Effect of Nutritional Flushing Using Long-Term Energy and Protein Supplementation on Growth Performance and Reproductive Parameters of Doyogena Ewes in Ethiopia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
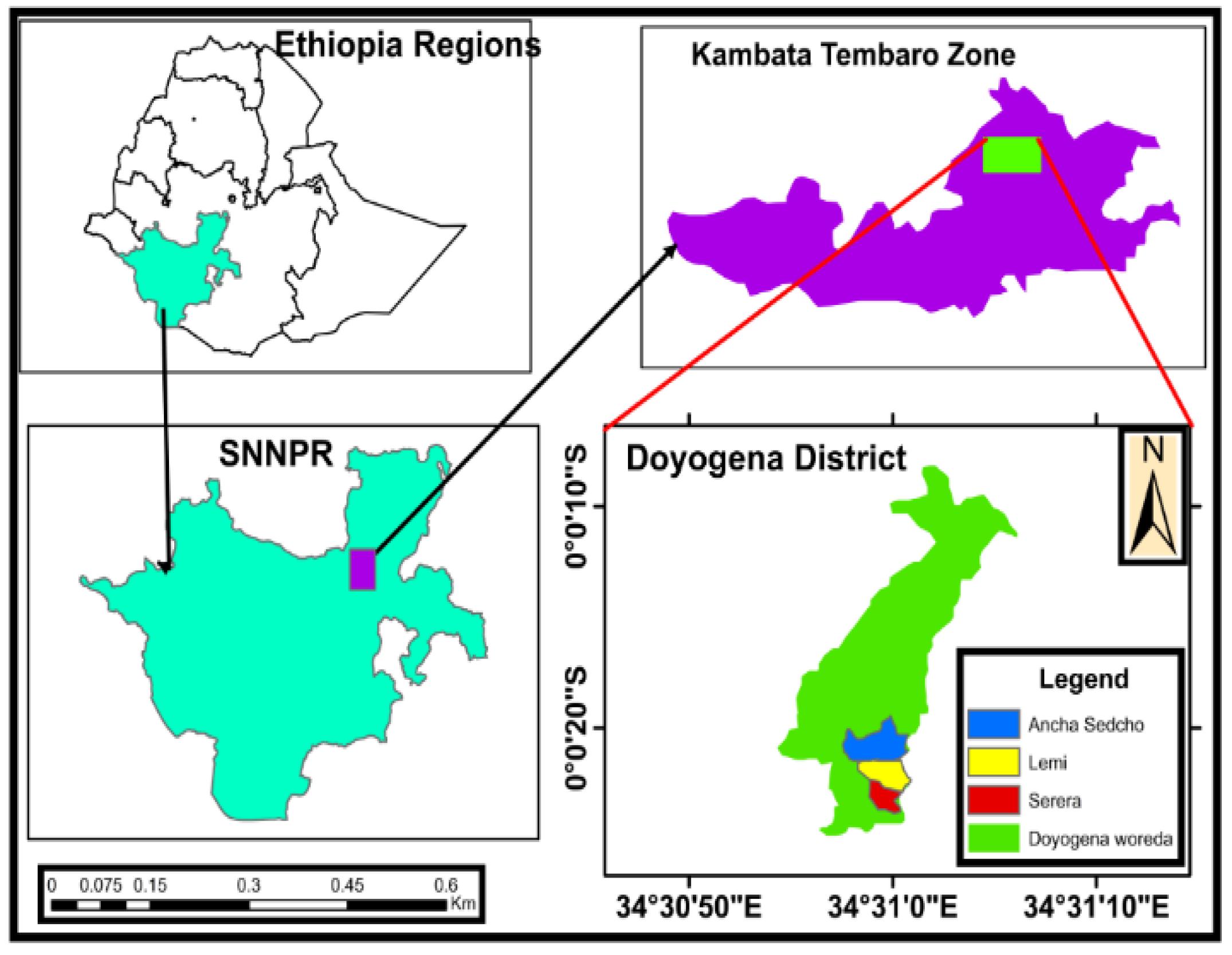
2.1. Study Area
2.2. Farmer Selection
2.3. Experimental Animals and Their Management
2.4. Experimental Feed Preparation and Management
2.5. Experimental Steup and Treatment Diets
2.6. Estrous Synchronization
2.7. Data Collection
2.7.1. Body Weight Measurements
2.7.2. Body Condition Score
2.7.3. Reproductive Traits
- ▪
- Estrous response (%) was determined by the number of ewes displaying standing estrous in each treatment group in relation to the total number of ewes.
