Conserved Domains in Variable Surface Lipoproteins A-G of Mycoplasma hyorhinis May Serve as Probable Multi-Epitope Candidate Vaccine: Computational Reverse Vaccinology Approach
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequences and Alignment and Prediction of Epitopes
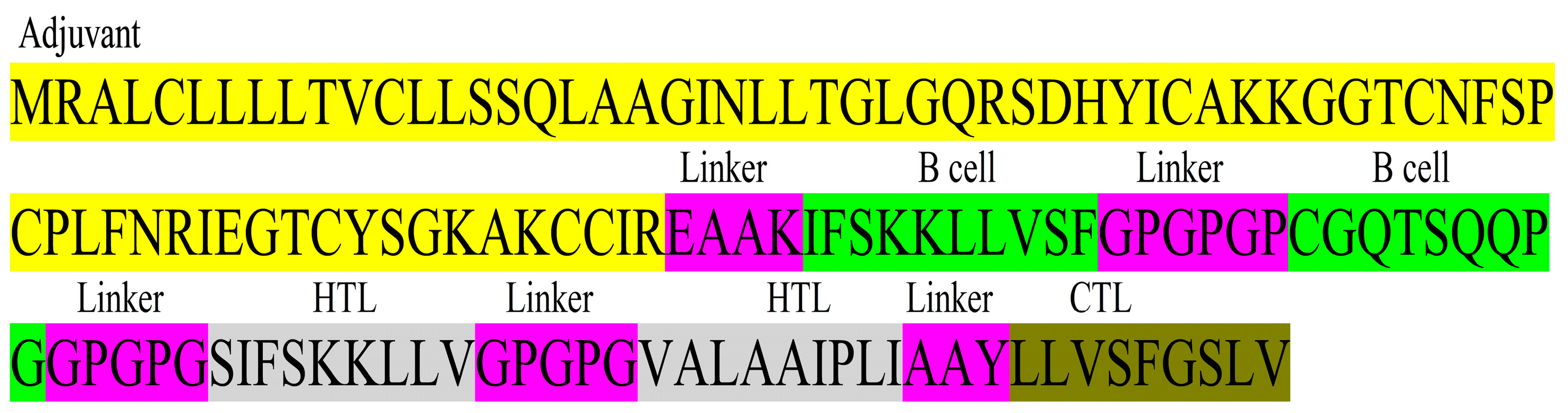
2.2. Construction of Multi-Epitope Vaccine
2.3. Prediction of Antigenicity
2.4. Physicochemical Properties
2.5. Prediction of Secondary Structure
2.6. Prediction and Validation of Tertiary Structure
2.7. Prediction of Discontinuous Epitopes
2.8. Molecular Docking
2.9. Molecular Dynamic Simulation
2.10. Immune Simulation
2.11. Codon Optimization and In Silico Cloning
3. Results
3.1. Multiple Sequence Alignment
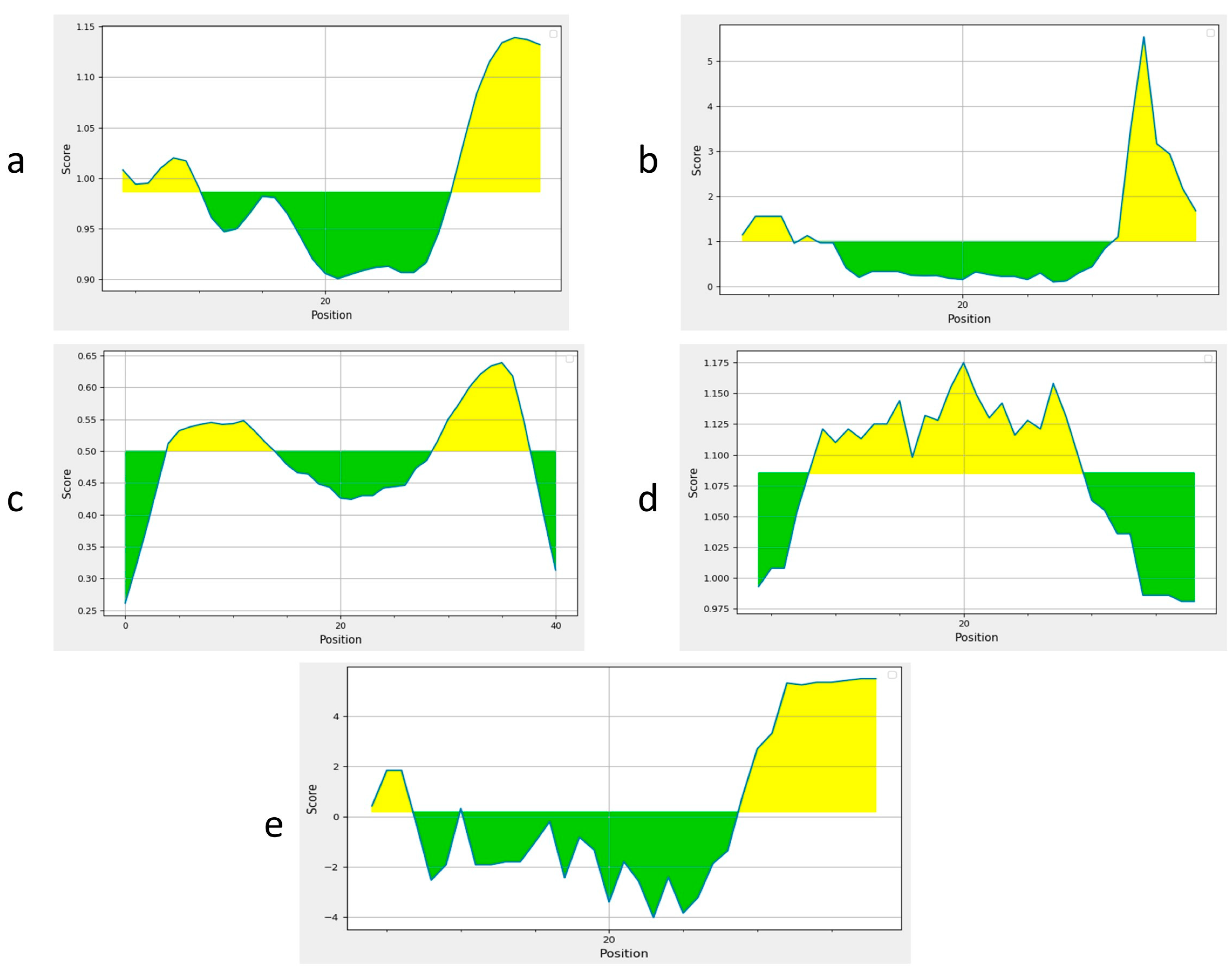
3.2. B Cell Epitopes
3.3. Prediction of CTL Epitopes
3.4. Prediction of HTL Epitopes
3.5. MEV Construct
3.6. Physicochemical Properties
3.7. Secondary Structure and Solubility
3.8. Prediction and Refinement of Tertiary Structure
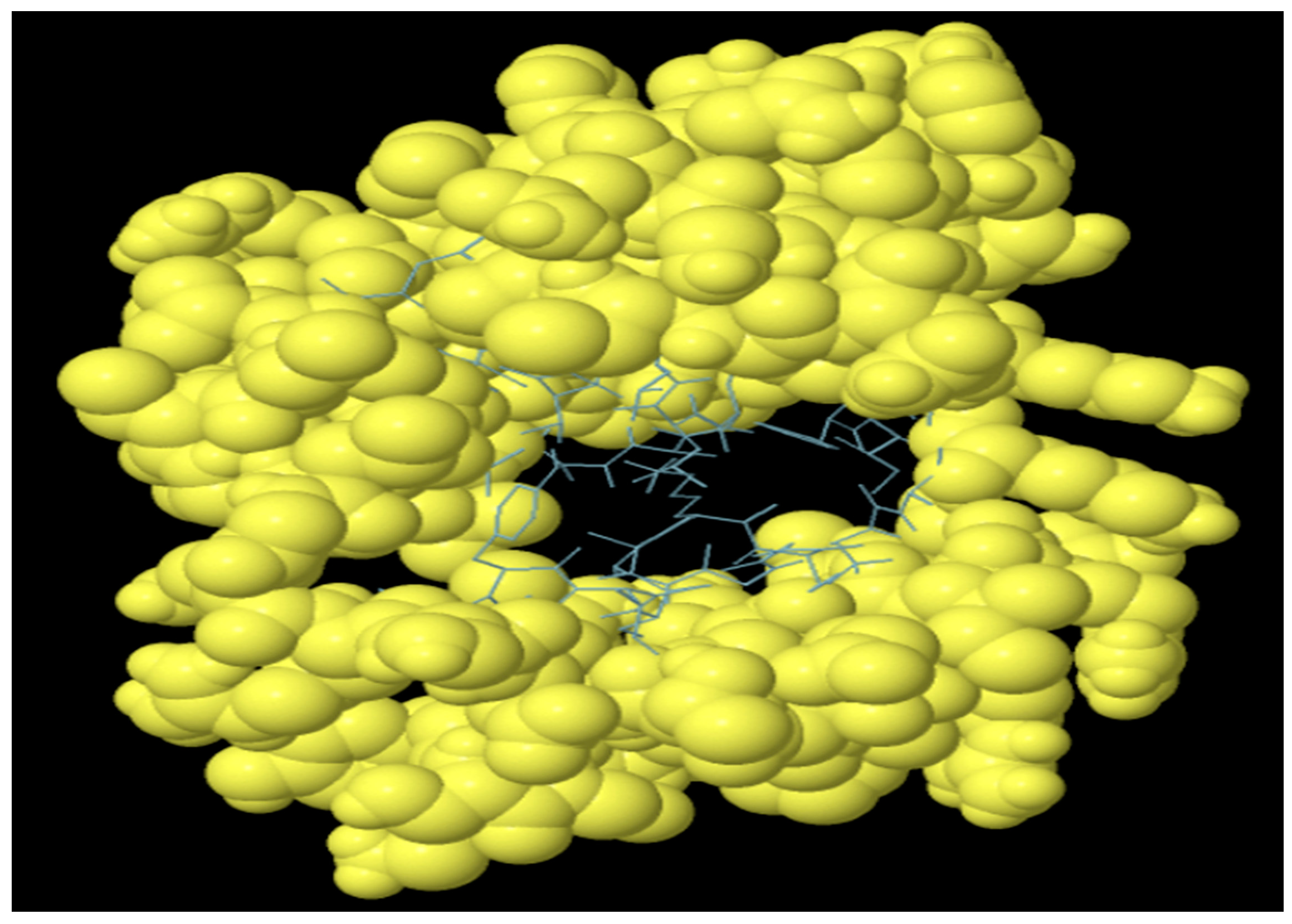
3.9. Discontinuous B Cell Epitopes
3.10. Molecular Docking
3.11. Molecular Dynamics Simulation
3.12. Molecular Cloning
3.13. Immune Simulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrarini, M.G.; Siqueira, F.M.; Mucha, S.G.; Palama, T.L.; Jobard, É.; Elena-Herrmann, B.; Ana, A.T.; Tardy, F.; Schrank, I.S.; Zaha, A.; et al. Insights on the virulence of swine respiratory tract mycoplasmas through genome-scale metabolic modeling. BMC Genom. 2016, 17, 353. [Google Scholar] [CrossRef]
- Kobisch, M.; Marois, C. Swine mycoplasmoses. Bull. Acad. Vet. Fr. 2008, 161, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Meng, L.; Jiang, B.; Zhang, J.; Yang, H.; Wu, J.; Shou, C. p37 from Mycoplasma hyorhinis promotes cancer cell invasiveness and metastasis through activation of MMP-2 and followed by phosphorylation of EGFR. Mol. Cancer Ther. 2008, 7, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Watson-McKown, R.; Droesse, M.; Wise, K.S. Gene families encoding phase- and size-variable surface lipoproteins of Mycoplasma hyorhinis. J. Bacteriol. 2000, 182, 1356–1363. