Basicranial Modular Organization. A Study in the Araucanian Horse of Colombia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
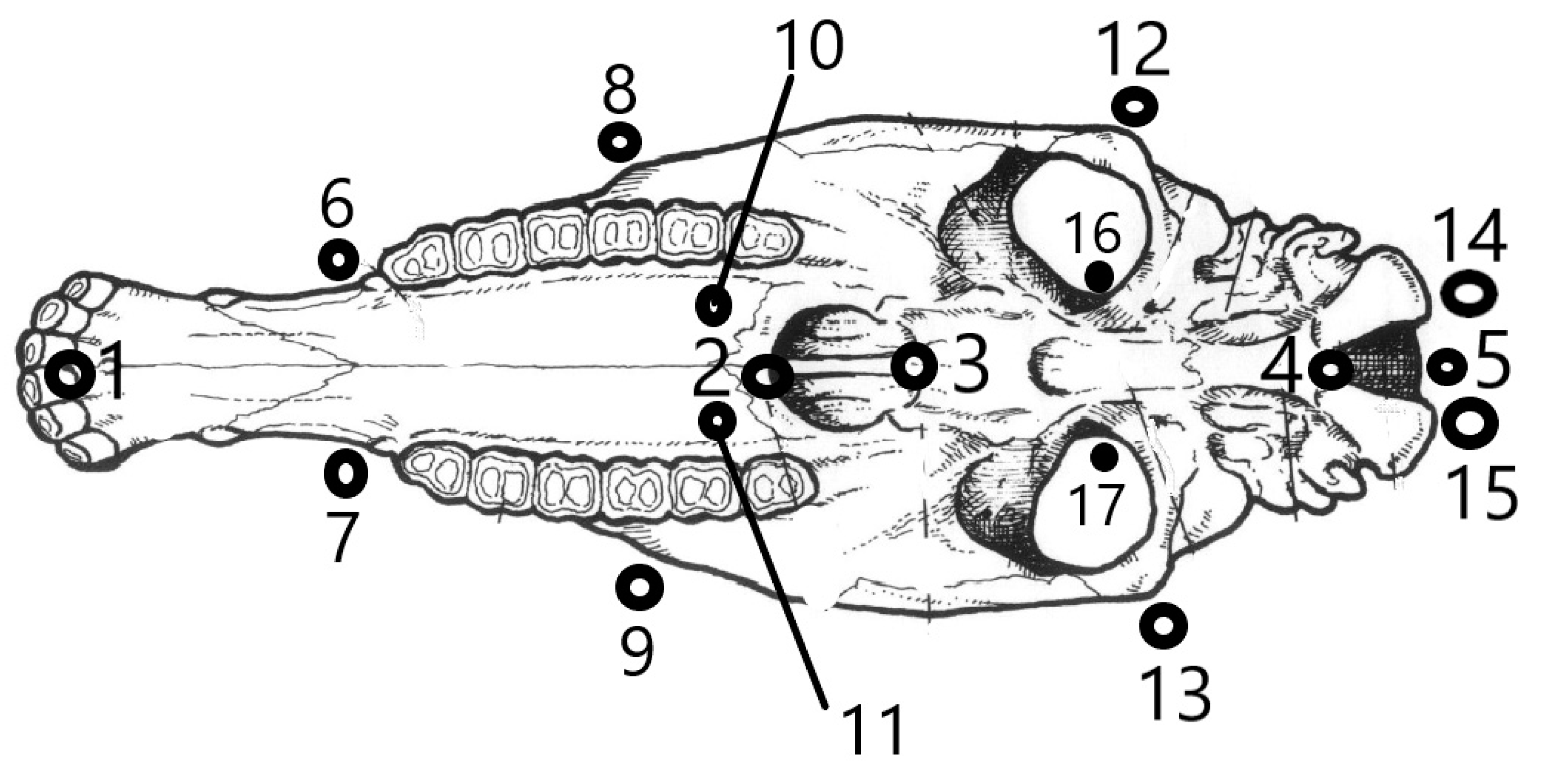
2.2. Primary Data Collection
2.3. Processing of the Primary Data
3. Results
3.1. Preliminary Analysis
3.2. Allometry
3.3. Principal Component Analysis
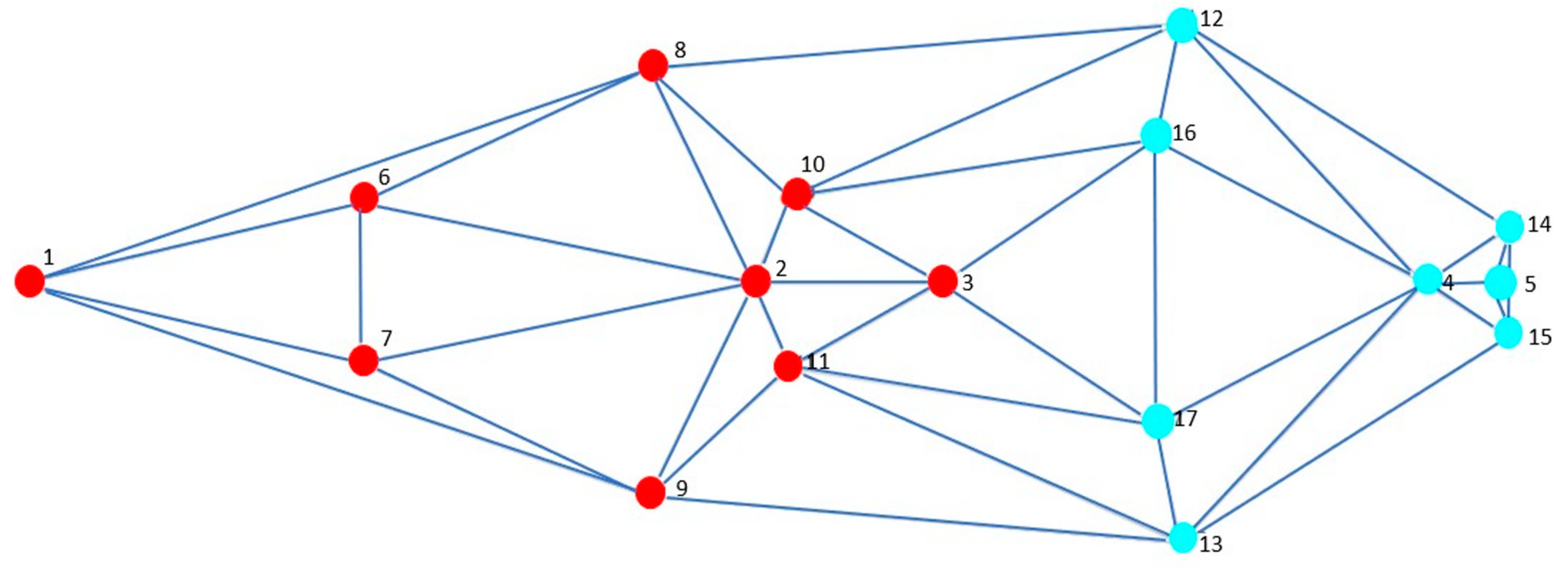
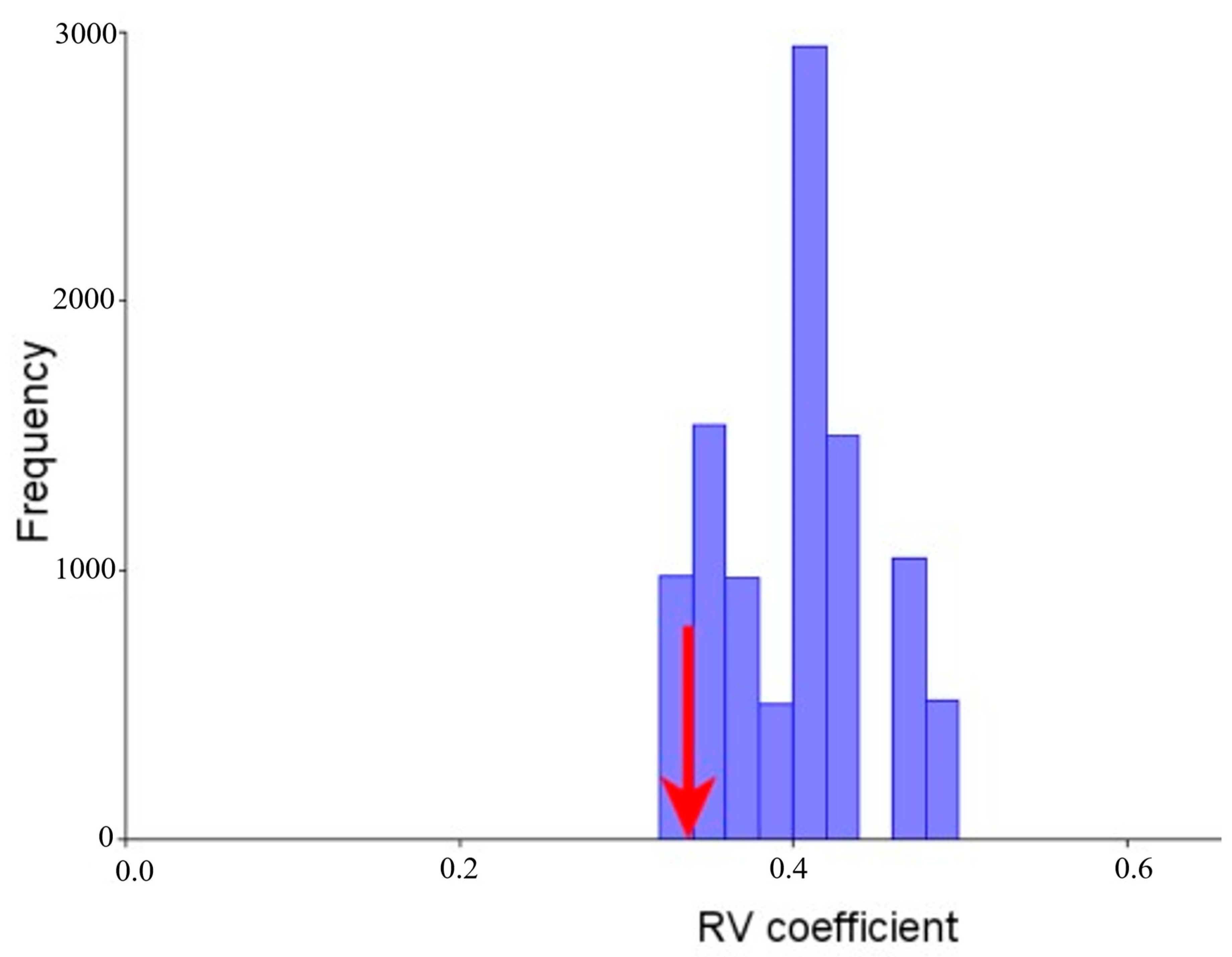
3.4. Modularity and Integration
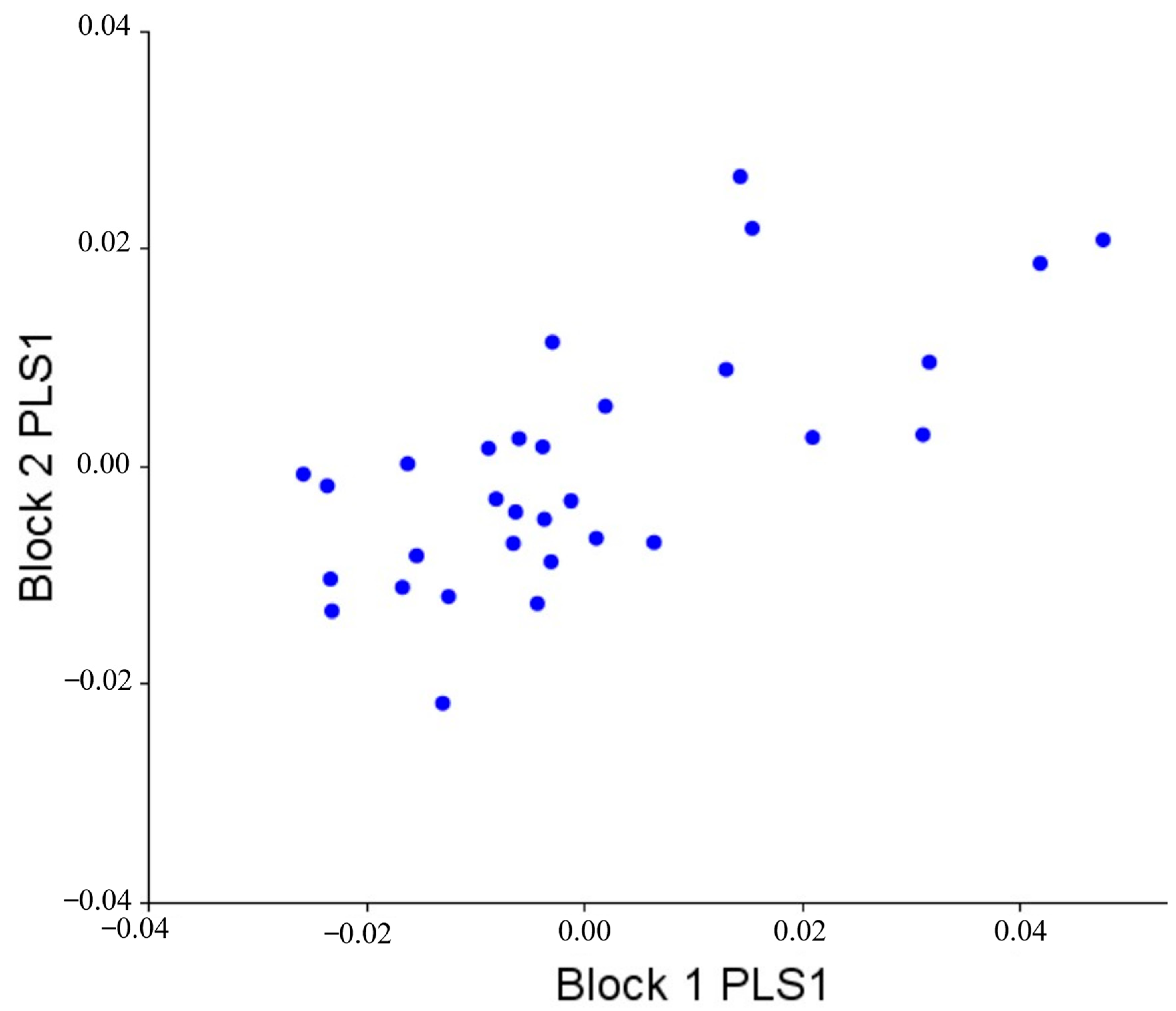
3.5. Morphological Integration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barone, R. Anatomie Comparée des Mamifères Domestiques. Tome 1, 5th ed.; Vigot Fréres: Paris, France, 1999. [Google Scholar]
- König, H.E.; Liebich, H.-G. Anatomía de los Animales Domésticos: Texto y Atlas en Color. Tomo 1. Aparato Locomotor, 3rd ed.; Medica Panamericana: Madrid, España, 2011; p. 424. [Google Scholar]
- Carlson, B. Human Embryology and Developmental Biology; Mosby Elsevier: Philadelphia, PA, USA, 1999. [Google Scholar]
- Campos Varela, I.Y. Desarrollo del cráneo y su importancia para la antropología forense. Morfolia 2017, 9, 16–28. [Google Scholar]
- Venditti, C.; Meade, A.; Pagel, M. Multiple routes to mammalian diversity. Nature 2011, 479, 393–396. [Google Scholar] [CrossRef]
- Olsson, J.; Svanbäck, R.; Eklöv, P. Growth rate constrain morphological divergence when driven by competition. Oikos 2006, 115, 15–22. [Google Scholar] [CrossRef]
