Retrobulbar Filling for Enophthalmos Treatment in Dogs: Technique, Description and Computed-Tomographic Evaluation. Preliminary Cadaveric Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol, Selection and Description of Subjects
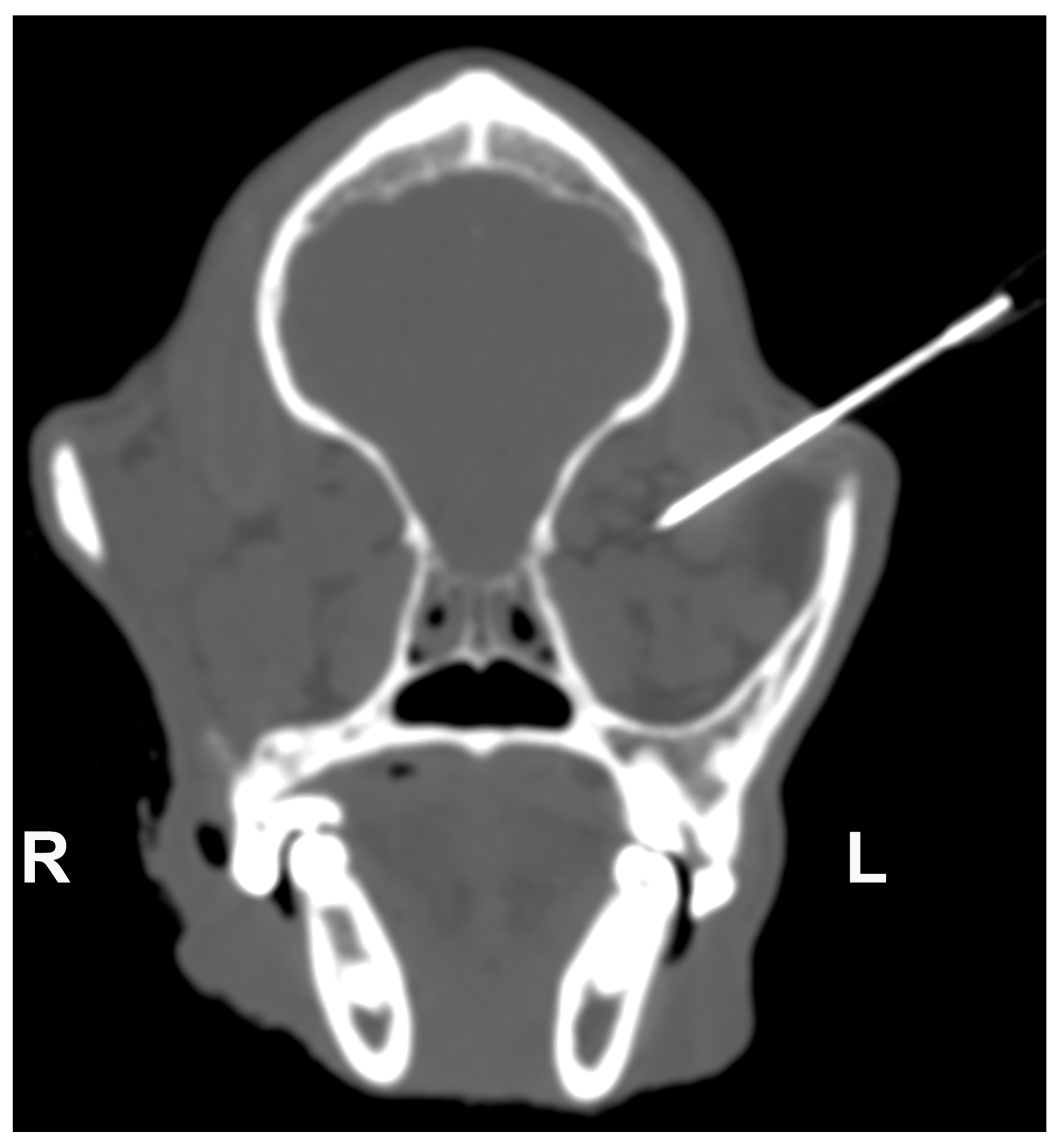
2.2. Retrobulbar Injections
2.3. Macroscopic and Histological Examination
2.4. CT Image Analysis
2.5. Statistical Analyses
3. Results
3.1. Population
3.2. Retrobulbar Injections Findings
3.3. Necropsy Macroscopic Examination and Histological Findings
3.4. CT Images Findings
3.5. Statistical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spiess, B.M.; Pot, S.A. Diseases and Surgery of the Canine Orbit. In Veterinary Ophthalmology, 5th ed.; Gelatt, K.N., Gilger, B.C., Kern, T.J., Eds.; John Wiley & Sons, Inc.: Oxford, UK, 2013; Volume II, pp. 793–831. [Google Scholar]
- Jones, B.R.; Studdert, V.P. Horner’s syndrome in the dog and cat as an aid to diagnosis. Aust. Vet. J. 1975, 51, 329–332. [Google Scholar] [CrossRef]
- Turner, S.M. Enophthalmos. In Saunders Solutions in Veterinary Practice: Small Animal Ophtalmology; Nind, F., Ed.; Saunders Solutions in Veterinary Practice; Elsevier: Amsterdam, The Netherlands, 2008; pp. 319–324. [Google Scholar]
- Rubin, L.F. Inherited Eye Diseases in Purebred Dogs; Williams & Wilkins: Philadelphia, PA, USA, 1989. [Google Scholar]
- Renwick, P.W.; Petersen-Jones, S.M. Orbital and ocular pain. In Small Animal Ophthalmology, 4th ed.; Peiffer, R.L., Petersen-Jones, S.M., Eds.; W.B. Saunders: Edinburgh, UK, 2009; pp. 203–252. [Google Scholar]
- Stades, F.; van der Woerdt, A. Diseases and Surgery of the Canine Eyelid. In Veterinary Ophthalmology, 5th ed.; Gelatt, K.N., Giler, B.C., Kern, T.J., Eds.; John Wiley & Sons Inc.: Oxford, UK, 2013; Volume II, pp. 832–893. [Google Scholar]
- Ye, L.; Zhang, L.; Zhu, Y.; Zhang, Y.; Wu, W.; Zhang, Y. Enophthalmos: Exploration of Quantitative Treatment With Retro-Orbital Fat Globules Injection. J. Craniofacial Surg. 2020, 31, 54–57. [Google Scholar] [CrossRef]
- Cervelli, D.; Gasparini, G.; Moro, A.; Grussu, F.; Boniello, R.; Pelo, S. Retrobulbar Lipofilling to Correct the Enophthalmos. J. Craniofacial Surg. 2011, 22, 1918–1922. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Qiu, Q.; Yang, Z. Autologous Fat Graft for the Treatment of Sighted Posttraumatic Enophthalmos and Sunken Upper Eyelid. Ophthalmic Plast. Reconstr. Surg. 2018, 34, 381–386. [Google Scholar] [CrossRef]