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Shao, J.; Li, Y.; Yu, Y.; Shao, G.; Feng, Z.; Xiong, Q. The variable lipoprotein family participates in the interaction of Mycoplasma hyorhinis with host extracellular matrix and plasminogen. Vet. Microbiol. 2022, 265, 109310. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, B.; Wang, J.; Ni, B.; Ji, Y.; Wei, Y.; Xiao, S.; Feng, Z.; Liu, M.; Shao, G. Characterization of the role in adherence of Mycoplasma hyorhinis variable lipoproteins containing different repeat unit copy numbers. Vet. Microbiol. 2016, 197, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Lirola, L.G.; Meiroz-De-Souza-Almeida, H.; Magtoto, R.L.; McDaniel, A.J.; Merodio, M.M.; Ferreyra, F.S.M.; Poonsuk, K.; Gatto, I.R.H.; Baum, D.H.; Ross, R.F.; et al. Early detection and differential serodiagnosis of Mycoplasma hyorhinis and Mycoplasma hyosynoviae infections under experimental conditions. PLoS ONE 2019, 14, e0223459. [Google Scholar] [CrossRef]
- Abbas, A.H.; Pereira, S.S.; D’Archivio, S.; Wickstead, B.; Morrison, L.J.; Hall, N.; Hertz-Fowler, C.; Darby, A.C.; Jackson, A.P. The structure of a conserved telomeric region associated with variant antigen loci in the blood parasite trypanosoma congolense. Genome Biol. Evol. 2018, 10, 2458–2473. [Google Scholar] [CrossRef]
- Liang, F.T.; Alvarez, A.L.; Gu, Y.; Nowling, J.M.; Ramamoorthy, R.; Philipp, M.T. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J. Immunol. 1999, 163, 5566–5573. [Google Scholar] [CrossRef]
- Xu, C.G.; Hao, Y.Q.; Zhang, L.; Hao, R.X.; Liu, X.L.; Huang, Z.Y. Molecular cloning and immune response analysis of putative variable lipoproteins from Mycoplasma mycoides subsp capri. Genet. Mol. Res. 2014, 13, 1527–1539. [Google Scholar] [CrossRef]
- Martinson, B.; Zoghby, W.; Barrett, K.; Bryson, L.; Christmas, R.; Minion, F.C.; Kroll, J. Efficacy of an inactivated Mycoplasma hyorhinis vaccine in pigs. Vaccine 2018, 36, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.W.; Zhu, H.Z.; Huang, L.P.; Xia, D.L.; Wu, H.L.; Bian, H.Q.; Feng, L.; Liu, C.M. Efficacy in pigs of a new inactivated vaccine combining porcine circovirus type 2 and Mycoplasma hyorhinis. Vet. Microbiol. 2020, 242, 108588. [Google Scholar] [CrossRef] [PubMed]
