Equine Placentitis in Mares Induces the Secretion of Pro-Inflammatory Cytokine eIL-1β and the Active Extracellular Matrix Metalloproteinase (MMP)-9
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Committee Approval
2.2. Animals and Experimental Design
2.3. Blood Samples
2.4. eIL-1β Assay
2.5. Zymography Gel Activity
2.6. Statistical Analysis
3. Results
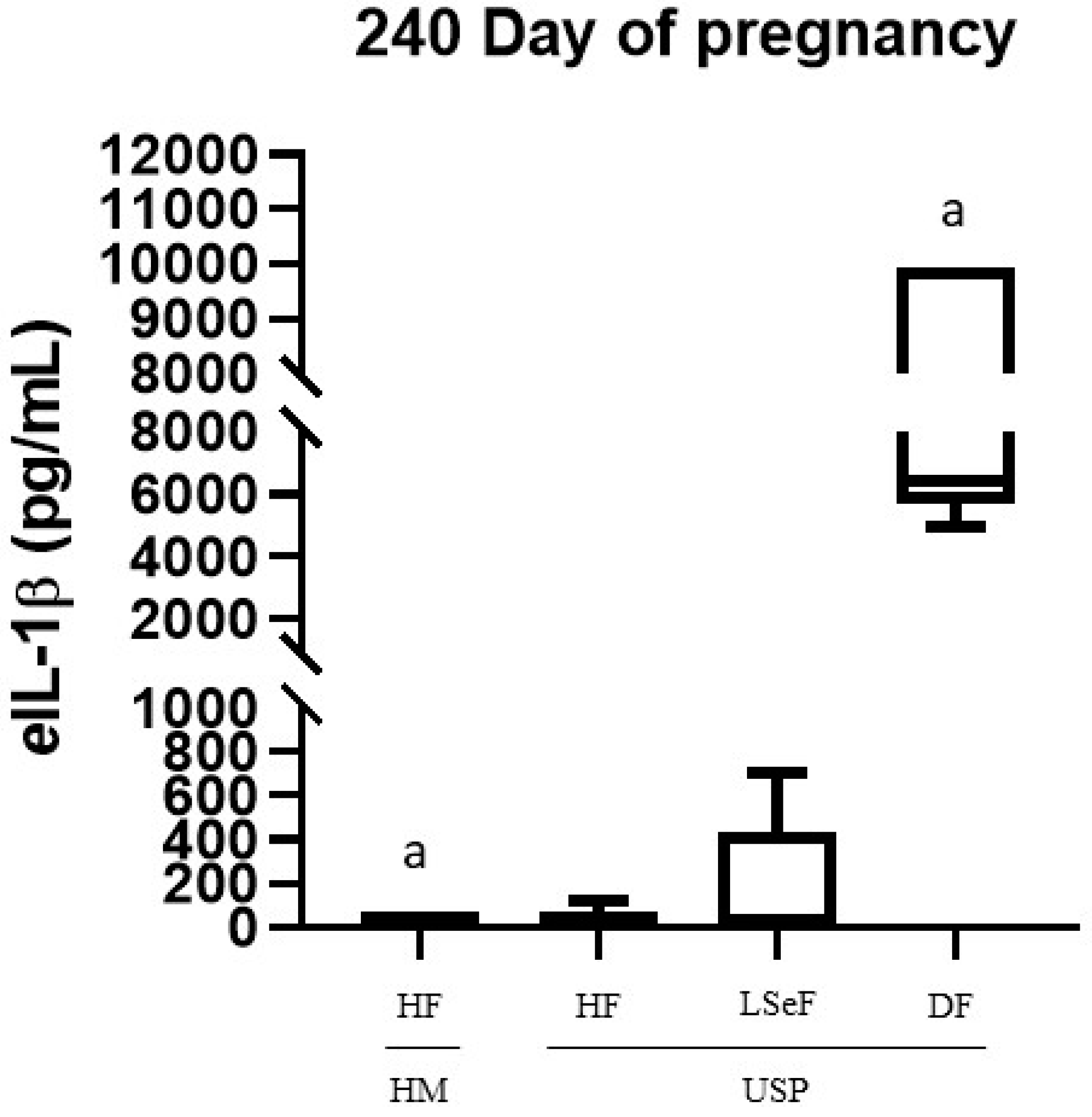
3.1. Concentration of eIL-1β
3.2. proMMP-2 Activity at 240 and 320 Days of Gestation
3.2.1. proMMP-2 Activity at 240 Days of Gestation
3.2.2. proMMP-2 Activity at 320 Days of Gestation
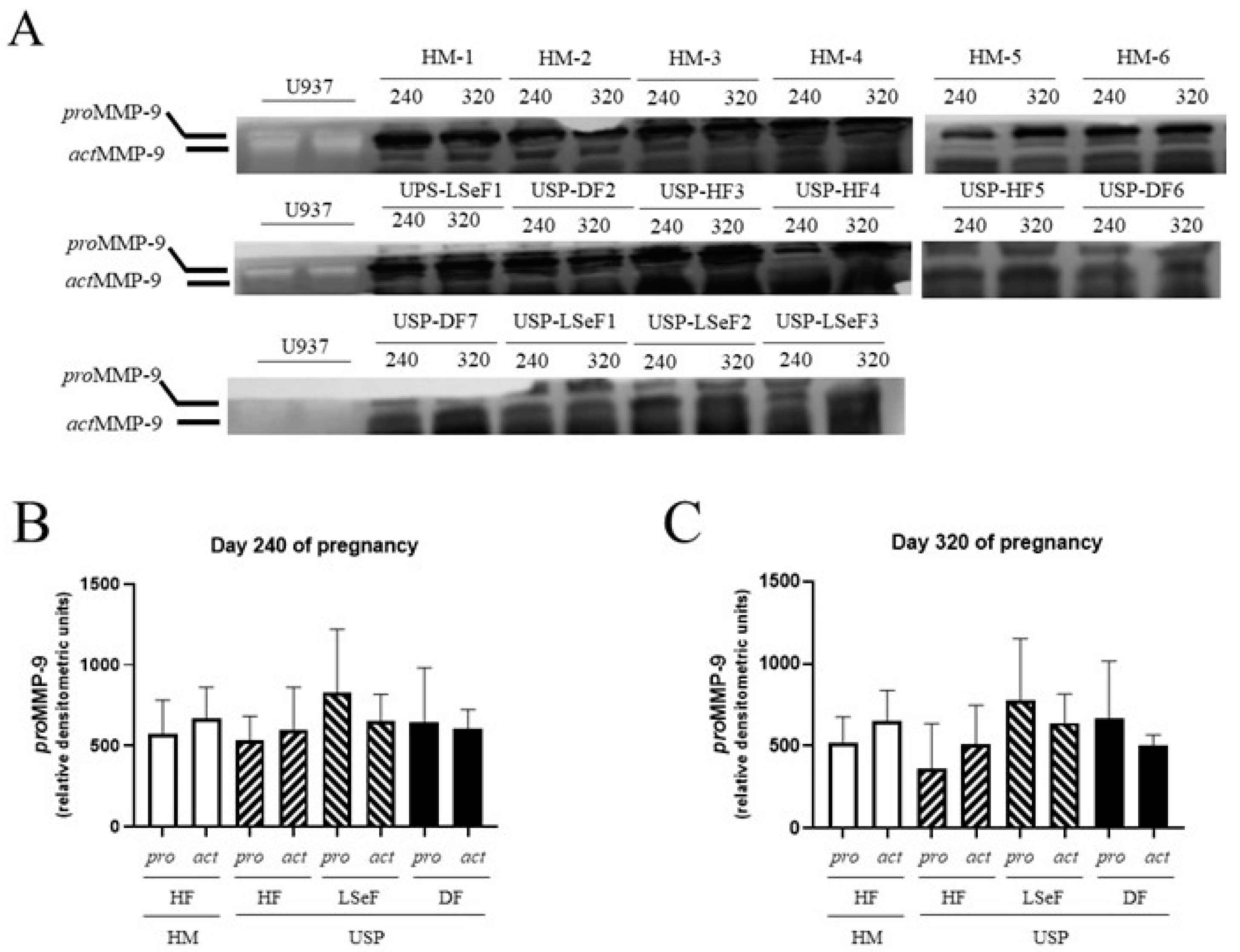
3.2.3. proMMP-9 Activity at 240 Days of Gestation
3.2.4. actMMP-9 Activity at 240 Days of Gestation
3.2.5. proMMP-9 Activity at 320 Days of Gestation
3.2.6. actMMP-9 Activity at 320 Days of Gestation
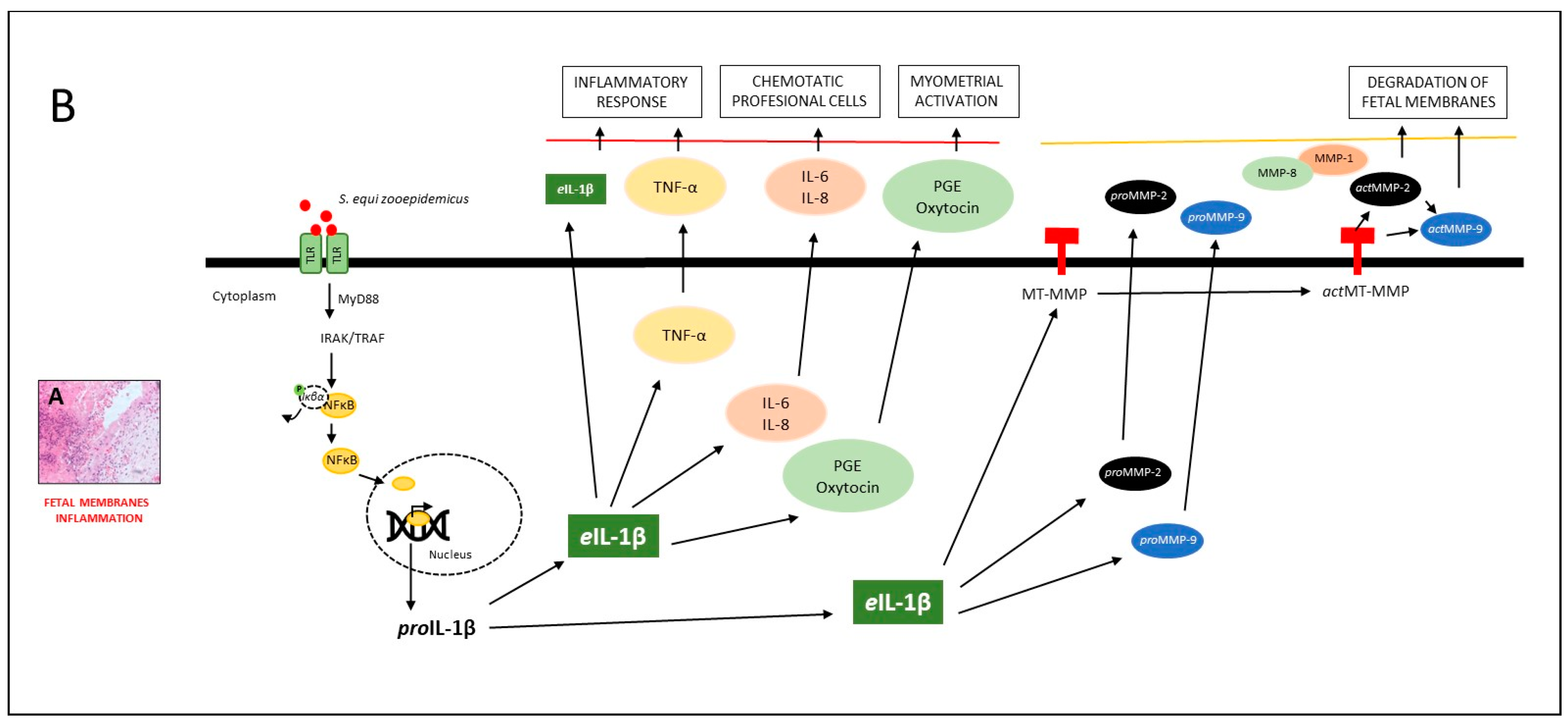
4. Discussion
4.1. The Inflammatory Response and the Activation of Labor
4.2. The Inflammatory Response to the Infectious Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donahue, J.M.; Williams, N.M. Emergent causes of placentitis and abortion. Vet. Clin. N. Am. Equine Pract. 2000, 16, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Fedorka, C.E.; Ball, B.A.; Scoggin, K.E.; Loux, S.C.; Troedsson, M.H.T.; Adams, A.A. The feto-maternal immune response to equine placentitis. Am. J. Reprod. Immunol. 2019, 82, e13179. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.; Carrington, S.; Fitzpatrick, E.; Duggan, V. Ascending placentitis in the mare: A review. Ir. Vet. J. 2008, 61, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.B.; Ball, B.A.; Loux, S.C.; Boakari, Y.L.; Scoggin, K.E.; El-Sheikh Ali, H.; Cogliati, B.; Esteller-Vico, A. Uterine cervix as a fundamental part of the pathogenesis of pregnancy loss associated with ascending placentitis in mares. Theriogenology 2020, 145, 167–175. [Google Scholar] [CrossRef]
- LeBlanc, M. Ascending placentitis in the mare: An update. Reprod. Domest. Anim. 2010, 45 (Suppl. S2), 28–34. [Google Scholar] [CrossRef]
- Peric, A.; Weiss, J.; Vulliemoz, N.; Baud, D.; Stojanov, M. Bacterial Colonization of the Female Upper Genital Tract. Int. J. Mol. Sci. 2019, 20, 3405. [Google Scholar] [CrossRef]
- Chopra, A.; Radhakrishnan, R.; Sharma, M. Porphyromonas gingivalis and adverse pregnancy outcomes: A review on its intricate pathogenic mechanisms. Crit. Rev. Microbiol. 2020, 46, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Constant, O.; Maarifi, G.; Blanchet, F.P.; Van de Perre, P.; Simonin, Y.; Salinas, S. Role of Dendritic Cells in Viral Brain Infections. Front. Immunol. 2022, 13, 862053. [Google Scholar] [CrossRef] [PubMed]
- Layman, Q.D.; Rezabek, G.B.; Ramachandran, A.; Love, B.C.; Confer, A.W. A retrospective study of equine actinobacillosis cases: 1999–2011. J. Vet. Diagn. Investig. 2014, 26, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, S. Specific immune response of mares and their newborn foals to Actinobacillus spp. present in the oral cavity. Acta Vet. Scand. 2001, 42, 237–242. [Google Scholar] [CrossRef]