- ▪
- The onset of estrous was defined as the midpoint between the last rejection and first acceptance by a mounting male, while the end of estrous was determined as halfway between the last acceptance and first rejection [15].
- ▪
- The duration of estrous was measured in hours, from the first to the last occurrence of standing estrous.
- ▪
- Conception rate (%) was calculated as the number of pregnant ewes divided by the number of ewes that exhibited estrous and were inseminated, multiplied by 100.
- ▪
- The lambing rate (%) was calculated as the number of ewes that gave birth divided by the number of pregnant ewes multiplied by 100.
- ▪
- The fecundity rate was calculated as the number of lambs born divided by the number of ewes inseminated, multiplied by 100.
- ▪
- The gestation period was defined as the duration between conception and parturition.
- ▪
- Litter size at birth was calculated as the number of lambs born divided by the number of ewes that gave birth.
- ▪
- The abortion rate was calculated as the number of ewes that were aborted divided by the number of ewes that were inseminated, multiplied by 100.
2.8. Chemical Analysis of Feeds
2.9. Data Analysis
3. Results
3.1. Effects of Nutritional Flushing on Body Weight of Doyogena Ewes
3.2. Effects of Nutritional Flushing on Body Condition Score
3.3. Effects of Nutritional Flushing on the Reproductive Performance
3.3.1. Effect of Nutritional Flushing on Estrous
3.3.2. Effect of Nutritional Flushing on Conception, Gestation, Abortion, and Fecundity
3.3.3. Effect of Nutritional Flushing on Lambing Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CSA (Central Statistical Agency). Agricultural Sample Survey, Livestock and Livestock Characteristics; CSA (Central Statistical Agency): Addis Ababa, Ethiopia, 2020. [Google Scholar]
- Scaramuzzi, R.J.; Campbell, B.K.; Downing, J.A.; Kendall, N.R.; Khalid, M.; Muñoz-Gutiérrez, M.; Somchit, A. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reprod. Nutr. Dev. 2006, 46, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Zabuli, J.; Tanaka, T.; Lu, W.; Kamomae, H. Intermittent nutritional stimulus by short-term treatment of high-energy diet promotes ovarian performance together with increases in blood levels of glucose and insulin in cycling goats. Anim. Reprod. Sci. 2010, 122, 28–29. [Google Scholar] [CrossRef]
- Archer, Z.A.; Rhind, S.M.; Findlay, P.A.; Kyle, C.E.; Thomas, L.; Marie, M.; Adam, C.L. Contrasting effects of different levels of food intake and adiposity on LH secretion and hypothalamic gene expression in sheep. J. Endocrinol. 2002, 175, 383–393. [Google Scholar] [CrossRef]
- Karikari, P.K.; Blasu, E.Y. Influence of Nutritional Flushing Prior to Mating on the Performance of West African Dwarf Goats Mated in the Rainy Season. Pak. J. Nutr. 2009, 8, 1068–1073. [Google Scholar] [CrossRef]
- Urrutia-Morales, J.; Meza-Herrera, C.A.; Tello-Varela, L.; Díaz-Gómez, M.O.; Beltrán, S.; Vatankhah, M.; Salehi, S.A. Genetic and non-genetic factors affecting Lori Bakhtiari ewe body weight and its relationship with productivity. Small Rumin. Res. 2010, 94, 98–102. [Google Scholar]