- Chua, B.Y.; Sekiya, T.; Jackson, D.C. Opinion: Making Inactivated and Subunit-Based Vaccines Work. Viral Immunol. 2018, 31, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Liljeroos, L.; Malito, E.; Ferlenghi, I.; Bottomley, M.J. Structural and Computational Biology in the Design of Immunogenic Vaccine Antigens. J. Immunol. Res. 2015, 2015, 156241. [Google Scholar] [CrossRef] [PubMed]
- Dar, H.A.; Ismail, S.; Waheed, Y.; Ahmad, S.; Jamil, Z.; Aziz, H.; Hetta, H.F.; Muhammad, K. Designing a multi-epitope vaccine against Mycobacteroides abscessus by pangenome-reverse vaccinology. Sci. Rep. 2021, 11, 11197. [Google Scholar] [CrossRef]
- Mahmood, M.; Javaid, A.; Shahid, F.; Ashfaq, U.A. Rational design of multimeric based subunit vaccine against Mycoplasma pneumonia: Subtractive proteomics with immunoinformatics framework. Infect. Genet. Evol. 2021, 91, 104795. [Google Scholar] [CrossRef] [PubMed]
- Moodley, A.; Fatoba, A.; Okpeku, M.; Chiliza, T.E.; Simelane, M.B.C.; Pooe, O.J. Reverse vaccinology approach to design a multi-epitope vaccine construct based on the Mycobacterium tuberculosis biomarker PE_PGRS17. Immunol. Res. 2022, 70, 501–517. [Google Scholar] [CrossRef]
- Urrutia-Baca, V.H.; Gomez-Flores, R.; De La Garza-Ramos, M.A.; Tamez-Guerra, P.; Lucio-Sauceda, D.G.; Rodríguez-Padilla, M.C. Immunoinformatics Approach to Design a Novel Epitope-Based Oral Vaccine Against Helicobacter pylori. J. Comput. Biol. 2019, 26, 1177–1190. [Google Scholar] [CrossRef]
- Gupta, N.; Kumar, A. Designing an efficient multi-epitope vaccine against Campylobacter jejuni using immunoinformatics and reverse vaccinology approach. Microb. Pathog. 2020, 147, 104398. [Google Scholar] [CrossRef]
- Heinson, A.I.; Woelk, C.H.; Newell, M.L. The promise of reverse vaccinology. Int. Health 2015, 7, 85–89. [Google Scholar] [CrossRef]
- Alsowayeh, N.; Albutti, A.; Al-Shouli, S.T. Reverse Vaccinology and Immunoinformatic Assisted Designing of a Multi-Epitopes Based Vaccine Against Nosocomial Burkholderia cepacia. Front. Microbiol. 2022, 13, 929400. [Google Scholar] [CrossRef] [PubMed]