- Gil, C.F.; Vázquez, J.J.; Soler, L.A.; Cárceles, R.C.; Solano, M.R.; Lomba, M.J. Estudios de restos de ganado porcino de 4500 años de antiguedad encontrados en el yacimiento calcolítico C/Marcsilla N° 12 (Lorca, Murcia). In Proceedings of the Libro de Actas del XXV Congreso Nacional y XVI Congreso Iberoamericano de Historia de la Veterinaria, Toledo, Spain, 15–17 November 2019; pp. 177–181. [Google Scholar]
- Klingenberg, C.P. Morphometric integration and modularity in configurations of landmarks: Tools for evaluating a priori hypotheses. Evol. Dev. 2009, 11, 405–421. [Google Scholar] [CrossRef] [Green Version]
- Goswami, A.; Polly, P.D. Methods for Studying Morphological Integration and Modularity. Paléontol. Soc. Pap. 2010, 16, 213–243. [Google Scholar] [CrossRef]
- Lieberman, D.E. Evolution of the Human Head; Harvard University Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Püschel, T. Modularidad e Integración Morfológica en Cráneos Humanos: Un Enfoque Morfométrico Geométrico. Int. J. Morphol. 2014, 32, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.L.; Taylor, W.T.T.; Belardi, J.B.; Neme, G.; Gil, A.; Roberts, P.; Thornhill, C.; Hodgins, G.W.L.; Orlando, L. Caballos y humanos en el nuevo mundo: Investigaciones arqueológicas en américa del Norte y perspectivas para Argentina. Anales Arqueol. Etnol. 2019, 74, 247–268. [Google Scholar]
