Exploring the Relationship between Neutrophil Activation and Different States of Canine L. infantum Infection: Nitroblue Tetrazolium Test and IFN-γ
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dogs and Clinical Data
2.2. Blood Collection
2.3. Antileishmanial Antibody Quantification via ELISA
2.4. Nitroblue Tetrazolium Reduction Test
2.5. Cytokine Release Whole Blood Assay and Determination of Canine IFN-γ
2.6. Statistical Analysis
3. Results
3.1. Dog Clinical Characteristics and Performed Tests
3.2. Age-Stratified Analysis of NBT Rate in Dogs with Papular Dermatitis (Stage I): Insights from Younger and Older Canine Cohorts
3.3. Follow-Up of Dogs with Papular Dermatitis (Stage I)
3.4. Total Leucocyte Concentration and Differential Leukocyte Concentration
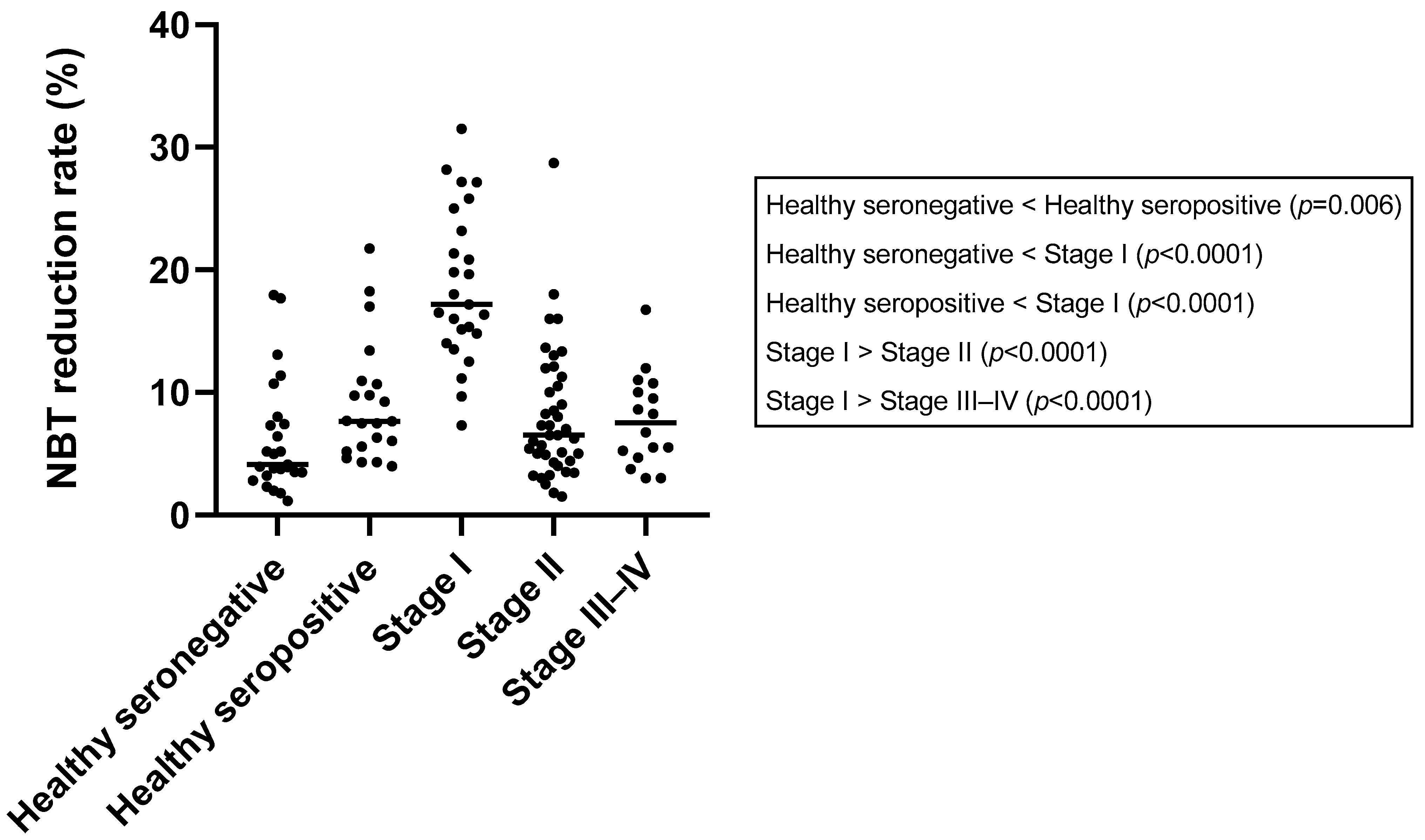
3.5. Nitroblue Tetrazolium Reduction Test
3.6. Leishmania Infantum-Specific Antibody Levels
3.7. IFN-γ Concentration
3.8. Correlation between Parameters Studied
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet Guidelines for the Practical Management of Canine Leishmaniosis. Parasites Vectors 2011, 4, 86. [Google Scholar] [CrossRef] [PubMed]
- Toepp, A.J.; Petersen, C.A. The Balancing Act: Immunology of Leishmaniosis. Res. Vet. Sci. 2020, 130, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Montserrat-Sangrà, S.; Ordeix, L.; Martínez-Orellana, P. Leishmania infantum-Specific Production of IFN-γ and IL-10 in Stimulated Blood from Dogs with Clinical Leishmaniosis. Parasites Vectors 2016, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Hosein, S.; Blake, D.P.; Solano-Gallego, L. Insights on Adaptive and Innate Immunity in Canine Leishmaniosis. Parasitology 2017, 144, 95–115. [Google Scholar] [CrossRef]
- Alvar, J.; Cañavate, C.; Molina, R.; Moreno, J.; Nieto, J. Canine Leishmaniasis. Adv. Parasitol. 2004, 57, 1–88. [Google Scholar] [CrossRef]
- Papadogiannakis, E.I.; Koutinas, A.F. Cutaneous Immune Mechanisms in Canine Leishmaniosis Due to Leishmania infantum. Vet. Immunol. Immunopathol. 2015, 163, 94–102. [Google Scholar] [CrossRef]
- Boggiatto, P.M.; Ramer-Tait, A.E.; Metz, K.; Kramer, E.E.; Gibson-corley, K.; Mullin, K.; Hostetter, J.M.; Gallup, J.M.; Jones, D.E.; Petersen, C.A. Immunologic Indicators of Clinical Progression during Canine Leishmania infantum Infection. Clin. Vaccine Immunol. 2010, 17, 267–273. [Google Scholar] [CrossRef]
- Venuprasad, K.; Chattopadhyay, S.; Saha, B. CD28 Signaling in Neutrophil Induces T-Cell Chemotactic Factor(s) Modulating T-Cell Response. Hum. Immunol. 2003, 64, 38–43. [Google Scholar] [CrossRef]