- Vilela Rodrigues, T.C.; Jaiswal, A.K.; Lemes, M.R.; da Silva, M.V.; Sales-Campos, H.; Alcântara, L.C.J.; de Tosta, S.F.O.; Kato, R.B.; Alzahrani, K.J.; Barh, D.; et al. An immunoinformatics-based designed multi-epitope candidate vaccine (mpme-VAC/STV-1) against Mycoplasma pneumoniae. Comput. Biol. Med. 2022, 142, 105194. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Ullah, I.; Zhu, B.; Adnan, M.; Liaqat, R.; Kong, W.B.; Niu, S. In silico analysis of epitope-based vaccine candidate against tuberculosis using reverse vaccinology. Sci. Rep. 2021, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R.; Heo, L.; Seok, C. Effective protein model structure refinement by loop modeling and overall relaxation. Proteins Struct. Funct. Bioinform. 2016, 84, 293–301. [Google Scholar] [CrossRef] [PubMed]
- De Vries, S.J.; Van Dijk, M.; Bonvin, A.M.J.J. The HADDOCK web server for data-driven biomolecular docking. Nat. Protoc. 2010, 5, 883–897. [Google Scholar] [CrossRef]
- López-Blanco, J.R.; Aliaga, J.I.; Quintana-Ortí, E.S.; Chacón, P. IMODS: Internal coordinates normal mode analysis server. Nucleic Acids Res. 2014, 42, 271–276. [Google Scholar] [CrossRef]
- Wang, J.; Hua, L.; Gan, Y.; Yuan, T.; Li, L.; Yu, Y.; Xie, Q.; Olaniran, A.O.; Chiliza, T.E.; Shao, G.; et al. Virulence and Inoculation Route Influence the Consequences of Mycoplasma hyorhinis Infection in Bama Miniature Pigs. Microbiol. Spectr. 2022, 10, e0249321. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Pan, L.; Li, J.; Yu, Y.; Liu, B.; Zubair, M.; Wei, Y.; Pillay, B.; Olaniran, A.O.; et al. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) moonlights as an adhesin in Mycoplasma hyorhinis adhesion to epithelial cells as well as a plasminogen receptor mediating extracellular matrix degradation. Vet. Res. 2021, 52, 80. [Google Scholar] [CrossRef]
- Gautier-Bouchardon, A.V.; Ferré, S.; Le Grand, D.; Paoli, A.; Gay, E.; Poumarat, F. Overall decrease in the susceptibility of Mycoplasma bovis to antimicrobials over the past 30 years in France. PLoS ONE 2014, 9, e87672. [Google Scholar] [CrossRef]
- Lee, J.A.; Hwang, M.A.; Han, J.H.; Cho, E.H.; Lee, J.B.; Park, S.Y.; Song, C.S.; Choi, I.S.; Lee, S.W. Reduction of mycoplasmal lesions and clinical signs by vaccination against Mycoplasma hyorhinis. Vet. Immunol. Immunopathol. 2018, 196, 14–17. [Google Scholar] [CrossRef]