- Salamanca, C.A.; Parés-Casanova, P.M.; Crosby, R.A.; Monroy, N. Biometric analysis of araucano criollo horse. Arch. Zootec. 2017, 66, 267–278. [Google Scholar] [CrossRef]
- Parés-Casanova, P.M.; Crosby-Granados, R.A.; Muñoz, F.; Salamanca-Carreño, A. Marked Directional Skull Asymmetry in the Araucan Horse. VCOT Open 2020, 03, e11–e18. [Google Scholar] [CrossRef] [Green Version]
- Salamanca-Carreño, A.; Jordana, J.; Crosby-Granados, R.A.; Bentez-Molano, J.; Parés-Casanova, P.M. Lineal Discrimination of Horses and Mules. A Sympatric Case from Arauca, Colombia. Animals 2020, 10, 679. [Google Scholar] [CrossRef]
- Sisson, S.; Grossman, J.D.; Getty, R. Anatomía de los Animales Domésticos; Salvat Editores: Barcelona, España, 1982. [Google Scholar]
- Webster, M.; Sheets, H.D. A Practical Introduction to Landmark-Based Geometric Morphometrics. Paléontol. Soc. Pap. 2010, 16, 163–188. [Google Scholar] [CrossRef] [Green Version]
- Toro Ibacache, M.V.; Manriquez Soto, G.; Suazo Galdames, I. Morfometría geométrica y el estudio de las formas biológicas: De la morfología descriptiva a la morfología cuantitativa geométrica. Int. J. Morphol. 2010, 28, 977–990. [Google Scholar] [CrossRef]
- Parés-Casanova, P.M.; Salamanca-Carreño, A.; Crosby-Granados, R.; Carolino, N.; Leite, J.V.; Dantas, R.; Lopes, S. Crecimiento postnatal diferenciado del neurocráneo y del viscerocráneo en equinos domésticos. Rev. Inv. Vet. Perú 2018, 29, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Rohlf, F.J. Digitalized Landmarks and Outlines (2.26); Stony Brook; Department of Ecology and Evolution, State University of New York: New York, NY, USA, 2010. [Google Scholar]