- El-Sheikh Ali, H.; Legacki, E.L.; Loux, S.C.; Esteller-Vico, A.; Dini, P.; Scoggin, K.E.; Conley, A.J.; Stanley, S.D.; Ball, B.A. Equine placentitis is associated with a downregulation in myometrial progestin signalingdagger. Biol. Reprod. 2019, 101, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Kahler, A.; McGonnell, I.M.; Smart, H.; Kowalski, A.A.; Smith, K.C.; Wathes, D.C.; Mestre, A.M. Fetal morphological features and abnormalities associated with equine early pregnancy loss. Equine Vet. J. 2021, 53, 530–541. [Google Scholar] [CrossRef] [PubMed]
- LeCuyer, T.E.; Rink, A.; Bradway, D.S.; Evermann, J.F.; Nicola, A.V.; Baszler, T.; Haldorson, G.J. Abortion in a Mediterranean miniature donkey (Equus asinus) associated with a gammaherpesvirus similar to Equid herpesvirus 7. J. Vet. Diagn. Investig. 2015, 27, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Murase, H.; Miyazawa, M.; Harada, T.; Ozawa, M.; Sato, F.; Hada, T. Aborted fetal sizes of Thoroughbred horses in Hidaka, Japan, between 2005 and 2015. J. Equine Sci. 2017, 28, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.; Bauquier, J. Causes of equine perinatal mortality. Vet. J. 2021, 273, 105675. [Google Scholar] [CrossRef] [PubMed]
- Grandolfo, E.; Parisi, A.; Ricci, A.; Lorusso, E.; de Siena, R.; Trotta, A.; Buonavoglia, D.; Martella, V.; Corrente, M. High mortality in foals associated with Salmonella enterica subsp. enterica Abortusequi infection in Italy. J. Vet. Diagn. Investig. 2018, 30, 483–485. [Google Scholar] [CrossRef]
- Marenzoni, M.L.; Bietta, A.; Lepri, E.; Proietti, P.C.; Cordioli, P.; Canelli, E.; Stefanetti, V.; Coletti, M.; Timoney, P.J.; Passamonti, F. Role of equine herpesviruses as co-infecting agents in cases of abortion, placental disease and neonatal foal mortality. Vet. Res. Commun. 2013, 37, 311–317. [Google Scholar] [CrossRef]
- Baumann, S.; Gurtner, C.; Marti, H.; Borel, N. Detection of Chlamydia species in 2 cases of equine abortion in Switzerland: A retrospective study from 2000 to 2018. J. Vet. Diagn. Investig. 2020, 32, 542–548. [Google Scholar] [CrossRef]
- Ryan, P.L.; Christiansen, D.L.; Hopper, R.M.; Walters, F.K.; Moulton, K.; Curbelo, J.; Greene, J.M.; Willard, S.T. HORSE SPECIES SYMPOSIUM: A novel approach to monitoring pathogen progression during uterine and placental infection in the mare using bioluminescence imaging technology and lux-modified bacteria1,2. J. Anim. Sci. 2011, 89, 1541–1551. [Google Scholar] [CrossRef]
- Marenzoni, M.L.; Stefanetti, V.; Danzetta, M.L.; Timoney, P.J. Gammaherpesvirus infections in equids: A review. Vet. Med. Res. Rep. 2015, 6, 91–101. [Google Scholar] [CrossRef]
