Comparative Evaluation of GS-441524, Teriflunomide, Ruxolitinib, Molnupiravir, Ritonavir, and Nirmatrelvir for In Vitro Antiviral Activity against Feline Infectious Peritonitis Virus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line
2.2. Antiviral Drugs
2.3. FIPV and Quantification of FIPV via qRT-PCR
2.4. The 50% Tissue Culture Infective Dose (TCID50) of FIPV Stock
2.5. Cytotoxicity of the Antiviral Drugs
2.6. Cell Viability Assay of the Antiviral Drugs
2.7. Antiviral Efficacies of the Drugs
2.8. Statistical Analysis
3. Results
3.1. Effect of Initial Inocula and Incubation Times on Anti-FIPV Efficacy of GS441524
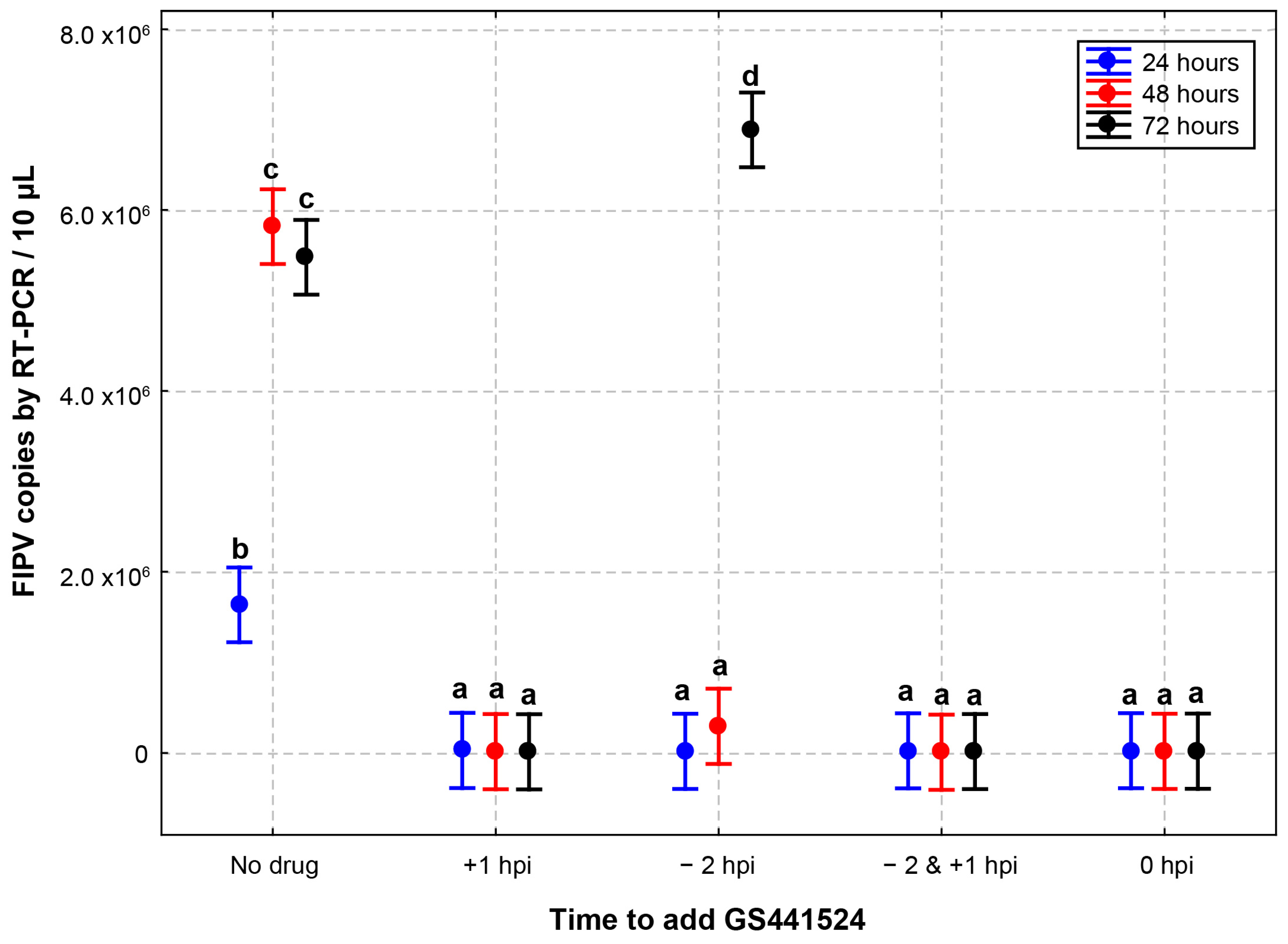
3.2. Effect of Different Addition and Removal Times of GS441524 on the Anti-FIPV Efficacy
3.3. Cytotoxicity and Cell Viability of Antiviral Drugs
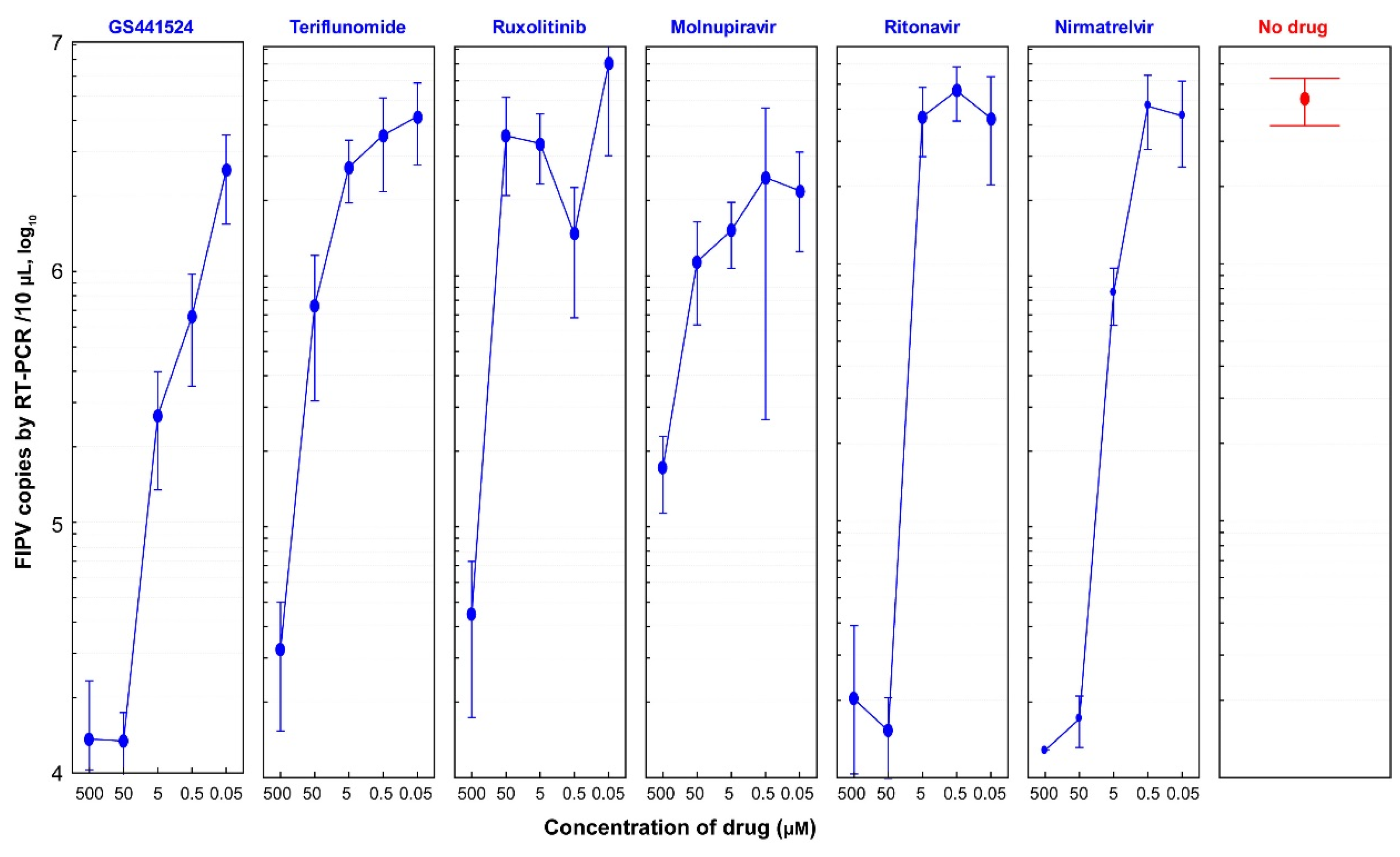
3.4. Antiviral Efficacies of Six Antiviral Drugs
3.5. Selectivity Index (SI) of Six Drugs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Addie, D.D.; Dennis, J.M.; Toth, S.; Callanan, J.J.; Reid, S.; Jarrett, O. Long-term impact on a closed household of pet cats of natural infection with feline coronavirus, feline leukaemia virus and feline immunodeficiency virus. Vet. Rec. 2000, 146, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Novicoff, W.; Nadeau, J.; Evans, S. Unlicensed GS-441524-Like Antiviral Therapy Can Be Effective for at-Home Treatment of Feline Infectious Peritonitis. Animals 2021, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.S.; Porter, E.; Matthews, D.; Kipar, A.; Tasker, S.; Helps, C.R.; Siddell, S.G. Genotyping coronaviruses associated with feline infectious peritonitis. J. Gen. Virol. 2015, 96, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C. An update on feline infectious peritonitis: Diagnostics and therapeutics. Vet. J. 2014, 201, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C.; Perron, M.; Bannasch, M.; Montgomery, E.; Murakami, E.; Liepnieks, M.; Liu, H. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J. Feline Med. Surg. 2019, 21, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Coggins, S.J.; Norris, J.M.; Malik, R.; Govendir, M.; Hall, E.J.; Kimble, B.; Thompson, M.F. Outcomes of treatment of cats with feline infectious peritonitis using parenterally administered remdesivir, with or without transition to orally administered GS-441524. J. Vet. Intern. Med. 2023. [Google Scholar] [CrossRef]
