Postnatal Changes of Somatostatin Expression in Hippocampi of C57BL/6 Mice; Modulation of Neuroblast Differentiation in the Hippocampus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Tissue Processing
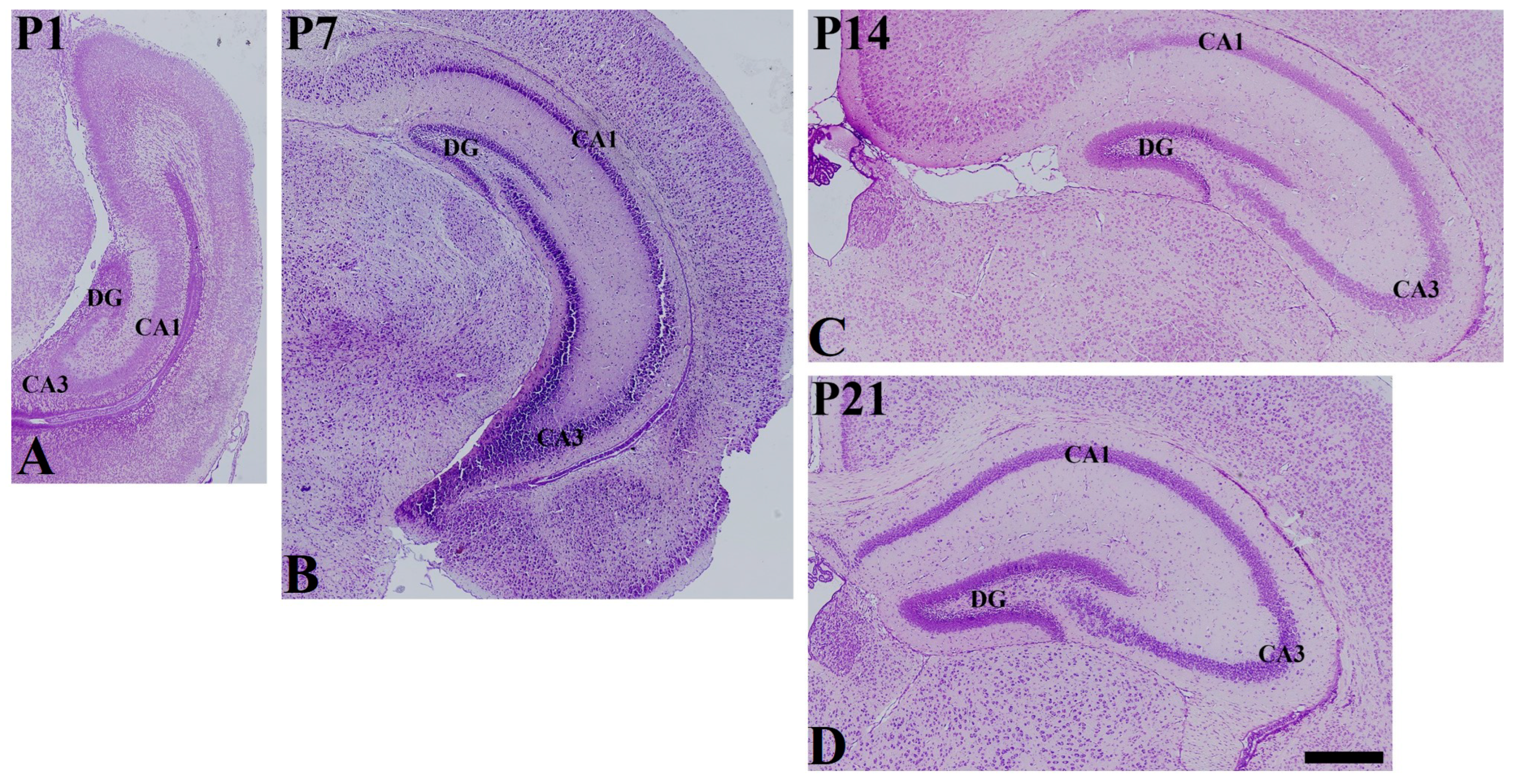
2.3. Cresyl Violet Staining
2.4. Immunohistochemistry for SST, Ki67, and Doublecortin
2.5. Double Immunofluorescence
2.6. Data Analysis
3. Results
3.1. Double Immunofluorescence
3.2. Postnatal Expression of SST in the Hippocampus
3.3. Double Immunofluorescence of NeuN or GFAP with SST at P14
3.4. Effects of SST on Cell Proliferation and Neuroblast Differentiation in the Hippocampal Dentate Gyrus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Squire, L.R. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992, 99, 195. [Google Scholar] [CrossRef] [PubMed]
- Jarrard, L.E. What does the hippocampus really do? Behav. Brain Res. 1995, 71, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.-M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H.; Kempermann, G.; Palmer, T.D.; Peterson, D.A.; Ray, J. Multipotent progenitor cells in the adult dentate gyrus. J. Neurobiol. 1998, 36, 249–266. [Google Scholar] [CrossRef]
- Bayer, S.A. Development of the hippocampal region in the rat I. Neurogenesis examined with 3H-thymidine autoradiography. J. Comp. Neurol. 1980, 190, 87–114. [Google Scholar] [CrossRef]
- Bayer, S.A.; Altman, J. Hippocampal development in the rat: Cytogenesis and morphogenesis examined with autoradiography and low-level X-irradiation. J. Comp. Neurol. 1974, 158, 55–79. [Google Scholar] [CrossRef]
- Altman, J.; Bayer, S. Postnatal development of the hippocampal dentate gyrus under normal and experimental conditions. In The Hippocampus; Springer: Berlin/Heidelberg, Germany, 1975; pp. 95–122. [Google Scholar]
- Sibbe, M.; Förster, E.; Basak, O.; Taylor, V.; Frotscher, M. Reelin and Notch1 cooperate in the development of the dentate gyrus. J. Neurosci. 2009, 29, 8578–8585. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Yoo, K.-Y.; Park, J.H.; Choi, J.W.; Kim, W.; Hwang, I.K.; Won, M.-H. Detailed differentiation of calbindin d-28k-immunoreactive cells in the dentate gyrus in C57BL/6 mice at early postnatal stages. Lab. Anim. Res. 2011, 27, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef]
- Patel, Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef]
- Cavanagh, M.E.; Parnavelas, J.G. Development of somatostatin immunoreactive neurons in the rat occipital cortex: A combined immunocytochemical—Autoradiographic study. J. Comp. Neurol. 1988, 268, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Forloni, G.; Hohmann, C.; Coyle, J.T. Developmental expression of somatostatin in mouse brain. I. Immunocytochemical studies. Dev. Brain Res. 1990, 53, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, G. Neurogenesis and neuronal regeneration in the adult fish brain. J. Comp. Physiol. A 2006, 192, 649–670. [Google Scholar] [CrossRef]
- Buckmaster, P.S.; Kunkel, D.D.; Robbins, R.J.; Schwartzkroin, P.A. Somatostatin-immunoreactivity in the hippocampus of mouse, rat, guinea pig, and rabbit. Hippocampus 1994, 4, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, J.; Guillou, J.-L.; Gastambide, F.; Hoyer, D.; Duron, E.; Viollet, C. Somatostatin, Alzheimer’s disease and cognition: An old story coming of age? Prog. Neurobiol. 2009, 89, 153–161. [Google Scholar] [CrossRef]