- Habibizad, J.; Riasi, A.; Kohram, H.; Rahmani, H.R. Effect of long-term or short-term supplementation of high energy or high energy-protein diets on ovarian follicles and blood metabolites and hormones in ewes. Small Rumin. Res. 2015, 132, 37–43. [Google Scholar] [CrossRef]
- Kebede, H.G. Estimates of Genetic Parameters and Genetic Trends for Productive and Reproductive Traits of Doyogena Sheep in Southern Ethiopia. MSc Thesis, Jimma University, Jimma, Ethiopia, 2019. [Google Scholar]
- Rekik, M.; Haile, A.; Abebe, D.; Muluneh, D.; Goshme, S.; Ben Salem, I.; Hilali, M.; Lassoued, N.; Chanyalew, Y.; Rischkowsky, B. GnRH and prostaglandin-based synchronization protocols as alternatives to progestogen-based treatments in sheep. Reprod. Domest. Anim. 2016, 51, 924–929. [Google Scholar] [CrossRef]
- ICARDA (International Center for Agricultural Research in Dry Areas). Field Solutions for Sheep Artificial Insemination; Technical Synthesis Report; ICARDA (International Center for Agricultural Research in Dry Areas): Beirut, Lebanon, 2016. [Google Scholar]
- Khan, K.; Meyer, H.H.; Thompson, J.M. Effect of prelambing supplementation and ewe body condition score on lamb survival and total weight of lamb weaned. Proc. West. Sect. Am. Soc. Anim. Sci. 1992, 43, 175. [Google Scholar]
- Holtz, W.; Sohnrey, B.; Gerland, M.; Driancourt, M.A. Ovsynchsynchronization and fixed time insemination in goats. Theriogenology 2008, 69, 785–792. [Google Scholar] [CrossRef]
- Ates, S.; Lucas, R.J.; Edwards, G.R. Stocking rate effects on liveweight gain of ewes and their twin lambs when grazing subterranean clover–perennial grass pastures. Grass Forage Sci. 2014, 70, 418–427. [Google Scholar] [CrossRef]
- Van Soest, P.; Robertson, J. Analysis of Forage and Fibrous Foods. A Laboratory Manual for Animal Science; Cornell University New York: New York, NY, USA, 1985. [Google Scholar]
- Menke, K.; Steingass, H. Estimating the energetic feed value from chemical composition and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Menke, K.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedstuff from the gas production when they are incubated with rumen liquor. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Sheep, 6th ed.; National Academy Press: Washington, DC, USA, 1985. [Google Scholar]
- Kearl, L. Nutrient Requirements of Ruminants in Developing Countries; Utah University: Salt Lake City, UT, USA, 1982; pp. 71–91. [Google Scholar]
- Nottle, M.B.; Seamark, R.F.; Setchell, B.P. Feeding lupin grain for six days prior to a cloprostenol induced luteolysis can increase ovulation rate in sheep irrespective of when in the oestrous cycle supplementation commences. Reprod. Fertil. Dev. 1990, 2, 189–192. [Google Scholar] [CrossRef]
- Downing, J.A.; Joss, J.; Scaramuzzi, R.J. A mixture of branched chain amino acids, leucine, isoleucine, and valine, increase ovulation rate when infused during the late luteal phase of the oestrus cycle: An effect that may be mediated by insulin. J. Endocrinol. 1995, 145, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Molle, G.; Branca, A.; Ligios, S.; Sitzia, M.; Casu, S.; Landau, S.; Zoref, Z. Effect of grazing background and flushing supplementation on reproductive performance in Sarda ewes. Small Rumin. Res. 1995, 17, 245–254. [Google Scholar] [CrossRef]