- Green, J.; Syme, H.; Tayler, S. Thirty-two cats with effusive or non-effusive feline infectious peritonitis treated with a combination of remdesivir and GS-441524. J. Vet. Intern. Med. 2023. [Google Scholar] [CrossRef]
- Roy, M.; Jacque, N.; Novicoff, W.; Li, E.; Negash, R.; Evans, S.J.M. Unlicensed Molnupiravir is an Effective Rescue Treatment Following Failure of Unlicensed GS-441524-like Therapy for Cats with Suspected Feline Infectious Peritonitis. Pathogens 2022, 11, 1209. [Google Scholar] [CrossRef]
- Murphy, B.G.; Perron, M.; Murakami, E.; Bauer, K.; Park, Y.; Eckstrand, C.; Liepnieks, M.; Pedersen, N.C. The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies. Vet. Microbiol. 2018, 219, 226–233. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Kim, Y.; Liu, H.; Galasiti Kankanamalage, A.C.; Eckstrand, C.; Groutas, W.C.; Bannasch, M.; Meadows, J.M.; Chang, K.O. Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J. Feline Med. Surg. 2018, 20, 378–392. [Google Scholar] [CrossRef]
- Dickinson, P.J.; Bannasch, M.; Thomasy, S.M.; Murthy, V.D.; Vernau, K.M.; Liepnieks, M.; Montgomery, E.; Knickelbein, K.E.; Murphy, B.; Pedersen, N.C. Antiviral treatment using the adenosine nucleoside analogue GS-441524 in cats with clinically diagnosed neurological feline infectious peritonitis. J. Vet. Intern. Med. 2020, 34, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Gunn-Moore, D.B.E.; Taylor, S.; Tasker, S.; Sorrell, S. An Update on Treatment of FIP in the UK. Vet Times 2022, 51, 8–11. [Google Scholar]
- Li, Y.; Cao, L.; Li, G.; Cong, F.; Li, Y.; Sun, J.; Luo, Y.; Chen, G.; Li, G.; Wang, P.; et al. Remdesivir Metabolite GS-441524 Effectively Inhibits SARS-CoV-2 Infection in Mouse Models. J. Med. Chem. 2022, 65, 2785–2793. [Google Scholar] [CrossRef]
- Yan, V.C.; Muller, F.L. Advantages of the Parent Nucleoside GS-441524 over Remdesivir for COVID-19 Treatment. ACS Med. Chem. Lett. 2020, 11, 1361–1366. [Google Scholar] [CrossRef]
- Arbel, R.; Wolff Sagy, Y.; Hoshen, M.; Battat, E.; Lavie, G.; Sergienko, R.; Friger, M.; Waxman, J.G.; Dagan, N.; Balicer, R.; et al. Nirmatrelvir Use and Severe COVID-19 Outcomes during the Omicron Surge. N. Engl. J. Med. 2022, 387, 790–798. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Zhou, S.; Graham, R.L.; Pruijssers, A.J.; Agostini, M.L.; Leist, S.R.; Schafer, A.; Dinnon, K.H., III; Stevens, L.J.; et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020, 12, eabb5883. [Google Scholar] [CrossRef]