- Brandonisio, O.; Panunzio, M.; Faliero, S.M.; Ceci, L.; Fasanella, A.; Puccini, V. Evaluation of Polymorphonuclear Cell and Monocyte Functions in Leishmania infantum-Infected Dogs. Vet. Immunol. Immunopathol. 1996, 53, 95–103. [Google Scholar] [CrossRef]
- Panaro, M.A.; Puccini, V.; Faliero, S.M.; Marzio, R.; Marangi, A.; Lisi, S.; Brandonisio, O. Leishmania donovani lipophosphoglycan (LPG) inhibits respiratory burst and chemotaxis of dog phagocytes. New Microbiol. 1996, 19, 107–112. [Google Scholar]
- van Zandbergen, G.; Klinger, M.; Mueller, A.; Dannenberg, S.; Gebert, A.; Solbach, W.; Laskay, T. Cutting Edge: Neutrophil Granulocyte Serves as a Vector for Leishmania Entry into Macrophages. J. Immunol. 2004, 173, 6521–6525. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Nauseef, W.M.; Borregaard, N. Neutrophils at Work. Nat. Immunol. 2014, 15, 602–611. [Google Scholar] [CrossRef]
- Mócsai, A. Diverse Novel Functions of Neutrophils in Immunity, Inflammation, and Beyond. J. Exp. Med. 2013, 210, 1289–1299. [Google Scholar] [CrossRef]
- Roos, D.; Van Bruggen, R.; Meischl, C. Oxidative Killing of Microbes by Neutrophils. Microbes Infect. 2003, 5, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. How Human Neutrophils Kill and Degrade Microbes: An Integrated View. Immunol. Rev. 2007, 219, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. The undefined Inside the Neutrophil Phagosome: Oxidants, Myeloperoxidase, and Bacterial Killing. Blood 1998, 92, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Ozinsky, A. Phagocytosis of Microbes: Complexity in Action. Annu. Rev. Immunol. 2002, 20, 825–852. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.; Kettle, A.; Hampton, M. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M. NADPH Oxidase: An Update. Blood 1999, 93, 1464–1476. [Google Scholar] [CrossRef]
- Babior, B.M. The Respiratory Burst Oxidase. Adv. Enzymol. Relat. Areas Mol. Biol. 2006, 65, 49–95. [Google Scholar] [CrossRef]
- Wardini, A.B.; Pinto-da-Silva, L.H.; Nadaes, N.R.; Nascimento, M.T.; Roatt, B.M.; Reis, A.B.; Viana, K.F.; Giunchetti, R.C.; Saraiva, E.M. Neutrophil Properties in Healthy and Leishmania infantum-Naturally Infected Dogs. Sci. Rep. 2019, 9, 6247. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.D.; Liang, Y.; Shelite, T.R.; Walker, D.H.; Melby, P.C.; Soong, L. Permissive and Protective Roles for Neutrophils in Leishmaniasis. Clin. Exp. Immunol. 2015, 182, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes-Costa, A.B.; Nascimento, M.T.; Froment, G.S.; Soares, R.P.; Morgado, F.N.; Conceicao-Silva, F.; Saraiva, E. Leishmania amazonensis Promastigotes Induce and Are Killed by Neutrophil Extracellular Traps. Proc. Natl. Acad. Sci. USA 2009, 106, 6748–6753. [Google Scholar] [CrossRef]