- Nurfeta, A.; Tolera, A.; Eik, L.; Sundstøl, F. The supplementary value of different parts of enset (Ensete ventricosum) to sheep-fed wheat straw and Desmodium intortum hay. Livest. Sci. 2008, 119, 22–30. [Google Scholar] [CrossRef]
- Rafiq, M.; Khan, M.F.; Aujla, K.M. Economic benefits of flushing and supplemental feeding of salt-range ewes on Pothwar Ranges of Pakistan. Pak. J. Biol. Sci. 2003, 6, 115–121. [Google Scholar] [CrossRef]
- Islam, R.; Bhat, A.S.; Sarkar, T.K.; Singh, P.K.; Khan, M.Z. Effect of flushing on reproductive performance of Corriedale ewes. Indian J. Small Rumin. 2007, 13, 55–60. [Google Scholar]
- Santos, G.M.G.; Silva, K.C.F.; Casimiro, T.R.; Costa, M.C.; Mori, R.M.; Mizubuti, I.Y.; Moriera, F.B.; Seneda, M.M. Reproductive performance of ewes mated in the spring when given nutritional supplements to enhance energy levels. Anim. Reprod. 2009, 6, 422–427. [Google Scholar]
- Kassem, R.; Owen, J.B.; Fadel, I.; Juha, H.; Whitaker, C.J. Aspects of fertility and lamb survival in Awassi sheep under semi-arid conditions. Res. Dev. Agric. 1989, 6, 161–168. [Google Scholar]
- Moghaddam, A.; Kohram, H.; Rahimi-Feyli, P.; Sohrabi, G. Effect of intravaginal device type and treatment length on estrus synchronization a reproductive performance of Farahani ewes out of breeding season. Songklanakarin J. Sci. Technol. 2019, 43, 80–86. [Google Scholar]
- Thomas, D.L.; Thomford, P.J.; Crickman, J.G.; Cobb, A.R.; Dziuk, P.J. Effects of plane of nutrition and phenobarbital during the pre-mating period on reproduction in ewes fed differentially during the summer and mated in the fall. J. Anim. Sci. 1987, 64, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Gunn, R.G.; Smith, W.F.; Senior, A.J.; Sim, D.A.; Hunter, E.A. Pre-mating herbage intake in reproductive performance of North Country Cheviot ewes in different levels of body condition. Anim. Prod. 1991, 52, 149–156. [Google Scholar] [CrossRef]
- Naqvi, S.M.K.; Sejian, V.; Karim, S.A. Effect of feed flushing during the summer season on growth, reproductive performance, and blood metabolites in Malpura ewes under semi-arid tropical environment. Trop. Anim. Health Prod. 2012, 45, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Danjuma, F.A.; Bawa, É.K.; Nwannenna, A.I.; Aluwong, T. Prostaglandin versus progestagen protocols in estrus synchronization in the Yankasa ewe. Sci. J. Anim. Sci. 2015, 4, 97–102. [Google Scholar]
- El-Shahat, K.; Abdel, M.U. Effects of dietary supplementation with vitamin E and selenium on metabolic and reproductive performance of Egyptian Baladi ewes under subtropical conditions. World Appl. Sci. J. 2011, 12, 1492–1499. [Google Scholar]
- Naqvi, S.M.K.; Gulyani, R.; Mittal, J.P. Estrus synchronization response in Kheri ewes treated with prostaglandin F2α. Indian J. Anim. Sci. 1997, 68, 564–565. [Google Scholar]