- Agostini, T.; Perello, R.; Arcuri, F.; Spinelli, G. Retroseptal Lipotransfer to Correct Enophthalmos in the Postraumatic Amaurotic Eye. Plast. Reconstr. Surg. 2014, 134, 989e–990e. [Google Scholar] [CrossRef]
- Hardy, T.G.; Joshi, N.; Kelly, M.H. Orbital volume augmentation with autologous micro-fat grafts. Ophthalmic Plast. Reconstr. Surg. 2007, 23, 445–449. [Google Scholar] [CrossRef]
- Hunter, P.D.; Baker, S.S. The treatment of enophthalmos by orbital injection of fat autograft. JAMA Otolaryngol. Head Neck Surg. 1994, 120, 835–839. [Google Scholar] [CrossRef]
- Fox, D.M. Orbital Fat Injection: Technique and 5-Year Follow-Up. Aesthetic Plast. Surg. 2019, 43, 123–132. [Google Scholar] [CrossRef]
- Grahn, B.H.; Stewart, W.A.; Towner, R.A.; Noseworthy, M.D. Magnetic resonance imaging of the canine and feline eye, orbit, and optic nerves and its clinical application. Can. Vet. J. 1993, 34, 418–424. [Google Scholar]
- Morgan, R.V.; Daniel, G.B.; Donnell, R.L. Magnetic Resonance Imaging of the normal eye and orbit of the dog and cat. Vet. Radiol. Ultrasound 1994, 35, 102–108. [Google Scholar] [CrossRef]
- Penninck, D.; Daniel, G.B.; Brawer, R.; Tidwell, A.S. Cross-sectional imaging techniques in veterinary ophthalmology. Clin. Tech. Small Anim. Pract. 2001, 16, 22–39. [Google Scholar] [CrossRef]
- Dubois, L.; Steenen, S.A.; Gooris, P.J.; Bos, R.R.; Becking, A.G. Controversies in orbital reconstruction—III. Biomaterials for orbital reconstruction: A review with clinical recommendations. Int. J. Oral Maxillofac. Surg. 2016, 45, 41–50. [Google Scholar] [CrossRef]
- Ramieri, G.; Spada, M.C.; Bianchi, S.D.; Berrone, S. Dimensions and volumes of the orbit and orbital fat in posttraumatic enophthalmos. Dentomaxillofacial Radiol. 2000, 29, 302–311. [Google Scholar] [CrossRef]
- Boroffka, S.A.E.B.; Verbruggen, A.-M.; Grinwis, G.C.M.; Voorhout, G.; Barthez, P.Y. Assessment of ultrasonography and computed tomography for the evaluation of unilateral orbital disease in dogs. J. Am. Vet. Med. Assoc. 2007, 230, 671–680. [Google Scholar] [CrossRef]
- Scrivani, P.V. Sense Organs, Circulatory System and Endocrine System. In Veterinary Head and Neck Imaging, 1st ed.; Scrivani, P.V., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2022; pp. 673–772. [Google Scholar]
- Boroffka, S.A.; Voorhout, G. Direct and reconstructed multiplanar computed tomography of the orbits of healthy dogs. Am. J. Vet. Res. 1999, 60, 1500–1507. [Google Scholar]