- Cook, S.; Wittenburg, L.; Yan, V.C.; Theil, J.H.; Castillo, D.; Reagan, K.L.; Williams, S.; Pham, C.D.; Li, C.; Muller, F.L.; et al. An Optimized Bioassay for Screening Combined Anticoronaviral Compounds for Efficacy against Feline Infectious Peritonitis Virus with Pharmacokinetic Analyses of GS-441524, Remdesivir, and Molnupiravir in Cats. Viruses 2022, 14, 2429. [Google Scholar] [CrossRef]
- Neubauer, A.; Johow, J.; Mack, E.; Burchert, A.; Meyn, D.; Kadlubiec, A.; Torje, I.; Wulf, H.; Vogelmeier, C.F.; Hoyer, J.; et al. The janus-kinase inhibitor ruxolitinib in SARS-CoV-2 induced acute respiratory distress syndrome (ARDS). Leukemia 2021, 35, 2917–2923. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Cook, S.E.; Vogel, H.; Castillo, D.; Olsen, M.; Pedersen, N.; Murphy, B.G. Investigation of monotherapy and combined anticoronaviral therapies against feline coronavirus serotype II in vitro. J. Feline Med. Surg. 2022, 24, 943–953. [Google Scholar] [CrossRef]
- Maghzi, A.H.; Houtchens, M.K.; Preziosa, P.; Ionete, C.; Beretich, B.D.; Stankiewicz, J.M.; Tauhid, S.; Cabot, A.; Berriosmorales, I.; Schwartz, T.H.W.; et al. COVID-19 in teriflunomide-treated patients with multiple sclerosis. J. Neurol. 2020, 267, 2790–2796. [Google Scholar] [CrossRef]
- Luetic, G.; Menichini, M.L.; Burgos, M.; Alonso, R.; Carnero Contentti, E.; Carra, A.; Deri, N.; Steinberg, J.; Rojas, J.I.; on behalf RelacoEM Study Group. COVID-19 in Argentine teriflunomide-treated multiple sclerosis patients: First national case series. Mult. Scler. Relat. Disord. 2021, 53, 103049. [Google Scholar] [CrossRef]
- Ciardi, M.R.; Zingaropoli, M.A.; Pasculli, P.; Perri, V.; Tartaglia, M.; Valeri, S.; Russo, G.; Conte, A.; Mastroianni, C.M. The peripheral blood immune cell profile in a teriflunomide-treated multiple sclerosis patient with COVID-19 pneumonia. J. Neuroimmunol. 2020, 346, 577323. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.; Kiladjian, J.J.; Al-Ali, H.K.; Gisslinger, H.; Waltzman, R.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.; Knoops, L.; et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med. 2012, 366, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, A.M. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N. Engl. J. Med. 2015, 372, 1670–1671. [Google Scholar] [CrossRef]