- Orellana-Guerrero, D.; Renaudin, C.; Edwards, L.; Rose, E.; Aleman, M.; Moore, P.F.; Dujovne, G. Fungal Placentitis Caused by Aspergillus terreus in a Mare: Case Report. J. Equine Vet. Sci. 2019, 83, 102799. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.L.; Hopkins, L.J.; Gangloff, M.; Bryant, C.E. The molecular basis for recognition of bacterial ligands at equine TLR2, TLR1 and TLR6. Vet. Res. 2013, 44, 50. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh Ali, H.; Boakari, Y.L.; Loux, S.C.; Dini, P.; Scoggin, K.E.; Esteller-Vico, A.; Kalbfleisch, T.; Ball, B.A. Transcriptomic analysis reveals the key regulators and molecular mechanisms underlying myometrial activation during equine placentitis†. Biol. Reprod. 2020, 102, 1306–1325. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh Ali, H.; Dini, P.; Scoggin, K.; Loux, S.; Fedorka, C.; Boakari, Y.; Norris, J.; Esteller-Vico, A.; Kalbfleisch, T.; Ball, B. Transcriptomic analysis of equine placenta reveals key regulators and pathways involved in ascending placentitis†. Biol. Reprod. 2020, 104, 638–656. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh Ali, H.; Loux, S.C.; Kennedy, L.; Scoggin, K.E.; Dini, P.; Fedorka, C.E.; Kalbfleisch, T.S.; Esteller-Vico, A.; Horohov, D.W.; Erol, E.; et al. Transcriptomic analysis of equine chori-oallantois reveals immune networks and molecular mechanisms involved in nocardioform placentitis. Vet. Res. 2021, 52, 103. [Google Scholar] [CrossRef]
- Figueiredo, M.D.; Vandenplas, M.L.; Hurley, D.J.; Moore, J.N. Differential induction of MyD88- and TRIF-dependent pathways in equine monocytes by Toll-like receptor agonists. Vet. Immunol. Immunopathol. 2009, 127, 125–134. [Google Scholar] [CrossRef]
- Domino, M.; Jasinski, T.; Kautz, E.; Juszczuk-Kubiak, E.; Ferreira-Dias, G.; Zabielski, R.; Sady, M.; Gajewski, Z. Expression of genes involved in the NF-kappaB-dependent pathway of the fibrosis in the mare endometrium. Theriogenology 2020, 147, 18–24. [Google Scholar] [CrossRef]
- Siemieniuch, M.J.; Szóstek, A.Z.; Gajos, K.; Kozdrowski, R.; Nowak, M.; Okuda, K. Type of Inflammation Differentially Affects Expression of Interleukin 1β and 6, Tumor Necrosis Factor-α and Toll-Like Receptors in Subclinical Endometritis in Mares. PLoS ONE 2016, 11, e0154934. [Google Scholar] [CrossRef]
- Leblanc, M.M.; Giguère, S.; Lester, G.D.; Brauer, K.; Paccamonti, D.L. Relationship between infection, inflammation and premature parturition in mares with experimentally induced placentitis. Equine Vet. J. 2012, 44, 8–14. [Google Scholar] [CrossRef]
- Lyle, S.K. Immunology of infective preterm delivery in the mare. Equine Vet. J. 2014, 46, 661–668. [Google Scholar] [CrossRef]
- McGlothlin, J.A.; Lester, G.D.; Hansen, P.J.; Thomas, M.; Pablo, L.; Hawkins, D.L.; LeBlanc, M.M. Alteration in uterine contractility in mares with experimentally induced placentitis. Reproduction 2004, 127, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Pinteaux, E.; Abdulaal, W.H.; Mufazalov, I.A.; Humphreys, N.E.; Simonsen-Jackson, M.; Francis, S.; Müller, W.; Waisman, A. Cell-specific conditional deletion of interleukin-1 (IL-1) ligands and its receptors: A new toolbox to study the role of IL-1 in health and disease. J. Mol. Med. 2020, 98, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.; Duchamp, G.; Gérard, N. In vivo effect of interleukin-1beta and interleukin-1RA on oocyte cytoplasmic maturation, ovulation, and early embryonic development in the mare. Reprod. Biol. Endocrinol. 