- Winer, J.N.; Verstraete, F.J.M.; Cissell, D.D.; Le, C.; Vapniarsky, N.; Good, K.L.; Gutierrez, C.J.; Arzi, B. Clinical Features and Computed Tomography Findings Are Utilized to Characterize Retrobulbar Disease in Dogs. Front. Vet. Sci. 2018, 5, 186. [Google Scholar] [CrossRef]
- LeCouteur, R.A.; Fike, J.R.; Scagliotti, R.H.; Cann, C.E. Computed tomography of orbital tumors in the dog. J. Am. Vet. Med. Assoc. 1982, 180, 910–913. [Google Scholar]
- Calia, C.M.; Kirschner, S.E.; Baer, K.E.; Stefanacci, J.D. The use of computed tomography scan for the evaluation of orbital disease in cats and dogs. Vet. Comp. Ophthalmol. 1994, 4, 24–30. [Google Scholar]
- Cakir, B.; Aygit, A.C.; Omur-Okten, O.; Yalcin, O. Retro-Orbital Intraconal Fat Injection: An Experimental Study in Rabbits. J. Oral Maxillofac. Surg. 2012, 70, 242–250. [Google Scholar] [CrossRef]
- Greco, A.; Costanza, D.; Senatore, A.; Bruzzese, D.; Micieli, F.; Chiavaccini, L.; Di Giancamillo, M.; Della Valle, G.; Vesce, G.; Brunetti, A.; et al. A computed tomography–based method for the assessment of canine retrobulbar cone volume for ophthalmic anaesthesia. Vet. Anaesth. Analg. 2021, 48, 759–766. [Google Scholar] [CrossRef]
- Chiavaccini, L.; Micieli, F.; Meomartino, L.; Duffee, L.R.; Vesce, G. A novel supra-temporal approach to retrobulbar anaesthesia in dogs: Preliminary study in cadavers. Vet. J. 2017, 223, 68–70. [Google Scholar] [CrossRef]
- Piegari, G.; Iovane, V.; Carletti, V.; Fico, R.; Costagliola, A.; De Biase, D.; Prisco, F.; Paciello, O. Assessment of Google Glass for Photographic Documentation in Veterinary Forensic Pathology: Usability Study. JMIR Mhealth Uhealth 2018, 6, e180. [Google Scholar] [CrossRef]
- Piegari, G.; De Biase, D.; d’Aquino, I.; Prisco, F.; Fico, R.; Ilsami, R.; Pozzato, N.; Genovese, A.; Paciello, O. Diagnosis of Drowning and the Value of the Diatom Test in Veterinary Forensic Pathology. Front. Vet. Sci. 2019, 6, 404. [Google Scholar] [CrossRef]
- Manson, P.N.; Grivas, A.; Rosenbaum, A.; Vannier, M.; Zinreich, J.; Iliff, N. Studies on enophthalmos: II. The measurement of orbital injuries and their treatment by quantitative computed tomography. Plast. Reconstr. Surg. 1986, 77, 203–214. [Google Scholar] [CrossRef]






| Mean (±SD) | 95% CI | Range (Min–Max) | |
|---|---|---|---|
| Pre-RT (n = 6) | 42.93 (±3.44) | 39.32–46.55 | 39.9–47.5 |
| Pre-LE (n 0 6) | 43.22 (±3.59) | 39.44–46.99 | 39.5–47.6 |