- Gozzetti, A.; Capochiani, E.; Bocchia, M. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19. Leukemia 2020, 34, 2815–2816. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Peng, W.Y.; Lee, W.H.; Lin, T.Y.; Yang, M.H.; Dalley, J.W.; Tsai, T.H. Biotransformation and brain distribution of the anti-COVID-19 drug molnupiravir and herb-drug pharmacokinetic interactions between the herbal extract Scutellaria formula-NRICM101. J. Pharm. Biomed. Anal. 2023, 234, 115499. [Google Scholar] [CrossRef] [PubMed]
- Painter, W.P.; Holman, W.; Bush, J.A.; Almazedi, F.; Malik, H.; Eraut, N.; Morin, M.J.; Szewczyk, L.J.; Painter, G.R. Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2. Antimicrob. Agents Chemother. 2021, 65, 10–128. [Google Scholar] [CrossRef]
- Fischer, W.A., Jr.; Eron, J.J., Jr.; Holman, W.; Cohen, M.S.; Fang, L.; Szewczyk, L.J.; Sheahan, T.P.; Baric, R.; Mollan, K.R.; Wolfe, C.R.; et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci. Transl. Med. 2022, 14, eabl7430. [Google Scholar] [CrossRef]
- Cook, S.E.; Vogel, H.; Castillo, D.; Oslen, M.; Pedersen, N.; Murphy, B.G. A rational approach to identifying effective combined anticoronaviral therapies against feline coronavirus. bioRxiv 2020, 2020-07. [Google Scholar] [CrossRef]
- Lamb, Y.N. Nirmatrelvir Plus Ritonavir: First Approval. Drugs 2022, 82, 585–591. [Google Scholar] [CrossRef]
- Vandyck, K.; Deval, J. Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection. Curr. Opin. Virol. 2021, 49, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.S.B. Novel Nitrile Peptidomimetics for Treating COVID-19. ACS Med. Chem. Lett. 2022, 13, 330–331. [Google Scholar] [CrossRef] [PubMed]
- White, J.M.; Schiffer, J.T.; Bender Ignacio, R.A.; Xu, S.; Kainov, D.; Ianevski, A.; Aittokallio, T.; Frieman, M.; Olinger, G.G.; Polyak, S.J. Drug Combinations as a First Line of Defense against Coronaviruses and Other Emerging Viruses. mBio 2021, 12, e0334721. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.J.; Diniz, P.P.V.; Juan, Y.C.; Bossong, F.; Collisson, E.W.; Drechsler, Y.; Kaltenboeck, B. Cross-sectional quantitative RT-PCR study of feline coronavirus viremia and replication in peripheral blood of healthy shelter cats in Southern California. J. Feline Med. Surg. 2018, 20, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the Calculation of TCID50 for Quantitation of Virus Infectivity. Virol. Sin. 2021, 36, 141–144. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Sathish, J.G.; Bhatt, S.; DaSilva, J.K.; Flynn, D.; Jenkinson, S.; Kalgutkar, A.S.; Liu, M.; Manickam, B.; Pinkstaff, J.; Reagan, W.J.; et al. Comprehensive Nonclinical Safety Assessment of Nirmatrelvir Supporting Timely Development of the SARS-CoV-2 Antiviral Therapeutic, Paxlovid. Int. J. Toxicol. 2022, 41, 276–290. [Google Scholar] [CrossRef]