- Vakili, B.; Nezafat, N.; Zare, B.; Erfani, N.; Akbari, M.; Ghasemi, Y.; Rahbar, M.R.; Hatam, G.R. A new multi-epitope peptide vaccine induces immune responses and protection against Leishmania infantum in BALB/c mice. Med. Microbiol. Immunol. 2020, 209, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide vaccine: Progress and challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef]
- Vartak, A.; Sucheck, S.J. Recent advances in subunit vaccine carriers. Vaccines 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Trincado, J.L.; Gomez-Perosanz, M.; Reche, P.A. Fundamentals and Methods for T- and B-Cell Epitope Prediction. J. Immunol. Res. 2017, 2017, 2680160. [Google Scholar] [CrossRef]
- Kumar, P.; Lata, S.; Shankar, U.N.; Akif, M. Immunoinformatics-Based Designing of a Multi-Epitope Chimeric Vaccine From Multi-Domain Outer Surface Antigens of Leptospira. Front. Immunol. 2021, 12, 735373. [Google Scholar] [CrossRef]
- Kazi, A.; Chuah, C.; Majeed, A.B.A.; Leow, C.H.; Lim, B.H.; Leow, C.Y. Current progress of immunoinformatics approach harnessed for cellular- and antibody-dependent vaccine design. Pathog. Glob. Health 2018, 112, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shu, J.; Jin, J.; Shu, J.; Feng, H.; Chen, J.; He, Y. Development of a Multi-Epitope Vaccine for Mycoplasma hyopneumoniae and Evaluation of Its Immune Responses in Mice and Piglets. Int. J. Mol. Sci. 2022, 23, 7899. [Google Scholar] [CrossRef] [PubMed]
- Mugunthan, S.P.; Mani Chandra, H. A Computational Reverse Vaccinology Approach for the Design and Development of Multi-Epitopic Vaccine Against Avian Pathogen Mycoplasma gallisepticum. Front. Vet. Sci. 2021, 8, 721061. [Google Scholar] [CrossRef]
- Jiang, Z.; Kang, X.; Song, Y.; Zhou, X.; Yue, M. Identification and Evaluation of Novel Antigen Candidates against Salmonella Pullorum Infection Using Reverse Vaccinology. Vaccines 2023, 11, 865. [Google Scholar] [CrossRef]
- Hajialibeigi, A.; Amani, J.; Gargari, S.L.M. Identification and evaluation of novel vaccine candidates against Shigella flexneri through reverse vaccinology approach. Appl. Microbiol. Biotechnol. 2021, 105, 1159–1173. [Google Scholar] [CrossRef]