- Pereira, M.; Valério-Bolas, A.; Santos-Mateus, D.; Alexandre-Pires, G.; Santos, M.; Rodrigues, A.; Rocha, H.; Santos, A.; Martins, C.; Tomas, A.; et al. Canine Neutrophils Activate Effector Mechanisms in Response to Leishmania infantum. Vet. Parasitol. 2017, 248, 10–20. [Google Scholar] [CrossRef]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the Host-Pathogen Interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef]
- Terrazas, C.; Oghumu, S.; Varikuti, S.; Martinez-Saucedo, D.; Beverley, S.M.; Satoskar, A.R. Uncovering Leishmania-Macrophage Interplay Using Imaging Flow Cytometry. J. Immunol. Methods 2015, 423, 93–98. [Google Scholar] [CrossRef]
- Pollard, K.M.; Cauvi, D.M.; Toomey, C.B.; Morris, K.V.; Kono, D.H. Interferon-γ and Systemic Autoimmunity. Discov. Med. 2013, 16, 123. [Google Scholar]
- Schoenborn, J.R.; Wilson, C.B. Regulation of Interferon-Gamma during Innate and Adaptive Immune Responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar] [CrossRef]
- Hertzog, P.; Forster, S.; Samarajiwa, S. Systems Biology of Interferon Responses. J. Interferon Cytokine Res. 2011, 31, 5–11. [Google Scholar] [CrossRef]
- Baccala, R.; Kono, D.H.; Theofilopoulos, A.N. Interferons as Pathogenic Effectors in Autoimmunity. Immunol. Rev. 2005, 204, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Ambruso, D.R.; Briones, N.J.; Baroffio, A.F.; Murphy, J.R.; Tran, A.D.; Gowan, K.; Sanford, B.; Ellison, M.; Jones, K.L. In Vivo Interferon-Gamma Induced Changes in Gene Expression Dramatically Alter Neutrophil Phenotype. PLoS ONE 2022, 17, e0263370. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Beaman, B.L. Interferon-Gamma Activation of Polymorphonuclear Neutrophil Function. Immunology 2004, 112, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ellison, M.A.; Gearheart, C.M.; Porter, C.C.; Ambruso, D.R. IFN-γ Alters the Expression of Diverse Immunity Related Genes in a Cell Culture Model Designed to Represent Maturing Neutrophils. PLoS ONE 2017, 12, e0185956. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ochoa, P.; Sabate, D.; Homedes, J.; Ferrer, L. Use of the Nitroblue Tetrazolium Reduction Test for the Evaluation of Domperidone Effects on the Neutrophilic Function of Healthy Dogs. Vet. Immunol. Immunopathol. 2012, 146, 97–99. [Google Scholar] [CrossRef]
- Gómez-Ochoa, P.; Lara, A.; Couto, G.; Marcen, J.M.; Peris, A.; Gascón, M.; Castillo, J.A. The Nitroblue Tetrazolium Reduction Test in Canine Leishmaniosis. Vet. Parasitol. 2010, 172, 135–138. [Google Scholar] [CrossRef]
- Esfandiari, N.; Sharma, R.K.; Saleh, R.A.; Thomas, A.J.; Agarwal, A. Utility of the Nitroblue Tetrazolium Reduction Test for Assessment of Reactive Oxygen Species Production by Seminal Leukocytes and Spermatozoa. J. Androl. 2003, 24, 862–870. [Google Scholar] [CrossRef]
