vaala W, Chappell DE, Schneider C, Craig B, Bain F, Barnett DC, Gaughan E, et al. Voluntary Biosurveillance of Streptococcus equi Subsp. equi in Nasal Secretions of 9409 Equids with Upper Airway Infection in the USA. Veterinary Sciences. 2023; 10(2):78. https://doi.org/10.3390/vetsci10020078
Chicago/Turabian StyleJaramillo-Morales, Camilo, Kaitlyn James, Samantha Barnum, Wendy Vaala, Duane E. Chappell, Chrissie Schneider, Bryant Craig, Fairfield Bain, D. Craig Barnett, Earl Gaughan, and et al. 2023. "Voluntary Biosurveillance of Streptococcus equi Subsp. equi in Nasal Secretions of 9409 Equids with Upper Airway Infection in the USA" Veterinary Sciences 10, no. 2: 78. https://doi.org/10.3390/vetsci10020078