- Zilch, T.J.; Lee, J.J.; Bressan, G.C.; McDonough, S.P.; Mohammed, H.O.; Divers, T.J.; Chang, Y.F. Evaluation of new leptospiral antigens for the diagnosis of equine leptospirosis: An approach using pan-genomic analysis, reverse vaccinology and antigenic selection. Equine Vet. J. 2021, 53, 13380. [Google Scholar] [CrossRef]
- Esmailnia, E.; Amani, J.; Gargari, S.L.M. Identification of novel vaccine candidate against Salmonella enterica serovar Typhi by reverse vaccinology method and evaluation of its immunization. Genomics 2020, 112, 3374–3381. [Google Scholar] [CrossRef]
- Chauhan, V.; Rungta, T.; Goyal, K.; Singh, M.P. Designing a multi-epitope based vaccine to combat Kaposi Sarcoma utilizing immunoinformatics approach. Sci. Rep. 2019, 9, 2517. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, F.; Shao, J.; Li, Y.; Li, S.; Chang, H.; Zhang, Y. Application of built-in adjuvants for epitope-based vaccines. PeerJ 2019, 2019, e6185. [Google Scholar] [CrossRef] [PubMed]
- Soltan, M.A.; Elbassiouny, N.; Gamal, H.; Elkaeed, E.B.; Eid, R.A.; Eldeen, M.A.; Al-karmalawy, A.A. In silico prediction of a multitope vaccine against moraxella catarrhalis: Reverse vaccinology and immunoinformatics. Vaccines 2021, 9, 669. [Google Scholar] [CrossRef]
- Jalal, K.; Khan, K.; Ahmad, D.; Hayat, A.; Basharat, Z.; Abbas, M.N.; Alghamdi, S.; Almehmadi, M.; Sahibzada, M.U.K. Pan-genome reverse vaccinology approach for the design of multi-epitope vaccine construct against escherichia albertii. Int. J. Mol. Sci. 2021, 22, 12814. [Google Scholar] [CrossRef] [PubMed]
- Muneta, Y.; Uenishi, H.; Kikuma, R.; Yoshihara, K.; Shimoji, Y.; Yamamoto, R.; Hamashima, N.; Yokomizo, Y.; Mori, Y. Porcine TLR2 and TLR6: Identification and Their Involvement in Mycoplasma hyopneumoniae Infection. J. Interf. Cytokine Res. 2003, 23, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Tawaratsumida, K.; Furuyashiki, M.; Katsumoto, M.; Fujimoto, Y.; Fukase, K.; Suda, Y.; Hashimoto, M. Characterization of N-terminal structure of TLR2-activating lipoprotein in Staphylococcus aureus. J. Biol. Chem. 2009, 284, 9147–9152. [Google Scholar] [CrossRef]