- Muniz-Junqueira, M.I.; de Paula-Coelho, V.N. Meglumine Antimonate Directly Increases Phagocytosis, Superoxide Anion and TNF-Alpha Production, but Only via TNF-Alpha It Indirectly Increases Nitric Oxide Production by Phagocytes of Healthy Individuals, in Vitro. Int. Immunopharmacol. 2008, 8, 1633–1638. [Google Scholar] [CrossRef]
- Martínez-Flórez, I.; Guerrero, M.J.; Dalmau, A.; Cabré, M.; Alcover, M.M.; Berenguer, D.; Good, L.; Fisa, R.; Riera, C.; Ordeix, L.; et al. Effect of Local Administration of Meglumine Antimoniate and Polyhexamethylene Biguanide Alone or in Combination with a Toll-like Receptor 4 Agonist for the Treatment of Papular Dermatitis Due to Leishmania infantum in Dogs. Pathogens 2023, 12, 821. [Google Scholar] [CrossRef]
- Baxarias, M.; Jornet-Rius, O.; Donato, G.; Mateu, C.; Alcover, M.M.; Pennisi, M.G.; Solano-Gallego, L. Signalment, Immunological and Parasitological Status and Clinicopathological Findings of Leishmania-Seropositive Apparently Healthy Dogs. Animals 2023, 13, 1649. [Google Scholar] [CrossRef]
- Riera, C.; Valladares, J.E.; Gállego, M.; Aisa, M.J.; Castillejo, S.; Fisa, R.; Ribas, N.; Carrió, J.; Alberola, J.; Arboix, M. Serological and Parasitological Follow-Up in Dogs Experimentally Infected with Leishmania infantum and Treated with Meglumine Antimoniate. Vet. Parasitol. 1999, 84, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Baxarias, M.; Solano-Gallego, L. Effect of Storage on Nitro Blue Tetrazolium Reduction Test in Dog Blood Samples. Vet. Clin. Pathol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Orellana, P.; González, N.; Baldassarre, A.; Álvarez-Fernández, A.; Ordeix, L.; Paradies, P.; Soto, M.; Solano-Gallego, L. Humoral Responses and Ex Vivo IFN-γ Production after Canine Whole Blood Stimulation with Leishmania infantum Antigen or KMP11 Recombinant Protein. Vet. Sci. 2022, 9, 116. [Google Scholar] [CrossRef]
- Carrillo, E.; Carrasco-Antón, N.; López-Medrano, F.; Salto, E.; Fernández, L.; San Martín, J.V.; Alvar, J.; Aguado, J.M.; Moreno, J. Cytokine Release Assays as Tests for Exposure to Leishmania, and for Confirming Cure from Leishmaniasis, in Solid Organ Transplant Recipients. PLoS Negl. Trop. Dis. 2015, 9, e0004179. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.; Pennisi, M.G.; Lupo, T.; Chicharro, C.; Solano-Gallego, L. Papular Dermatitis Due to Leishmania infantum Infection in Seventeen Dogs: Diagnostic Features, Extent of the Infection and Treatment Outcome. Parasites Vectors 2014, 7, 120. [Google Scholar] [CrossRef]