2005, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, L.; Brayboy, L. Macrophages: An indispensable piece of ovarian health. Biol. Reprod. 2021, 104, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, T.; Zdrojkowski, Ł.; Kautz, E.; Juszczuk-Kubiak, E.; Ferreira-Dias, G.; Domino, M. The NF-κB-signalling pathway in mare′s endometrium infiltrated with the inflammatory cells. Reprod. Domest. Anim. 2022, 57, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Katila, T.; Ferreira-Dias, G. Evolution of the Concepts of Endometrosis, Post Breeding Endometritis, and Susceptibility of Mares. Animals 2022, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.K.; Richter, I.G.; Ahrens, T.; Merle, R.; Alalwani, A.; Lilge, S.; Purschke, K.; Barnewitz, D.; Gehlen, H. MMP-9 Concentration in Peritoneal Fluid Is a Valuable Biomarker Associated with Endotoxemia in Equine Colic. Mediat. Inflamm. 2021, 2021, 9501478. [Google Scholar] [CrossRef]
- Jaworska, J.; Ropka-Molik, K.; Piórkowska, K.; Szmatoła, T.; Kowalczyk-Zięba, I.; Wocławek-Potocka, I.; Siemieniuch, M. Transcriptome Profiling of the Retained Fetal Membranes—An Insight in the Possible Pathogenesis of the Disease. Animals 2021, 11, 675. [Google Scholar] [CrossRef]
- Read, J.E.; Cabrera-Sharp, V.; Offord, V.; Mirczuk, S.M.; Allen, S.P.; Fowkes, R.C.; de Mestre, A.M. Dynamic changes in gene expression and signalling during trophoblast development in the horse. Reproduction 2018, 156, 313–330. [Google Scholar] [CrossRef]
- Vagnoni, K.E.; Ginther, O.J.; Lunn, D.P. Metalloproteinase Activity has a Role in Equine Chorionic Girdle Cell Invasion1. Biol. Reprod. 1995, 53, 800–805. [Google Scholar] [CrossRef]
- El-Sheikh Ali, H.; Scoggin, K.E.; Ruby, R.; Loynachan, A.; Boakari, Y.; Fernandes, C.; Dini, P.; Fedorka, C.E.; Loux, S.C.; Esteller-Vico, A.; et al. Equine cervical remodeling during placentitis and the prepartum period: A transcriptomic approach. Reproduction 2021, 161, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Safari, Z.; Soudi, S.; Jafarzadeh, N.; Hosseini, A.Z.; Vojoudi, E.; Sadeghizadeh, M. Promotion of angiogenesis by M13 phage and RGD peptide in vitro and in vivo. Sci. Rep. 2019, 9, 11182. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; He, Y.; Li, L.; Mao, W.; Chen, X.; Ni, H.; Dong, Y.; Lyu, F. Exosomal MMP2 derived from mature osteoblasts promotes angiogenesis of endothelial cells via VEGF/Erk1/2 signaling pathway. Exp. Cell Res. 2019, 383, 111541. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Han, C.-H.; Hu, F.-F.; Wang, Y.-B.; Cao, Y.-J. The correlation analysis of human embryonic MMP-9 secretion and embryo quality. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2354–2358. [Google Scholar] [PubMed]