- Liguz-Lecznar, M.; Urban-Ciecko, J.; Kossut, M. Somatostatin and somatostatin-containing neurons in shaping neuronal activity and plasticity. Front. Neural Circuits 2016, 10, 48. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, A.; Silva, D.; Magano, S.; Pereira, P.A.; Andrade, J.P. Old-onset caloric restriction effects on neuropeptide Y-and somatostatin-containing neurons and on cholinergic varicosities in the rat hippocampal formation. Age 2014, 36, 9737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piatti, V.C.; Espósito, M.S.; Schinder, A.F. The timing of neuronal development in adult hippocampal neurogenesis. Neuroscientist 2006, 12, 463–468. [Google Scholar] [CrossRef]
- Loepke, A.W.; McCann, J.C.; Kurth, C.D.; McAuliffe, J.J. The physiologic effects of isoflurane anesthesia in neonatal mice. Anesth. Analg. 2006, 102, 75–80. [Google Scholar] [CrossRef]
- Navarro, K.L.; Huss, M.; Smith, J.C.; Sharp, P.; Marx, J.O.; Pacharinsak, C. Mouse Anesthesia: The art and science. ILAR J 2021, 62, 238–273. [Google Scholar] [CrossRef]
- Yager, J.Y.; Ashwal, S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr. Neurol. 2009, 40, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frahm, K.A.; Tobet, S.A. Development of the blood–brain barrier within the paraventricular nucleus of the hypothalamus: Influence of fetal glucocorticoid excess. Brain Struct. Funct. 2015, 220, 2225–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.Y.; Yim, H.S.; Yoo, D.Y.; Kim, J.W.; Chung, J.Y.; Seong, J.K.; Yoon, Y.S.; Kim, D.W.; Hwang, I.K. Postnatal changes in glucose transporter 3 expression in the dentate gyrus of the C57BL/6 mouse model. Lab. Anim. Res. 2016, 32, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Katona, L.; Lapray, D.; Viney, T.J.; Oulhaj, A.; Borhegyi, Z.; Micklem, B.R.; Klausberger, T.; Somogyi, P. Sleep and movement differentiates actions of two types of somatostatin-expressing GABAergic interneuron in rat hippocampus. Neuron 2014, 82, 872–886. [Google Scholar] [CrossRef] [Green Version]
- Sloviter, R.S.; Nilaver, G. Immunocytochemical localization of GABA-, cholecystokinin-, vasoactive intestinal polypeptide-, and somatostatin-like immunoreactivity in the area dentata and hippocampus of the rat. J. Comp. Neurol. 1987, 256, 42–60. [Google Scholar] [CrossRef]
- Ludvigsen, E.; Carlsson, C.; Tiensuu Janson, E.; Sandler, S.; Stridsberg, M. Somatostatin receptor 1–5; expression profiles during rat development. Upsala J. Med. Sci. 2015, 120, 157–168. [Google Scholar] [CrossRef]
- Bendotti, C.; Hohmann, C.; Forloni, G.; Reeves, R.; Coyle, J.T.; Oster-Granite, M.L. Developmental expression of somatostatin in mouse brain. II. In situ hybridization. Dev. Brain Res. 1990, 53, 26–39. [Google Scholar] [CrossRef]
- Harris, K.M.; Jensen, F.E.; Tsao, B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: Implications for the maturation of synaptic physiology and long-term potentiation [published erratum appears in J Neurosci 1992 Aug; 12 (8): Following table of contents]. J. Neurosci. 1992, 12, 2685–2705. [Google Scholar]