- Jiao, Z.; Yan, Y.; Chen, Y.; Wang, G.; Wang, X.; Li, L.; Yang, M.; Hu, X.; Guo, Y.; Shi, Y.; et al. Adaptive Mutation in the Main Protease Cleavage Site of Feline Coronavirus Renders the Virus More Resistant to Main Protease Inhibitors. J. Virol. 2022, 96, e0090722. [Google Scholar] [CrossRef]
- Kim, Y.; Liu, H.; Galasiti Kankanamalage, A.C.; Weerasekara, S.; Hua, D.H.; Groutas, W.C.; Chang, K.O.; Pedersen, N.C. Reversal of the Progression of Fatal Coronavirus Infection in Cats by a Broad-Spectrum Coronavirus Protease Inhibitor. PLoS Pathog. 2016, 12, e1005531. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martin-Quiros, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Wahl, A.; Gralinski, L.E.; Johnson, C.E.; Yao, W.; Kovarova, M.; Dinnon, K.H., III; Liu, H.; Madden, V.J.; Krzystek, H.M.; De, C.; et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 2021, 591, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hill, C.S.; Sarkar, S.; Tse, L.V.; Woodburn, B.M.D.; Schinazi, R.F.; Sheahan, T.P.; Baric, R.S.; Heise, M.T.; Swanstrom, R. beta-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells. J. Infect. Dis. 2021, 224, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients. Clin. Infect. Dis. 2022, 76, e342–e349. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhou, X.; Wu, Q.; Zhang, X.; Feng, Z.; et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis. Ann. Med. 2022, 54, 516–523. [Google Scholar] [CrossRef] [PubMed]



| Drug Name | Drug Category | Viral Target | Investigated CoVs * | References |
|---|---|---|---|---|
| GS441524 | Adenosine nucleotide analog | Inhibition of RNA systhesis | FIPV, SARS-CoV-2, MERS-CoV | [5,6,9,13,14] |
| Nirmatrelvir | 3C-like Protease inhibitor | Inhibition of RNA systhesis | FIPV, SARS-CoV-2 | [15] |
| Molnupiravir | Isopropyl ester cytidine analog | Inhibition of RNA systhesis | FIPV, SARS-CoV-2, MERS-CoV | [8,16,17] |
| Ruxolitinib | Kinase inhibitor | Inhibition of viral entry | SARS-CoV-2 | [18] |
| Ritonavir | Antiretroviral protease inhibitor | Cleavage of viral polyproteins | FIPV, SARS-CoV-2 | [19,20] |