| Post-RT (n = 6) | 45.03 (±2.97) | 41.91–48.15 | 41.6–48.5 |
| Post-LE (n = 6) | 44.45 (±3.61) | 40.66–48.24 | 39–48 |
| Pre-Grouped (n = 12) | 43.08 (±3.36) | 40.94–45.21 | 39.5–47.6 |
| Post-Grouped (n = 12) | 44.74 (±3.16) | 42.73–46.75 | 39–48.5 |
| Displacement | Mean (±SD) | 95% CI | Range (Min–Max) | |
|---|---|---|---|---|
| Pre-RT (n = 6) | LD | 0.63 (±0.37) | 0.24–1.02 | 0.27–1.25 |
| RD | 0.72 (±0.24) | 0.46–0.98 | 0.47–1.09 | |
| Pre-LE (n = 6) | LR | 0.62 (±032) | 0.28–0.96 | 0.31–1.19 |
| RD | 0.76 (±0.21) | 0.53–0.98 | 0.55–1.02 | |
| Post-RT (n = 6) | LD | 0.78 (±0.33) | 0.43–1.13 | 0.46–1.39 |
| RD | 0.87 (±0.26) | 0.60–1.15 | 0.53–1.19 | |
| Post-LE (n = 6) | LD | 0.77 (±0.31) | 0.44–1.1 | 0.43–1.33 |
| RD | 0.85 (±0.18) | 0.66–1.04 | 0.61–1.12 | |
| Pre-Grouped (n = 12) | LD | 0.63 (±0.33) | 0.42–0.84 | 0.27–1.25 |
| RD | 0.74 (±0.22) | 0.60–0.88 | 0.47–1.09 | |
| Post—Grouped (n = 12) | LD | 0.77 (±0.30) | 0.58–0.97 | 0.43–1.39 |
| RD | 0.86 (±0.21) | 0.73–1 | 0.53–1.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanza, D.; Meomartino, L.; Lamagna, B.; Castiello, E.; Coluccia, P.; Piegari, G.; D’Aquino, I.; Lamagna, F.; Greco, A. Retrobulbar Filling for Enophthalmos Treatment in Dogs: Technique, Description and Computed-Tomographic Evaluation. Preliminary Cadaveric Study. Vet. Sci. 2023, 10, 267. https://doi.org/10.3390/vetsci10040267
Costanza D, Meomartino L, Lamagna B, Castiello E, Coluccia P, Piegari G, D’Aquino I, Lamagna F, Greco A. Retrobulbar Filling for Enophthalmos Treatment in Dogs: Technique, Description and Computed-Tomographic Evaluation. Preliminary Cadaveric Study. Veterinary Sciences. 2023; 10(4):267. https://doi.org/10.3390/vetsci10040267
Chicago/Turabian StyleCostanza, Dario, Leonardo Meomartino, Barbara Lamagna, Erica Castiello, Pierpaolo Coluccia, Giuseppe Piegari, Ilaria D’Aquino, Francesco Lamagna, and Adelaide Greco. 2023. "Retrobulbar Filling for Enophthalmos Treatment in Dogs: Technique, Description and Computed-Tomographic Evaluation. Preliminary Cadaveric Study" Veterinary Sciences 10, no. 4: 267. https://doi.org/10.3390/vetsci10040267
APA StyleCostanza, D., Meomartino, L., Lamagna, B., Castiello, E., Coluccia, P., Piegari, G., D’Aquino, I., Lamagna, F., & Greco, A. (2023). Retrobulbar Filling for Enophthalmos Treatment in Dogs: Technique, Description and Computed-Tomographic Evaluation. Preliminary Cadaveric Study. Veterinary Sciences, 10(4), 267. https://doi.org/10.3390/vetsci10040267