- Morales-Hernández, F.V.; Bautista-Bautista, G.; Acuña-González, R.J.; Vázquez-Cárdenas, P.; López-Canales, J.S.; Lozano-Cuenca, J.; Osorio-Caballero, M.; Flores-Herrera, H. Differential proMMP-2 and proMMP-9 secretion in human pre-implantation embryos at day 5 of development. Acta Biochim. Pol. 2022, 69, 683–689. [Google Scholar] [CrossRef]
- Barton, A.K.; Shety, T.; Bondzio, A.; Einspanier, R.; Gehlen, H. Metalloproteinases and their inhibitors are influenced by inhalative glucocorticoid therapy in combination with environmental dust reduction in equine recurrent airway obstruction. BMC Vet. Res. 2016, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Clutterbuck, A.L.; Harris, P.; Allaway, D.; Mobasheri, A. Matrix metalloproteinases in inflammatory pathologies of the horse. Vet. J. 2010, 183, 27–38. [Google Scholar] [CrossRef]
- Bradley, L.M.; Douglass, M.F.; Chatterjee, D.; Akira, S.; Baaten, B.J.G. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLoS Pathog. 2012, 8, e1002641. [Google Scholar] [CrossRef] [PubMed]
- Rossi, H.S.; Koho, N.M.; Ilves, M.; Rajamäki, M.M.; Mykkänen, A.K. Expression of extracellular matrix metalloproteinase inducer and matrix metalloproteinase-2 and -9 in horses with chronic airway inflammation. Am. J. Vet. Res. 2017, 78, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Loftus, J.P.; Johnson, P.J.; Belknap, J.K.; Pettigrew, A.; Black, S.J. Leukocyte-derived and endogenous matrix metalloproteinases in the lamellae of horses with naturally acquired and experimentally induced laminitis. Vet. Immunol. Immunopathol. 2009, 129, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Raulo, S.M.; Sorsa, T.; Tervahartiala, T.; Pirila, E.; Maisi, P. MMP-9 as a marker of inflammation in tracheal epithelial lining fluid (TELF) and in bronchoalveolar fluid (BALF) of COPD horses. Equine Vet. J. 2001, 33, 128–136. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.; Baclawska, A.; Okuda, K.; Skarzynski, D. Effect of proinflammatory cytokines on endometrial collagen and metallopeptidase expression during the course of equine endometrosis. Cytokine 2019, 123, 154767. [Google Scholar] [CrossRef] [PubMed]
- Fugler, L.A.; Eades, S.C.; Moore, R.M.; Koch, C.E.; Keowen, M.L. Plasma matrix metalloproteinase activity in horses after intravenous infusion of lipopolysaccharide and treatment with matrix metalloproteinase inhibitors. Am. J. Vet. Res. 2013, 74, 473–480. [Google Scholar] [CrossRef]
- Woessner, J.F. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991, 5, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Kargozaran, H.; Yuan, S.Y.; Breslin, J.W.; Watson, K.D.; Gaudreault, N.; Breen, A.; Wu, M.H. A role for endothelial-derived matrix metalloproteinase-2 in breast cancer cell transmigration across the endothelial-basement membrane barrier. Clin. Exp. Metastasis 2007, 24, 495–502. [Google Scholar] [CrossRef]