- Minkwitz, H.; Holz, L. The ontogenetic development of pyramidal neurons in the hippocampus (CA1) of the rat. J. Hirnforsch. 1975, 16, 37–54. [Google Scholar]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 3, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Nicola, Z.; Fabel, K.; Kempermann, G. Development of the adult neurogenic niche in the hippocampus of mice. Front. Neuroanat. 2015, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Naus, C.C. Development of somatostatin-like immunoreactivity in the hippocampal formation of normal and reeler mice. Neurosci. Lett. 1989, 96, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M. Ontogeny of glutamic acid decarboxylase, tyrosine hydroxylase, choline acetyltransferase, somatostatin and substance P in monkey cerebellum. Dev. Brain Res. 1987, 32, 181–186. [Google Scholar] [CrossRef]
- Cebada-Sánchez, S.; Insausti, R.; González-Fuentes, J.; Arroyo-Jiménez, M.; Rivas-Infante, E.; Lagartos, M.; Martínez-Ruiz, J.; Lozano, G.; Marcos, P. Distribution of peptidergic populations in the human dentate gyrus (somatostatin [SOM-28, SOM-12] and neuropeptide Y [NPY]) during postnatal development. Cell Tissue Res. 2014, 358, 25–41. [Google Scholar] [CrossRef]
- Malatesta, P.; Hartfuss, E.; Gotz, M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 2000, 127, 5253–5263. [Google Scholar] [CrossRef]
- Ferjoux, G.; Bousquet, C.; Cordelier, P.; Benali, N.; Lopez, F.; Rochaix, P.; Buscail, L.; Susini, C. Signal transduction of somatostatin receptors negatively controlling cell proliferation. J. Physiol. 2000, 94, 205–210. [Google Scholar] [CrossRef]
- Buscail, L.; Vernejoul, F.; Faure, P.; Torrisani, J.; Susini, C. Regulation of cell proliferation by somatostatin. Ann. Endocrinol. 2002, 63, 2S13–8. [Google Scholar]
- Ruscica, M.; Arvigo, M.; Gatto, F.; Dozio, E.; Feltrin, D.; Culler, M.D.; Minuto, F.; Motta, M.; Ferone, D.; Magni, P. Regulation of prostate cancer cell proliferation by somatostatin receptor activation. Mol. Cell. Endocrinol. 2010, 315, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Margheri, M.; Pacini, N.; Tani, A.; Nosi, D.; Squecco, R.; Dama, A.; Masala, E.; Francini, F.; Zecchi-Orlandini, S.; Formigli, L. Combined effects of melatonin and all-trans retinoic acid and somatostatin on breast cancer cell proliferation and death: Molecular basis for the anticancer effect of these molecules. Eur. J. Pharmacol. 2012, 681, 34–43. [Google Scholar] [CrossRef]
- Kasprzak, A. Somatostatin and its receptor system in colorectal cancer. Biomedicines 2021, 9, 1743. [Google Scholar] [CrossRef]
- Leroux, P.; Bodenant, C.; Bologna, E.; Gonzalez, B.; Vaudry, H. Transient expression of somatostatin receptors in the brain during development. In Ciba Foundation Symposium 190—Somatostatin and its Receptors: Somatostatin and its Receptors: Ciba Foundation Symposium 190; Ciba Foundation: Amsterdam, The Netherlands, 2007; pp. 127–141. [Google Scholar]
- Cha, C.I.; Lee, Y.I.; Lee, E.Y.; Park, K.H.; Baik, S.H. Age-related changes of VIP, NPY and somatostatin-immunoreactive neurons in the cerebral cortex of aged rats. Brain Res. 1997, 753, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Albers, A.R.; O’Dorisio, M.S.; Balster, D.A.; Caprara, M.; Gosh, P.; Chen, F.; Hoeger, C.; Rivier, J.; Wenger, G.D.; O’Dorisio, T.M. Somatostatin receptor gene expression in neuroblastoma. Regul. Pept. 2000, 88, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Leung, K.N. Tryptanthrin induces growth inhibition and neuronal differentiation in the human neuroblastoma LA-N-1 cells. Chem.-Biol. Interact. 2013, 203, 512–521. [Google Scholar] [CrossRef]