- Ceballos, C.P.; Valenzuela, N. The Role of Sex-specific Plasticity in Shaping Sexual Dimorphism in a Long-lived Vertebrate, the Snapping Turtle Chelydra serpentina. Evol. Biol. 2011, 38, 163–181. [Google Scholar] [CrossRef]
- Brachetta-Aporta, N.; Gonzalez, P.N.; Bernal, V. Integrating data on bone modeling and morphological ontogenetic changes of the maxilla in modern humans. Ann. Anat. 2019, 222, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Adams, D.C. Methods for shape analysis of landmark data from articulated structures. Evol. Ecol. Res. 1999, 1, 959–970. [Google Scholar]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D. Geometric Morphometrics for Biologists: A Primer; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Savriama, Y.; Neustupa, J.; Klingenberg, C. Geometric morphometrics of symmetry and allometry in Micrasterias rotata (Zygnemophyceae, Viridiplantae). Nova Hedwigia, Beih. 2010, 136, 43–54. [Google Scholar] [CrossRef]
- Rohlf, F.J. The tps series of software. Hystrix 2015, 26, 9–12. [Google Scholar] [CrossRef]
- Lebrun, R.; Perier, A.; Masters, J.; Marivaux, L.; Couette, S. Lower Levels of Vestibular Developmental Stability in Slow-Moving than Fast-Moving Primates. Symmetry 2021, 13, 2305. [Google Scholar] [CrossRef]