- Venter, J.L.; Greyling, J.P.C. Effect of different flushing periods and synchronized mating on body weight, blood glucose and reproductive performance in spring-mated ewes. Small Rumin. Res. 1994, 13, 257–261. [Google Scholar] [CrossRef]
- Naqvi, S.M.K.; Soren, N.M.; Karim, S.A. Effect of concentrate supplementation on performance, ovarian response, and some biochemical profile of Malpura ewes. Trop. Anim. Health Prod. 2011, 43, 905–913. [Google Scholar] [CrossRef]
- Farrag, B. Productive characteristics and reproductive responses to estrus synchronization and flushing in Abou-Delik ewes grazing in arid rangelands in Halaieb Shalateen—Abouramad Triangle of Egypt. World Vet. J. 2019, 9, 201–210. [Google Scholar] [CrossRef]
- Sabra, H.A.; Hassan, S.G. Effect of new regime of nutritional flushing on reproductive performance of Egyptian Barki Ewes. Glob. Vet. 2008, 2, 28–31. [Google Scholar]
- Kridli, R.T.; Husein, M.Q.; Humphrey, W.D. Effect of royal jelly and GnRH on the estrus synchronization and gestation rate in ewes using intravaginal sponges. Small Rumin. Res. 2003, 49, 25–30. [Google Scholar] [CrossRef]
- Shafiullah, S.; Ashok, K.; Amit, K.; Purohit, G. Effect of flushing of ewes on body weight, estrous response, and conception rate. Indian J. Small Rumin. 2022, 28, 218–220. [Google Scholar]
- Huseyin, N.; Orhan, Y.; Huseyin, D. Effects of flushing during the normal breeding season on reproductive performance and birth weights of lambs in Akkaraman ewes. J. Health Sci. 2006, 4, 92–99. [Google Scholar]
- Abdulkareem, T.A.; Eidan, S.M.; Al-Maliki, L.A.; Al-Saidy, F.K.; Mahdi, M.R. Reproductive performance of Iraqi Awassi ewes owned by sheep owners and extension farms in response to flushing and estrus synchronization regimes. Iraqi J. Agric. Sci. 2014, 45, 328–334. [Google Scholar]
- Lassoued, N.; Rekik, M.; Ben Salem, H. Influence of supplementary feeding and the ram effect on conception rate of Barbarine ewes during spring mating. Nutr. Foraging Ecol. Sheep Goats 2014, 52, 117–125. [Google Scholar]
- Ravindranath, B.M.; Krishnaswamy, A.; Chandrashekara, M. Conception rate and frequency of single and multiple births in estrus synchronized nari suwarna ewes maintained under two different feeding strategies. Int. J. Livest. Res. 2014, 4, 288–293. [Google Scholar] [CrossRef]
- El-Hawy, A.S.; El-Bassiony, M.F.; Abo Bakr, S.; Gawish, H.A.; Badawy, M.T.; Gado, H.M. Productive and Reproductive Performance and Metabolic Profile of Barki Ewes Supplemented with Two Forms of Probiotics as Feed Additives. World Vet. J. 2019, 9, 135–145. [Google Scholar] [CrossRef]
- Blumer, S.E.; Gardner, G.E.; Ferguson, M.B.; Thompson, A.N. Environmental and genetic factors influence the live weight of adult Merino and Border Leicester X Merino ewes across multiple sites and years. Anim. Prod. Sci. 2015, 56, 775–788. [Google Scholar] [CrossRef]
- Sejian, V.; Maurya, V.P.; Kumar, K.; Naqvi, S.M.K. Effect of multiple stresses (thermal, nutritional, and walking stress) on growth, physiological response, blood biochemical and endocrine responses in Malpura ewes under semi-arid tropical environment. Trop. Anim. Health Prod. 2013, 45, 107–116. [Google Scholar] [CrossRef]