- Allport, J.R.; Lim, Y.C.; Shipley, J.M.; Senior, R.M.; Shapiro, S.D.; Matsuyoshi, N.; Vestweber, D.; Luscinskas, F.W. Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J. Leukoc. Biol. 2002, 71, 821–828. [Google Scholar] [CrossRef]
- Nawrocki-Raby, B.; Gilles, C.; Polette, M.; Martinella-Catusse, C.; Bonnet, N.; Puchelle, E.; Foidart, J.-M.; van Roy, F.; Birembaut, P. E-Cadherin mediates mmp down-regulation in highly invasive bronchial tumor cells. Am. J. Pathol. 2003, 163, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta BBA Mol. Cell Res. 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Renaudin, C.; Troedsson, M.; Gillis, C.; King, V.; Bodena, A. Ultrasonographic evaluation of the equine placenta by transrectal and transabdominal approach in the normal pregnant mare. Theriogenology 1997, 47, 559–573. [Google Scholar] [CrossRef]
- McAfoos, J.L.; Ellerbrock, R.E.; Canisso, I.F. Fetal Death Associated With Premature Mammary Gland Development and Lactation in a Mare Treated With Weekly Injections of Long-Acting Progesterone. J. Equine Vet. Sci. 2019, 81, 102783. [Google Scholar] [CrossRef] [PubMed]
- Borba, L.D.A.; Nogueira, C.E.W.; Bruhn, F.R.P.; da Silva, G.C.; Feijó, L.S.; Canisso, I.F.; Curcio, B.D.R. Peripheral blood markers of sepsis in foals born from mares with experimentally induced ascending placentitis. Vet. Rec. 2020, 187, 29. [Google Scholar] [CrossRef] [PubMed]
- Koterba, A.M.; Brewer, B.D.; Tarplee, F.A. Clinical and clinicopathological characteristics of the septicaemic neonatal foal: Review of 38 cases. Equine Vet. J. 1984, 16, 376–382. [Google Scholar] [CrossRef]
- Taylor, S. A review of equine sepsis. Equine Vet. Educ. 2015, 27, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.M.; Ruby, R.E.; Dembek, K.A.; Barr, B.S.; Reuss, S.M.; Magdesian, K.G.; Olsen, E.; Burns, T.; Slovis, N.M.; Wilkins, P.A. Evaluation of updated sepsis scoring systems and systemic inflammatory response syndrome criteria and their association with sepsis in equine neonates. J. Vet. Intern. Med. 2018, 32, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Flores-Herrera, H.; García-López, G.; Díaz, N.; Molina-Hernández, A.; Osorio-Caballero, M.; Soriano-Becerril, D.; Zaga-Clavellina, V. An experimental mixed bacterial infection induced differential secretion of proinflammatory cytokines (IL-1β, TNFα) and proMMP-9 in human fetal membranes. Placenta 2012, 33, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, L.; Kähäri, V.-M. Matrix metalloproteinases in inflammation. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Takechi, M.; Uchida-Fujii, E.; Miyazawa, K.; Nukada, T.; Niwa, H. Ten cases of Mycobacterium avium subsp. hominissuis infections linked to equine abortions in Japan, 2018–2019. Vet. Med. Sci. 2021, 7, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.M.; Foote, A.K.; Smith, K.C.; Verheyen, K.L.; Mestre, A.M. Incidence and causes of pregnancy loss after Day 70 of gestation in Thoroughbreds. Equine Vet. J. 2021, 53, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.B.; Donahue, J.M.; Giles, R.C.; Petrites-Murphy, M.B., Jr.; Poonacha, K.B.; Roberts, A.W.; Smith, B.J.; Tramontin, R.R.; Tuttle, P.A.; Swerczek, T.W. Etiology and pathology of equine placentitis. J. Vet. Diagn. Investig. 