- Ordeix, L.; Rodríguez, A.; Martínez-Orellana, P.; Montserrat-Sangrà, S.; Solano-Gallego, L. Clinical follow up of a series of dogs with papular dermatitis due to Leishmania infantum. In Proceedings of the 29th Annual Congress of the ESVD-ECVD, Lausanne, Switzerland, 7–9 September 2017. [Google Scholar]
- Ordeix, L.; Solano-Gallego, L.; Fondevila, D.; Ferrer, L.; Fondati, A. Papular Dermatitis Due to Leishmania Spp. Infection in Dogs with Parasite-Specific Cellular Immune Responses. Vet. Dermatol. 2005, 16, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Bottero, E.; Poggi, M.; Viglione, M. Lesioni Papulari Indotte Da Leishmania spp. in 8 Cani Giovani. Veterinaria 2006, 20, 33–36. [Google Scholar]
- Noli, C.; Cornegliani, L. Leishmaniosi Bottoniforme. Descrizione Di Cinque Casi Italiani e Confronto Con La Letteratura. Quad. Dermatol. 2006, 1, 23–26. [Google Scholar]
- Almeida, B.F.M.; Narciso, L.G.; Bosco, A.M.; Pereira, P.P.; Braga, E.T.; Avanço, S.V.; Marcondes, M.; Ciarlini, P.C. Neutrophil Dysfunction Varies with the Stage of Canine Visceral Leishmaniosis. Vet. Parasitol. 2013, 196, 6–12. [Google Scholar] [CrossRef]
- Anwar, S.; Whyte, M.K.B. Neutrophil Apoptosis in Infectious Disease. Exp. Lung Res. 2007, 33, 519–528. [Google Scholar] [CrossRef]
- Silva, A.C.R.A.; de Almeida, B.F.M.; Soeiro, C.S.; Ferreira, W.L.; de Lima, V.M.F.; Ciarlini, P.C. Oxidative Stress, Superoxide Production, and Apoptosis of Neutrophils in Dogs with Chronic Kidney Disease. Can. J. Vet. Res. 2013, 77, 136. [Google Scholar] [PubMed]
- Barbosa, T.S.; Mori, C.K.; Ciarlini, P.C. Efeito Inibidor Do Soro Urêmico Sobre o Metabolismo Oxidativo Dos Neutrófilos de Cães. Arq. Bras. Med. Veterinária Zootec. 2010, 62, 1352–1358. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative Stress and Apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Kennedy, A.D.; Deleo, F.R. Neutrophil Apoptosis and the Resolution of Infection. Immunol. Res. 2009, 43, 25–61. [Google Scholar] [CrossRef] [PubMed]
- Abbehusen, M.M.C.; Almeida, D.A.V.; Solcà, S.M.; Da Silva Pereira, L.; Costa, D.J.; Gil-Santana, L.; Bozza, P.T.; Fraga, D.B.M.; Veras, P.S.T.; Dos-Santos, W.L.C.; et al. Clinical and Immunopathological Findings during Long Term Follow-up in Leishmania infantum Experimentally Infected Dogs. Sci. Rep. 2017, 7, 15914. [Google Scholar] [CrossRef]
- Reis, A.B.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Carvalho, M.G.; Mayrink, W.; França-Silva, J.C.; Giunchetti, R.C.; Genaro, O.; Corrêa-Oliveira, R. Parasite Density and Impaired Biochemical/Hematological Status Are Associated with Severe Clinical Aspects of Canine Visceral Leishmaniasis. Res. Vet. Sci. 2006, 81, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Almeida, V.; Lima, I.; Fraga, D.; Carrillo, E.; Moreno, J.; Dos-Santos, W.L.C. Hematological Changes in Dogs with Visceral Leishmaniasis Are Associated with Increased Ifn-γ and Tnf Gene Expression Levels in the Bone Marrow. Microorganisms 2021, 9, 1618. [Google Scholar] [CrossRef]