- Salamanca-Carreño, A.; Parés-Casanova, P.M.; Vélez-Terranova, O.M.; Monroy-Ochoa, N.I.; Crosby-Granados, R.A. Modularity among horse mandibles: A study in the Araucan breed. J. Appl. Anim. Res. 2022, 50, 322–326. [Google Scholar] [CrossRef]
- Escoufier, Y. Le Traitement des Variables Vectorielles. Biometrics 1973, 29, 751. [Google Scholar] [CrossRef]
- Adams, D.C. Evaluating modularity in morphometric data: Challenges with the RV coefficient and a new test measure. Methods Ecol. Evol. 2016, 7, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Lobón, J.M. Disparidad e Integración en el Cráneo de Archosauria: Aplicaciones de la Morfología Teórica y la Morfometría Geométrica en Macroevolución. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2007. [Google Scholar]
- Andrés, J.B. Integración Ontogenética en la Morfología Craneofacial Humana. Ph.D. Thesis; Universidad Nacional de la Plata: La Plata, Argentina, 2014. [Google Scholar]
- Barbeito-Andrés, J.; Sardi, M.L.; Anzelmo, M.; Pucciarelli, H.M. Matrices funcionales e integración morfológica. Un estudio ontogenico de la bóveda y el maxilar. Rev. Argent. Antropol. Biol. 2012, 14, 79–87. [Google Scholar]
- Reeve, E.C.R.; Murray, P.D.F. Evolution in the Horse’s Skull. Nature 1942, 150, 402–403. [Google Scholar] [CrossRef]
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| x1 | −0.7767 | 0.1055 | 0.1788 |
| y1 | 0.0000 | 0.0000 | 0.0000 |