- Chen, M.; Deng, H.; Zhao, Y.; Miao, X.; Gu, H.; Bi, Y.; Zhu, Y.; Guo, Y.; Shi, S.; Xu, J.; et al. Toll-Like Receptor 2 Modulates Pulmonary Inflammation and TNF-α Release Mediated by Mycoplasma pneumoniae. Front. Cell. Infect. Microbiol. 2022, 12, 824027. [Google Scholar] [CrossRef]
- Hasebe, A.; Mu, H.H.; Cole, B.C. A potential pathogenic factor from Mycoplasma hominis is a TLR2-dependent, macrophage-activating, P50-related adhesin. Am. J. Reprod. Immunol. 2014, 72, 285–295. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, Y.; Wang, Y.; Li, Y.; Zhang, L.; Xin, J. TLR2/MyD88/NF-κB signaling pathway regulates IL-1β production in DF-1 cells exposed to Mycoplasma gallisepticum LAMPs. Microb. Pathog. 2018, 117, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gianni, T.; Campadelli-Fiume, G. The Epithelial αvβ3-Integrin Boosts the MYD88-Dependent TLR2 Signaling in Response to Viral and Bacterial Components. PLoS Pathog. 2014, 10, e1004477. [Google Scholar] [CrossRef] [PubMed]







| No. | Start | End | Peptide | Length |
|---|---|---|---|---|
| 1 | 5 | 14 | IFSKKLLVSF | 10 |
| 2 | 30 | 38 | CGQTSQQPG | 9 |
| Peptide Sequence | Binding Affinity | Affinity Rescaled | Cleavage | TAP | Combined Score |
|---|---|---|---|---|---|
| LLVSFGSLV | 0.0872 | 0.3701 | 0.9251 | 0.5170 | 0.5347 |
| Peptide Sequence | Start | End | Score |
|---|---|---|---|
| SIFSKKLLV | 4 | 12 | 0.194 |
| VALAAIPLI | 18 | 26 | 0.24 |
| Physicochemical Properties | Score |
|---|---|
| Number of amino acids | 138 |
| Molecular weight | 14,153.88 |
| Theoretical pI | 9.42 |
| Total number of negatively charged residues (Asp + Glu) | 3 |
| Total number of positively charged residues (Arg + Lys) | 13 |
| Ext. coefficient | 4970 |
| Estimated half-life | >20 h (yeast, in vivo) >10 h (Escherichia coli, in vivo) |
| Instability index (II) | 26.53 (Stable) |
| Aliphatic index | 103.26 |
| Grand average of hydropathicity (GRAVY) | 0.484 (Polar protein) |
| Scaled solubility | 0.707 |
| Number of amino acids | 138 |
| No. | Residues | Number of Residues | Score |
|---|---|---|---|