- Gueirard, P.; Laplante, A.; Rondeau, C.; Milon, G.; Desjardins, M. Trafficking of Leishmania donovani Promastigotes in Non-Lytic Compartments in Neutrophils Enables the Subsequent Transfer of Parasites to Macrophages. Cell. Microbiol. 2008, 10, 100–111. [Google Scholar] [CrossRef]
- Brandonisio, O.; Panaro, M.A.; Marzio, R.; Marangi, A.; Faliero, S.M.; Jirillo, E. Impairment of the Human Phagocyte Oxidative Responses Caused by Leishmania Lipophosphoglycan (LPG): In Vitro Studies. FEMS Immunol. Med. Microbiol. 1994, 8, 57–62. [Google Scholar] [CrossRef]
- Vuotto, M.L.; De Luna, R.; Ielpo, M.T.L.; De Sole, P.; Moscatiello, V.; Simeone, I.; Gradoni, L.; Mancino, D. Chemiluminescence Activity in Whole Blood Phagocytes of Dogs Naturally Infected with Leishmania infantum. Luminescence 2000, 15, 251–255. [Google Scholar] [CrossRef]
- Ciarlini, P.C.; Valadares, T.C.; Ikeda-Garcia, F.A.; Marcondes, M.; De Lima, V.M.F. Leucograma E Metabolismo Oxidativo dos Neutrófilos de Cães com Leishmaniose Visceral Antes e Após O Tratamento com Antimoniato de Meglumina e Alopurinol. Ciência Anim. Bras. 2010, 11, 369–375. [Google Scholar] [CrossRef]
- Santos-Gomes, G.M.; Rosa, R.; Leandro, C.; Cortes, S.; Romão, P.; Silveira, H. Cytokine Expression during the Outcome of Canine Experimental Infection by Leishmania infantum. Vet. Immunol. Immunopathol. 2002, 88, 21–30. [Google Scholar] [CrossRef]
- do Nascimento, P.R.P.; Martins, D.R.A.; Monteiro, G.R.G.; Queiroz, P.V.; Freire-Neto, F.P.; Queiroz, J.W.; Morais Lima, Á.L.; Jeronimo, S.M.B. Association of Pro-Inflammatory Cytokines and Iron Regulatory Protein 2 (IRP2) with Leishmania Burden in Canine Visceral Leishmaniasis. PLoS ONE 2013, 8, e73873. [Google Scholar] [CrossRef] [PubMed]
- Aslan, H.; Oliveira, F.; Meneses, C.; Castrovinci, P.; Gomes, R.; Teixeira, C.; Derenge, C.A.; Orandle, M.; Gradoni, L.; Oliva, G.; et al. New Insights Into the Transmissibility of Leishmania infantum from Dogs to Sand Flies: Experimental Vector-Transmission Reveals Persistent Parasite Depots at Bite Sites. J. Infect. Dis. 2016, 213, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Orellana, P.; Marí-Martorell, D.; Montserrat-Sangrà, S.; Ordeix, L.; Baneth, G.; Solano-Gallego, L. Leishmania infantum-Specific IFN-γ Production in Stimulated Blood from Dogs with Clinical Leishmaniosis at Diagnosis and during Treatment. Vet. Parasitol. 2017, 248, 39–47. [Google Scholar] [CrossRef]