1993, 5, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Loux, S.; Ball, B. The proteome of fetal fluids in mares with experimentally-induced placentitis. Placenta 2018, 64, 71–78. [Google Scholar] [CrossRef]
- Pinho, M.D.; Erol, E.; Ribeiro-Gonçalves, B.; Mendes, C.I.; Carriço, J.A.; Matos, S.C.; Preziuso, S.; Luebke-Becker, A.; Wieler, L.H.; Melo-Cristino, J.; et al. Beta-hemolytic Streptococcus dysgalactiae strains isolated from horses are a genetically distinct population within the Streptococcus dysgalactiae taxon. Sci. Rep. 2016, 6, 31736. [Google Scholar] [CrossRef]
- Macpherson, M.L.; Giguere, S.; Pozor, M.A.; Burden, C.A.; Berghaus, L.J.; Berghaus, R.D.; Varner, J.C.; Hayna, J.T.; SuBenson, M.; Randell, S.A.; et al. Evidence for anti-inflammatory effects of firocoxib administered to mares with experimentally induced placentitis. Am. J. Reprod. Immunol. 2021, 86, e13396. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.K.; Shety, T.; Klier, J.; Geis, S.; Einspanier, R.; Gehlen, H. Metalloproteinases and their Inhibitors under the Course of Immunostimulation by CPG-ODN and Specific Antigen Inhalation in Equine Asthma. Mediat. Inflamm. 2019, 2019, 7845623. [Google Scholar] [CrossRef]
- Ellero, N.; Lanci, A.; Ferlizza, E.; Andreani, G.; Mariella, J.; Isani, G.; Castagnetti, C. Activities of matrix metalloproteinase-2 and -9 in amniotic fluid at parturition in mares with normal and high-risk pregnancy. Theriogenology 2021, 172, 116–122. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh Ali, H.; Scoggin, K.; Linhares Boakari, Y.; Dini, P.; Loux, S.; Fedorka, C.; Esteller-Vico, A.; Ball, B. Kinetics of placenta-specific 8 (PLAC8) in equine placenta during pregnancy and placentitis. Theriogenology 2021, 160, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Aubone, A.M.P.; Bisiau, C.M.; McCue, P.M.; Bouma, G.J. Presence of Clock genes in equine full-term placenta. J. Anim. Sci. 2020, 98, skaa094. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Taylor, B.D. Exploring Inflammatory Mediators in Fetal and Maternal Compartments During Human Parturition. Obstet. Gynecol. 2019, 134, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.M.; Bryant, A.H.; Rees, A.; Down, B.; Jones, R.H.; Thornton, C.A. Production and regulation of interleukin-1 family cytokines at the materno-fetal interface. Cytokine 2017, 99, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.; Janowski, T. Expression of proinflammatory cytokines IL-1β, IL-6 and TNFα in the retained placenta of mares. Theriogenology 2019, 126, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Naskou, M.C.; Norton, N.A.; Copland, I.B.; Galipeau, J.; Peroni, J.F. Innate immune responses of equine monocytes cultured in equine platelet lysate. Vet. Immunol. Immunopathol. 