| x2 | 0.1595 | −0.1770 | 0.1973 |
| y2 | 0.0000 | 0.0000 | 0.0000 |
| x3 | 0.1258 | −0.2369 | −0.1501 |
| y3 | 0.0000 | 0.0000 | 0.0000 |
| x4 | −0.1203 | −0.2064 | 0.0537 |
| y4 | 0.0000 | 0.0000 | 0.0000 |
| x5 | 0.0039 | 0.2589 | −0.0458 |
| y5 | 0.0000 | 0.0000 | 0.0000 |
| x6 | 0.1263 | 0.1706 | −0.2882 |
| y6 | 0.0356 | 0.0436 | −0.0970 |
| x7 | 0.1263 | 0.1706 | −0.2882 |
| y7 | −0.0356 | −0.0436 | 0.0970 |
| x8 | 0.1853 | 0.0183 | −0.1422 |
| y8 | −0.0258 | 0.1779 | 0.1118 |
| x9 | 0.1853 | 0.0183 | −0.1422 |
| y9 | 0.0258 | −0.1779 | −0.1118 |
| x10 | 0.1349 | −0.3320 | 0.3613 |
| y10 | −0.0653 | 0.0173 | 0.0998 |
| x11 | 0.1349 | −0.3320 | 0.3613 |
| y11 | 0.0653 | −0.0173 | −0.0998 |
| x12 | 0.0326 | 0.3179 | 0.1407 |
| y12 | −0.0735 | 0.1390 | 0.1781 |
| x13 | 0.0326 | 0.3179 | 0.1407 |
| y13 | 0.0735 | −0.1390 | −0.1781 |
| x14 | −0.2708 | −0.2414 | −0.3096 |
| y14 | −0.0408 | 0.0772 | −0.1520 |
| x15 | −0.2708 | −0.2414 | −0.3096 |
| y15 | 0.0408 | −0.0772 | 0.1520 |
| x16 | 0.0956 | 0.1945 | 0.1211 |
| y16 | −0.0719 | −0.0013 | −0.0030 |
| x17 | 0.0956 | 0.1945 | 0.1211 |
| y17 | 0.0719 | 0.0013 | 0.0030 |
| Single Value | p-Value (Perm.) | % Total Covariación | Correlation | p-Value (Perm.) | |
|---|---|---|---|---|---|
| PLS1 | 0.00015 | 0.008 | 78.198 | 0.69529 | 0.404 |
| PLS2 | 5.98 × 10−5 | 0.148 | 12.477 | 0.55855 | 0.296 |
| PLS3 | 4.1 × 10−5 | 0.016 | 5.858 | 0.71101 | 0.012 |