| Teriflunomide | Dihydroorotate dehydrogenase inhibitor | Inhibition of RNA systhesis | SARS-CoV-2 | [21] |
| FIPV Inoculum | 48 h | 72 h | ||||
|---|---|---|---|---|---|---|
| No Drug | GS441524 | Fold Reduced | No Drug | GS441524 | Fold Reduced | |
| 2.50 × 104 * | 5.91 × 106 | 2.76 × 103 | 2.14 × 103 | 4.67 × 106 | 3.24 × 103 | 1.44 × 103 |
| 2.50 × 103 | 1.17 × 107 | 3.48 × 102 | 3.36 × 104 | 5.18 × 106 | 2.09 × 102 | 2.47 × 104 |
| 2.50 × 102 | 1.23 × 107 | 4.60 × 101 | 2.67 × 105 | 9.05 × 106 | 2.36 × 101 | 3.83 × 105 |
| Treatments * | Increase in Viral Load | |||||
|---|---|---|---|---|---|---|
| 24 h | Fold Increase ** | 48 h | Fold Increase | 72 h | Fold Increase | |
| −2 hpi *** | 1.97 × 104 | 0.79 | 2.94 × 105 | 11 | 6.89 × 106 | 275 |
| −2 & +1 hpi | 2.47 × 104 | 0.99 | 1.03 × 104 | 0.41 | 1.62 × 104 | 0.65 |
| 0 hpi | 2.58 × 104 | 1.03 | 1.97 × 104 | 0.79 | 2.04 × 104 | 0.82 |
| +1 hpi | 2.84 × 104 | 1.14 | 1.52 × 104 | 0.61 | 1.39 × 104 | 0.56 |
| No drug | 1.63 × 106 | 65 | 5.82 × 106 | 232 | 5.48 × 106 | 219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barua, S.; Kaltenboeck, B.; Juan, Y.-C.; Bird, R.C.; Wang, C. Comparative Evaluation of GS-441524, Teriflunomide, Ruxolitinib, Molnupiravir, Ritonavir, and Nirmatrelvir for In Vitro Antiviral Activity against Feline Infectious Peritonitis Virus. Vet. Sci. 2023, 10, 513. https://doi.org/10.3390/vetsci10080513
Barua S, Kaltenboeck B, Juan Y-C, Bird RC, Wang C. Comparative Evaluation of GS-441524, Teriflunomide, Ruxolitinib, Molnupiravir, Ritonavir, and Nirmatrelvir for In Vitro Antiviral Activity against Feline Infectious Peritonitis Virus. Veterinary Sciences. 2023; 10(8):513. https://doi.org/10.3390/vetsci10080513
Chicago/Turabian StyleBarua, Subarna, Bernhard Kaltenboeck, Yen-Chen Juan, Richard Curtis Bird, and Chengming Wang. 2023. "Comparative Evaluation of GS-441524, Teriflunomide, Ruxolitinib, Molnupiravir, Ritonavir, and Nirmatrelvir for In Vitro Antiviral Activity against Feline Infectious Peritonitis Virus" Veterinary Sciences 10, no. 8: 513. https://doi.org/10.3390/vetsci10080513
APA StyleBarua, S., Kaltenboeck, B., Juan, Y.-C., Bird, R. C., & Wang, C. (2023). Comparative Evaluation of GS-441524, Teriflunomide, Ruxolitinib, Molnupiravir, Ritonavir, and Nirmatrelvir for In Vitro Antiviral Activity against Feline Infectious Peritonitis Virus. Veterinary Sciences, 10(8), 513. https://doi.org/10.3390/vetsci10080513