- Zanouny, A.; Marzouk, K.M.; El Zanouny, A.I.; Kaoud, M.A. Flushing vs. Hormonal treatment, which better improve the reproductive performance of Ossimi ewes. Egypt. J. Sheep Goat Sci. 2018, 13, 18–25. [Google Scholar]
- Baheg, R.M.A.; El-Bahrawy, K.A.; El-Azrak, K.M.; Samak, M.A.; Sallam, S.M.A. Effect of condensed tannins and saponin supplementation on reproductive performance in Barki ewes. Egypt. J. Nutr. Feed. 2017, 20, 197–210. [Google Scholar]
- Dhahir, N.N.; Ismaeel, M.A.; AL-Noori, Z.T. Effect of adding carrots as fed supplementation on reproductive performances in Awassi ewes. Iraqi J. Vet. Sci. 2022, 36, 413–417. [Google Scholar] [CrossRef]
- Njoya, A.; Awa, D.N.; Chupamom, J. The effects of a strategic supplementation and prophylaxis on the reproductive performance of primiparous Fulbe ewes in the semi-arid zone of Cameroon. Small Rumin. Res. 2002, 56, 21–29. [Google Scholar] [CrossRef]
- Shambel, G. Evaluation of Estrus Response and Fertility in Ewes with and without Flushing and Mated to Begait Rams under Traditional Management in Eastern Tigray, Northern Ethiopia. Master’s Thesis, Mekelle University, Mekelle, Ethiopia, 2017. [Google Scholar]
- Abdalla, E.; Farrag, B.; Hashem, A.; Khalil, F.; Abdel-Fattah, M. Effect of progestagen, PGF2α, PMSG, and GnRH on estrus synchronization and some reproductive and productive traits in Barki ewes. J. Agroaliment. Process. Technol. 2015, 20, 93–101. [Google Scholar]
- Khan, T.H.; Becka, N.F.G.; Khalid, M. The effect of hCG treatment on Day 12 post-mating on ovarian function and reproductive performance of ewes and ewe lambs. Anim. Reprod. Sci. 2009, 116, 162–168. [Google Scholar] [CrossRef]
- Habtegiorgis, K.; Haile, A.; Getachew, T.; Kirmani, M.A.; Gemiyo, D. Analysis of genetic parameters and genetic trends for early growth and reproductive traits of Doyogena sheep managed under community-based breeding program. Heliyon 2022, 8, e09749. [Google Scholar] [CrossRef]
| Nutrients | Natural Pasture | Supplements | |
|---|---|---|---|
| Enset Leaf | Commercial Concentrate | ||
| DM (%) | 93.56 | 92.53 | 92.99 |
| ASH | 10.98 | 6.34 | 11.30 |
| CP (% DM) | 8.72 | 13.21 | 17.55 |
| NDF (% DM) | 65.85 | 61.56 | 33.93 |
| ADF (% DM) | 40.23 | 38.14 | 19.38 |
| ADL (% DM) | 5.94 | 3.34 | 6.20 |
| ME (MJ/kg) | 6.34 | 7.74 | 7.95 |
| IVOMD | 45.67 | 56.31 | 55.21 |
| Treatment (T) | Supplemental Diet | Total (% DM) | CP (% DM) | ME (MJ/kg) | |||
|---|---|---|---|---|---|---|---|
| BW1 | BW2 | BW1 | BW2 | BW1 | BW2 | ||
| T0 | NP (control) | 1.10 | 1.46 | 9.52 | 12.7 | 6.9 | 9.2 |
| T1 | NP + (250 g EL + 500 g CC/day) | 1.78 | 2.15 | 15.04 | 13.4 | 12.4 | 14.7 |
| T2 | NP + (400 g EL + 500 g CC/day) | 1.93 | 2.29 | 14.89 | 14.6 | 13.5 | 15.8 |
| T3 | NP + (500 g EL + 400 g CC/day) | 1.93 | 2.29 | 14.68 | 14.5 | 13.5 | 15.8 |
| T4 | NP + (500 g EL + 250 g CC/day) | 1.78 | 2.15 | 14.45 | 13.4 | 12.4 | 14.7 |
| Treatments (kg, Means) | ||||||
|---|---|---|---|---|---|---|
| Parameters | T0 | T1 | T2 | T3 | T4 | p-Value |