| 1 | A:M1, A:R2, A:A3, A:L4, A:C5, A:L6, A:L7, A:L8, A:L9, A:T10, A:V11, A:L14, A:S15, A:S16, A:Q17, A:L18, A:A20, A:G21, A:L24, A:L25, A:T26, A:G27, A:L28, A:G29, A:Q30, A:R31, A:S32, A:D33, A:C44, A:N45, A:F46, A:S47, A:P48, A:C49, A:P50, A:L51, A:F52, A:N53, A:R54, A:I55, A:E56, A:G57, A:T58, A:C59, A:Y60, A:S61, A:G62, A:K63, A:A64, A:K65, A:C66, A:C67, A:I68, A:R69, A:E70, A:K73, A:K77, A:L80, A:V81, A:S82, A:F83, A:G84, A:P85, A:G86, A:P87, A:G88, A:P89, A:C90, A:G91, A:Q92, A:T93, A:S94, A:Q95, A:Q96, A:P97, A:G98, A:G99, A:P100, A:G101, A:P102, A:G103, A:S104, A:I105, A:F106, A:S107, A:K108, A:K109, A:V112, A:G113, A:P114, A:G115, A:P116, A:A121, A:A122, A:I123, A:P124, A:L125, A:I126, A:S133, A:F134, A:G135, A:S136, A:L137, A:V138 | 104 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubair, M.; Wang, J.; Yu, Y.; Rasheed, M.A.; Faisal, M.; Dawood, A.S.; Ashraf, M.; Shao, G.; Feng, Z.; Xiong, Q. Conserved Domains in Variable Surface Lipoproteins A-G of Mycoplasma hyorhinis May Serve as Probable Multi-Epitope Candidate Vaccine: Computational Reverse Vaccinology Approach. Vet. Sci. 2023, 10, 557. https://doi.org/10.3390/vetsci10090557
Zubair M, Wang J, Yu Y, Rasheed MA, Faisal M, Dawood AS, Ashraf M, Shao G, Feng Z, Xiong Q. Conserved Domains in Variable Surface Lipoproteins A-G of Mycoplasma hyorhinis May Serve as Probable Multi-Epitope Candidate Vaccine: Computational Reverse Vaccinology Approach. Veterinary Sciences. 2023; 10(9):557. https://doi.org/10.3390/vetsci10090557
Chicago/Turabian StyleZubair, Muhammad, Jia Wang, Yanfei Yu, Muhammad Asif Rasheed, Muhammad Faisal, Ali Sobhy Dawood, Muhammad Ashraf, Guoqing Shao, Zhixin Feng, and Qiyan Xiong. 2023. "Conserved Domains in Variable Surface Lipoproteins A-G of Mycoplasma hyorhinis May Serve as Probable Multi-Epitope Candidate Vaccine: Computational Reverse Vaccinology Approach" Veterinary Sciences 10, no. 9: 557. https://doi.org/10.3390/vetsci10090557
APA StyleZubair, M., Wang, J., Yu, Y., Rasheed, M. A., Faisal, M., Dawood, A. S., Ashraf, M., Shao, G., Feng, Z., & Xiong, Q. (2023). Conserved Domains in Variable Surface Lipoproteins A-G of Mycoplasma hyorhinis May Serve as Probable Multi-Epitope Candidate Vaccine: Computational Reverse Vaccinology Approach. Veterinary Sciences, 10(9), 557. https://doi.org/10.3390/vetsci10090557