- Esch, K.J.; Juelsgaard, R.; Martinez, P.A.; Jones, D.E.; Petersen, C.A. PD-1-Mediated T Cell Exhaustion during Visceral Leishmaniasis Impairs Phagocyte Function. J. Immunol. 2013, 191, 5542. [Google Scholar] [CrossRef]
- Scott, P.; Riley, E.M. Acquired Immunity to Intracellular Protozoa. Immune Response Infect. 2014, 24, 301–311. [Google Scholar] [CrossRef]



| Qualitative Characteristics | Healthy Seronegative (n = 25) | Healthy Seropositive (n = 21) | Stage I (n = 25) | Stage II (n = 41) | Stage III–IV (n = 16) | p-Value (Chi-Square, df) | |
|---|---|---|---|---|---|---|---|
| Breed (n = 128) | Crossbred (n = 54) | 9 (36.00%) | 11 (52.38%) | 14 (56.00%) | 16 (39.02%) | 4 (25.00%) | 0.25 (5.30, 4) |
| Purebred (n = 74) | 16 (64.00%) | 10 (47.62%) | 11 (44.00%) | 25 (60.98%) | 12 (75.00%) | ||
| Sex (n = 128) | Male (n = 74) | 12 (48.00%) | 10 (47.62%) | 16 (64.00%) | 23 (56.10%) | 13 (81.25%) | 0.2 (5.90, 4) |
| Female (n = 54) | 13 (52.00%) | 11 (52.38%) | 9 (36.00%) | 18 (43.90%) | 3 (18.75%) | ||
| NBT (n = 128) | Increased (n = 46) | 4 (16.00%) | 5 (23.81%) | 23 (92.00%) | 10 (24.39%) | 3 (18.75%) | <0.0001 * (44.60, 4) |
| WNL (n = 82) | 21 (84.00%) | 16 (76.19%) | 2 (8.00%) | 31 (75.61%) | 13 (81.25%) | ||
| Serology (n = 128) | Positive (n = 84) | 0 (0.00%) | 21 (100.00%) | 6 (24.00%) | 41 (100.00%) | 16 (100.00%) | <0.0001 * (107.80, 4) |
| Negative (n = 44) | 25 (100.00%) | 0 (0.00%) | 19 (76.00%) | 0 (0.00%) | 0 (0.00%) | ||
| IFN-γ (n = 97) | Producers (n = 34) | 0 (0.00%) | 9 (60.00%) | 12 (50.00%) | 9 (26.47%) | 4 (30.77%) | 0.01 * (12.54, 4) |
| Non-prod. (n = 63) | 10 (100.00%) | 6 (40.00%) | 12 (50.00%) | 25 (73.53%) | 9 (69.23%) | ||
| Quantitative Characteristics Median (Min–Max) | Healthy Seronegative (n = 25) | Healthy Seropositive (n = 21) | Stage I (n = 25) | Stage II (n = 41) | Stage III–IV (n = 16) | p-Value (Kruskal-Wallis Test) |
|---|---|---|---|---|---|---|
| Age (years) | 1.30 (1.00–1.60) | 5.00 (1.00–13.00) | 0.50 (0.25–7.00) | 4.00 (1.00–13.00) | 7.50 (1.00–11.00) | <0.0001 * |
| NBT (%) | 4.13 (1.15–17.96) | 7.65 (3.98–21.74) | 17.17 (7.33–31.50) | 6.50 (1.50–28.70) | 7.50 (3.00–16.75) | <0.0001 * |
| IFN-γ (pg/mL) | 0 (0–109.50) | 204.10 (0–4763.00) | 127.90 (0–3998.00) | 9.00 (0–5086.00) | 3.48 (0–548.80) | 0.001 * |
| Serology (ELISA units) | 5.50 (0.30–28.49) | 133.90 (75.79–956.30) | 21.42 (2.76–227.10) | 1894.00 (57.16–10,293.00) | 1857.00 (96.79–11,114.00) | <0.0001 * |
| NBT (%) | NBT Status (Cut-Off = 10.8) | IFN-γ | IFN-γ Status | Serology | Serological Status | Clinical Evolution | |
|---|---|---|---|---|---|---|---|