- Kogner, P.; Borgström, P.; Bjellerup, P.; Schilling, F.H.; Refai, E.; Jonsson, C.; Dominici, C.; Wassberg, E.; Bihl, H.; Jacobsson, H.; et al. Somatostatin in neuroblastoma and ganglioneuroma. Eur. J. Cancer 1997, 33, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Somvanshi, R.K.; Paik, S.; Kumar, U. Colocalization of cannabinoid receptor 1 with somatostatin and neuronal nitric oxide synthase in rat brain hypothalamus. J. Mol. Neurosci. 2015, 55, 480–491. [Google Scholar] [CrossRef]
- Fournier, N.M.; Lee, B.; Banasr, M.; Elsayed, M.; Duman, R.S. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK-and PI3K/Akt-dependent signaling. Neuropharmacology 2012, 63, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Dong, X.; Huang, X.; Huang, X.-J.; Liu, H.; Wang, Y.; Ye, W.-C.; Shi, L. A natural diarylheptanoid promotes neuronal differentiation via activating ERK and PI3K-Akt dependent pathways. Neuroscience 2015, 303, 389–401. [Google Scholar] [CrossRef]
- Ampofo, E.; Nalbach, L.; Menger, M.D.; Laschke, M.W. Regulatory mechanisms of somatostatin expression. Int. J. Mol. Sci. 2020, 21, 4170. [Google Scholar] [CrossRef]
- Podgorny, O.V.; Gulyaeva, N.V. Glucocorticoid-mediated mechanisms of hippocampal damage: Contribution of subgranular neurogenesis. J. Neurochem. 2021, 157, 370–392. [Google Scholar] [CrossRef]
- Lin, L.-C.; Sibille, E. Reduced brain somatostatin in mood disorders: A common pathophysiological substrate and drug target? Front. Pharmacol. 2013, 4, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, G.S.; Leon-Palmer, N.E.; Townsend, K.L. Bone morphogenetic proteins (BMPs) in the central regulation of energy balance and adult neural plasticity. Metabolism 2021, 123, 154837. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, N.; Otsuka, F.; Miyoshi, T.; Yamanaka, R.; Inagaki, K.; Yamashita, M.; Otani, H.; Takeda, M.; Suzuki, J.; Ogura, T. Effects of bone morphogenetic protein (BMP) on adrenocorticotropin production by pituitary corticotrope cells: Involvement of up-regulation of BMP receptor signaling by somatostatin analogs. Endocrinology 2010, 151, 1129–1141. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, D.Y.; Kim, W.; Jung, H.Y.; Hwang, I.K. Postnatal Changes of Somatostatin Expression in Hippocampi of C57BL/6 Mice; Modulation of Neuroblast Differentiation in the Hippocampus. Vet. Sci. 2023, 10, 81. https://doi.org/10.3390/vetsci10020081
Yoo DY, Kim W, Jung HY, Hwang IK. Postnatal Changes of Somatostatin Expression in Hippocampi of C57BL/6 Mice; Modulation of Neuroblast Differentiation in the Hippocampus. Veterinary Sciences. 2023; 10(2):81. https://doi.org/10.3390/vetsci10020081
Chicago/Turabian StyleYoo, Dae Young, Woosuk Kim, Hyo Young Jung, and In Koo Hwang. 2023. "Postnatal Changes of Somatostatin Expression in Hippocampi of C57BL/6 Mice; Modulation of Neuroblast Differentiation in the Hippocampus" Veterinary Sciences 10, no. 2: 81. https://doi.org/10.3390/vetsci10020081