| PLS4 | 2.61 × 10−5 | 0.060 | 2.366 | 0.61453 | 0.020 |
| PLS5 | 1.37 × 10−5 | 0.556 | 0.650 | 0.44533 | 0.188 |
| PLS6 | 9.85 × 10−5 | 0.256 | 0.338 | 0.46873 | 0.060 |
| PLS7 | 0.000005 | 0.464 | 0.087 | 0.40127 | 0.012 |
| PLS8 | 2.71 × 10−6 | 0.280 | 0.026 | 0.09417 | 0.536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanca-Carreño, A.; Parés-Casanova, P.M.; Crosby-Granados, R.A.; Vélez-Terranova, M.; Bentez-Molano, J. Basicranial Modular Organization. A Study in the Araucanian Horse of Colombia. Vet. Sci. 2023, 10, 255. https://doi.org/10.3390/vetsci10040255
Salamanca-Carreño A, Parés-Casanova PM, Crosby-Granados RA, Vélez-Terranova M, Bentez-Molano J. Basicranial Modular Organization. A Study in the Araucanian Horse of Colombia. Veterinary Sciences. 2023; 10(4):255. https://doi.org/10.3390/vetsci10040255
Chicago/Turabian StyleSalamanca-Carreño, Arcesio, Pere M. Parés-Casanova, René Alejandro Crosby-Granados, Mauricio Vélez-Terranova, and Jannet Bentez-Molano. 2023. "Basicranial Modular Organization. A Study in the Araucanian Horse of Colombia" Veterinary Sciences 10, no. 4: 255. https://doi.org/10.3390/vetsci10040255
APA StyleSalamanca-Carreño, A., Parés-Casanova, P. M., Crosby-Granados, R. A., Vélez-Terranova, M., & Bentez-Molano, J. (2023). Basicranial Modular Organization. A Study in the Araucanian Horse of Colombia. Veterinary Sciences, 10(4), 255. https://doi.org/10.3390/vetsci10040255