| Group PD-A (n = 22) | Median: 17.59 (min: 9.67, max: 31.50) | 22/22 Over cutoff | Median: 171.90 (min: 0, max: 3998.00) | 10/10 Producers | Median: 18.21 (min: 2.76, max: 227.10) | 5/22 positive | Partial or total improvement |
| Group PD-B (n = 3) | Median: 8.80 (min: 7.33, max: 20.83) | 2/3 WNL | Median: 7.03 (min: 0, max: 234.20) | 1/3 Producer | Median: 27.41 (min: 22.34, max: 79.90) | 1/3 positive | - |
| Dog PD-B.1 | 8.80 | WNL | 7.03 | Non-producer | 79.90 | Positive | Worsening of cutaneous clinical signs: diffuse worsening. |
| Dog PD-B.2 | 7.33 | WNL | 234.24 | Producer | 27.41 | Negative | Worsening of cutaneous clinical signs: ulcerative lesion at the nose bridge. |
| Dog PD-B.3 | 20.83 | Over cutoff | 0 | Non-producer | 22.34 | Negative | Worsening of cutaneous clinical signs: ulcerative lesion at the level of the right lower eyelid. |
| Median (Min–Max) | Healthy Seronegative | Healthy Seropositive | Stage I | Stage II | Stage III–IV | p-Value |
|---|---|---|---|---|---|---|
| Leucocytes (cell/µL) | 11,330 (6390–15,020) | 9710 (3360–16,920) | 11,180 (6200–20,540) | 10,470 (3990–19,430) | 9650 (3420–13,630) | 0.115 |
| Neutrophils (cell/µL) | 6675 (3706–9913) | 5889 (2755–11,844) | 6939 (3300–17,860) | 7171 (3032–14,378) | 6482 (2633–10,223) | 0.936 |
| Lymphocytes (cell/µL) | 3006 (1968–3930) | 1711 (235–3849) | 3590 (900–5800) | 1921 (518–4696) | 1472 (616–2385) | <0.0001 * |
| Eosinophils (cell/µL) | 409 (300–934) | 514 (63–2369) | 500 (20–1100) | 479 (0–2176) | 386 (0–1204) | 0.541 |
| Monocytes (cell/µL) | 668 (313–1502) | 413 (34–1157) | 750 (100–1310) | 517 (129–1269) | 560 (171–1204) | 0.003 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blasi-Brugué, C.; Martínez-Flórez, I.; Baxarias, M.; del Rio-Velasco, J.; Solano-Gallego, L. Exploring the Relationship between Neutrophil Activation and Different States of Canine L. infantum Infection: Nitroblue Tetrazolium Test and IFN-γ. Vet. Sci. 2023, 10, 572. https://doi.org/10.3390/vetsci10090572
Blasi-Brugué C, Martínez-Flórez I, Baxarias M, del Rio-Velasco J, Solano-Gallego L. Exploring the Relationship between Neutrophil Activation and Different States of Canine L. infantum Infection: Nitroblue Tetrazolium Test and IFN-γ. Veterinary Sciences. 2023; 10(9):572. https://doi.org/10.3390/vetsci10090572
Chicago/Turabian StyleBlasi-Brugué, Carles, Icíar Martínez-Flórez, Marta Baxarias, Joan del Rio-Velasco, and Laia Solano-Gallego. 2023. "Exploring the Relationship between Neutrophil Activation and Different States of Canine L. infantum Infection: Nitroblue Tetrazolium Test and IFN-γ" Veterinary Sciences 10, no. 9: 572. https://doi.org/10.3390/vetsci10090572