2018, 195, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; Fortier, M.A.; Bernal, A.L. Prostaglandin pathway gene expression in human placenta, amnion and choriodecidua is differentially affected by preterm and term labour and by uterine inflammation. BMC Pregnancy Childbirth 2014, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Hadley, E.E.; Richardson, L.S.; Torloni, M.R.; Menon, R. Gestational tissue inflammatory biomarkers at term labor: A systematic review of literature. Am. J. Reprod. Immunol. 2018, 79, e12776. [Google Scholar] [CrossRef]



| HEALTHY MARES (n = 6) | USP (n = 10) | ||||
|---|---|---|---|---|---|
| Variable | HM-HF (n = 6) | USP-HF (n = 3) | USP-LSeF (n = 4) | USP-DF (n = 3) | |
| Mares | |||||
| Age, years (Rank) | 12.5 ± 4.8 (9–22) | 9.3 ± 4.2 (6–14) | 10.5 ± 3.7 (6–14) | 14.3 ± 5.5 (8–10) | |
| CTUP, mm | 240 days | 3.98 ± 0.55 | 5.0 ± 1.3 | 5.7 ± 0.98 * | 5.8 ± 1.0 * |
| 320 days | 8.56 ± 1.93 | 9.0 ± 0.5 | 10.8 ± 0.8 | 12.1 ± 0.59 * | |
| External signs of placentitis | Normal | Normal | Normal | Premature udder and vaginal discharge | |
| Delivery, days (Rank) | 344.8 ± 5.3 (338–350) | 337. ± 11.9 (329–351) | 334.3 ± 11.5 (325–351) | 321.7 ± 11.9 a (308–330) | |
| Placental lesions | Normal | Ascending lesions | Ascending lesions | Ascending lesions, with necrosis | |
| Foal | |||||
| Sex | Male, n (%) | 4 (66.7) | 2 (66.7) | 0 | 2 (66.7) |
| Female, n (%) | 2 (33.3) | 1 (33.3) | 4 (100) | 1 (33.3) | |
| Weight, kg (rank) | 51.3 ± 5.2 (44–57) | 44.3 ± 7.6 (32–50) | 41.3 ± 7.3 (31–49) | 44.0 ± 1.0 (43–45) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Vázquez, M.M.; Meza-Serrano, E.; Lara-Pereyra, I.; Acuña-González, R.J.; Alonso-Morales, R.; Hayen-Valles, S.; Boeta, A.M.; Zarco, L.; Lozano-Cuenca, J.; López-Canales, J.S.; et al. Equine Placentitis in Mares Induces the Secretion of Pro-Inflammatory Cytokine eIL-1β and the Active Extracellular Matrix Metalloproteinase (MMP)-9. Vet. Sci. 2023, 10, 532. https://doi.org/10.3390/vetsci10090532
Morales-Vázquez MM, Meza-Serrano E, Lara-Pereyra I, Acuña-González RJ, Alonso-Morales R, Hayen-Valles S, Boeta AM, Zarco L, Lozano-Cuenca J, López-Canales JS, et al. Equine Placentitis in Mares Induces the Secretion of Pro-Inflammatory Cytokine eIL-1β and the Active Extracellular Matrix Metalloproteinase (MMP)-9. Veterinary Sciences. 2023; 10(9):532. https://doi.org/10.3390/vetsci10090532
Chicago/Turabian StyleMorales-Vázquez, María Margarita, Europa Meza-Serrano, Irlando Lara-Pereyra, Ricardo Josué Acuña-González, Rogelio Alonso-Morales, Sergio Hayen-Valles, Ana Myriam Boeta, Luis Zarco, Jair Lozano-Cuenca, Jorge Skiold López-Canales, and et al. 2023. "Equine Placentitis in Mares Induces the Secretion of Pro-Inflammatory Cytokine eIL-1β and the Active Extracellular Matrix Metalloproteinase (MMP)-9" Veterinary Sciences 10, no. 9: 532. https://doi.org/10.3390/vetsci10090532