| BW-I | 27.14 | 27.70 | 28.29 | 28.00 | 28.14 | 0.9106 |
| BW-AI | 29.14 b | 32.86 a | 34.14 a | 33.07 a | 31.86 a | 0.0054 |
| BW-P | 34.93 b | 37.14 a | 39.07 a | 38.93 a | 37.64 a | 0.0036 |
| BW-L | 31.14 c | 34.43 ab | 36.14 a | 35.57 a | 32.79 c | 0.0011 |
| Treatments | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | T0 | T1 | T2 | T3 | T4 | SEM | p-Value |
| BCS-I | 2.53 | 2.51 | 2.56 | 2.54 | 2.53 | 0.17 | 0.9996 |
| BCS-B | 2.57 b | 2.90 a | 3.07 a | 3.00 a | 2.79 ab | 0.18 | 0.0100 |
| BCS-G | 2.71 c | 2.97 abc | 3.16 a | 3.03 ab | 2.86 bc | 0.16 | 0.0232 |
| BCS-L | 2.79 b | 3.00 ab | 3.17 a | 3.04 ab | 2.83 b | 0.13 | 0.0227 |
| Treatments (Means ± SEM) | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | T0 | T1 | T2 | T3 | T4 | SEM | p-Value |
| Estrous response rate (%) Onset of estrous (hours) | 71.4 c 46.65 ± 3.77 a | 100 a 35.39 ± 3.93 bc | 100 a 32.43 ± 5.77 c | 85.7 b 34.14 ± 6.08 c | 71.4 c 39.35 ± 4.54 b | 0.82542 | 0.001 0.001 |
| Estrous duration (hours) | 24.66 ± 2.53 c | 30.33 ± 2.92 b | 35.46 ± 3.47 a | 34.63 ± 3.26 a | 29.04 ± 2.70 b | 0.001 | |
| N | 7 | 7 | 7 | 7 | 7 | ||
| Treatments | ||||||
|---|---|---|---|---|---|---|
| Parameters | T0 | T1 | T2 | T3 | T4 | p-Value |
| N | 7 | 7 | 7 | 7 | 7 | |
| Conception rate (%) Gestation (days) | 66.67 c 151.43 a | 80 b 150.29 a | 100 a 150.00 a | 100 a 149.07 a | 100 a 150.57 a | 0.0001 0.9061 |
| Fecundity rate (%) | 60 e | 100 d | 157.1 a | 133.3 b | 120 c | 0.0001 |
| Treatments | ||||||
|---|---|---|---|---|---|---|
| Parameters | T0 | T1 | T2 | T3 | T4 | p-Value |
| Lambing rate (%) Litter size (mean ± SEM) | 60.0 d 1.50 ± 0.20 c | 71.4 b 1.75 ± 0.11 ab | 85.7 a 1.83 ± 0.06 a | 83.3 a 1.60 ± 0.22 bc | 66.67 c 1.50 ± 0.24 c | 0.0001 0.0052 |
| Litter weight (kg, mean ± SEM) | 2.73 ± 0.088 c | 3.04 ± 0.117 bc | 3.39 ± 0.097 a | 3.19 ± 0.106 ab | 2.93 ± 0.091 bc | 0.0021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesfaye, A.; Asmare, B.; Abiso, T.; Wamatu, J. Effect of Nutritional Flushing Using Long-Term Energy and Protein Supplementation on Growth Performance and Reproductive Parameters of Doyogena Ewes in Ethiopia. Vet. Sci. 2023, 10, 368. https://doi.org/10.3390/vetsci10060368
Tesfaye A, Asmare B, Abiso T, Wamatu J. Effect of Nutritional Flushing Using Long-Term Energy and Protein Supplementation on Growth Performance and Reproductive Parameters of Doyogena Ewes in Ethiopia. Veterinary Sciences. 2023; 10(6):368. https://doi.org/10.3390/vetsci10060368
Chicago/Turabian StyleTesfaye, Asfaw, Bimrew Asmare, Tesfaye Abiso, and Jane Wamatu. 2023. "Effect of Nutritional Flushing Using Long-Term Energy and Protein Supplementation on Growth Performance and Reproductive Parameters of Doyogena Ewes in Ethiopia" Veterinary Sciences 10, no. 6: 368. https://doi.org/10.3390/vetsci10060368
APA StyleTesfaye, A., Asmare, B., Abiso, T., & Wamatu, J. (2023). Effect of Nutritional Flushing Using Long-Term Energy and Protein Supplementation on Growth Performance and Reproductive Parameters of Doyogena Ewes in Ethiopia. Veterinary Sciences, 10(6), 368. https://doi.org/10.3390/vetsci10060368






