Invasive Bacterial Infections of the Musculoskeletal and Central Nervous System during Pig Rearing: Detection Frequencies of Different Pathogens and Specific Streptococcus suis Genotypes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Background Data and Gross Examination
2.3. Tissue Selection
2.4. Bacteriology
2.5. cps Typing and Virulence-Associated Gene Profiling
2.6. Histopathology
2.7. Statistical Analysis
3. Results
3.1. Gross Lesions and Background Data
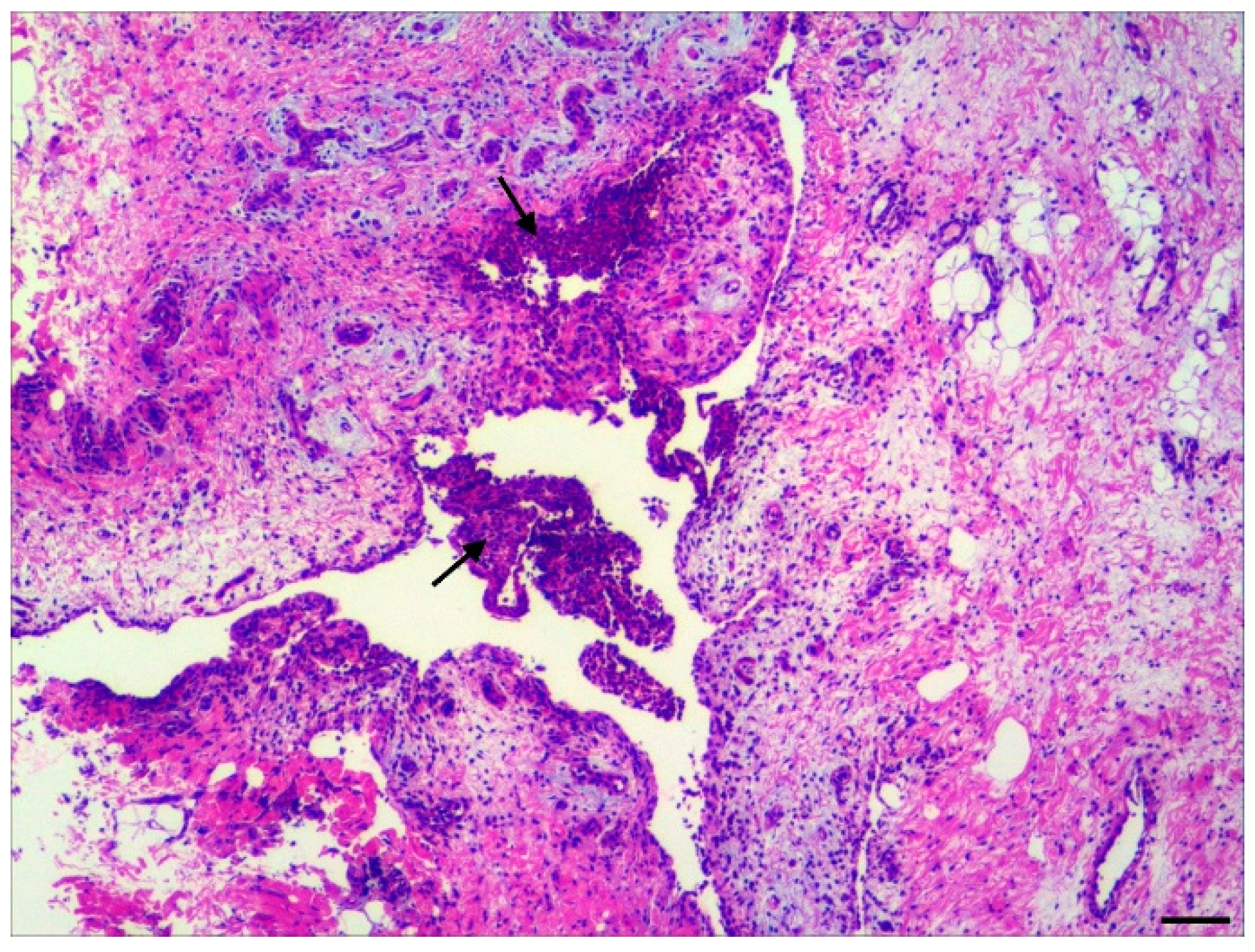
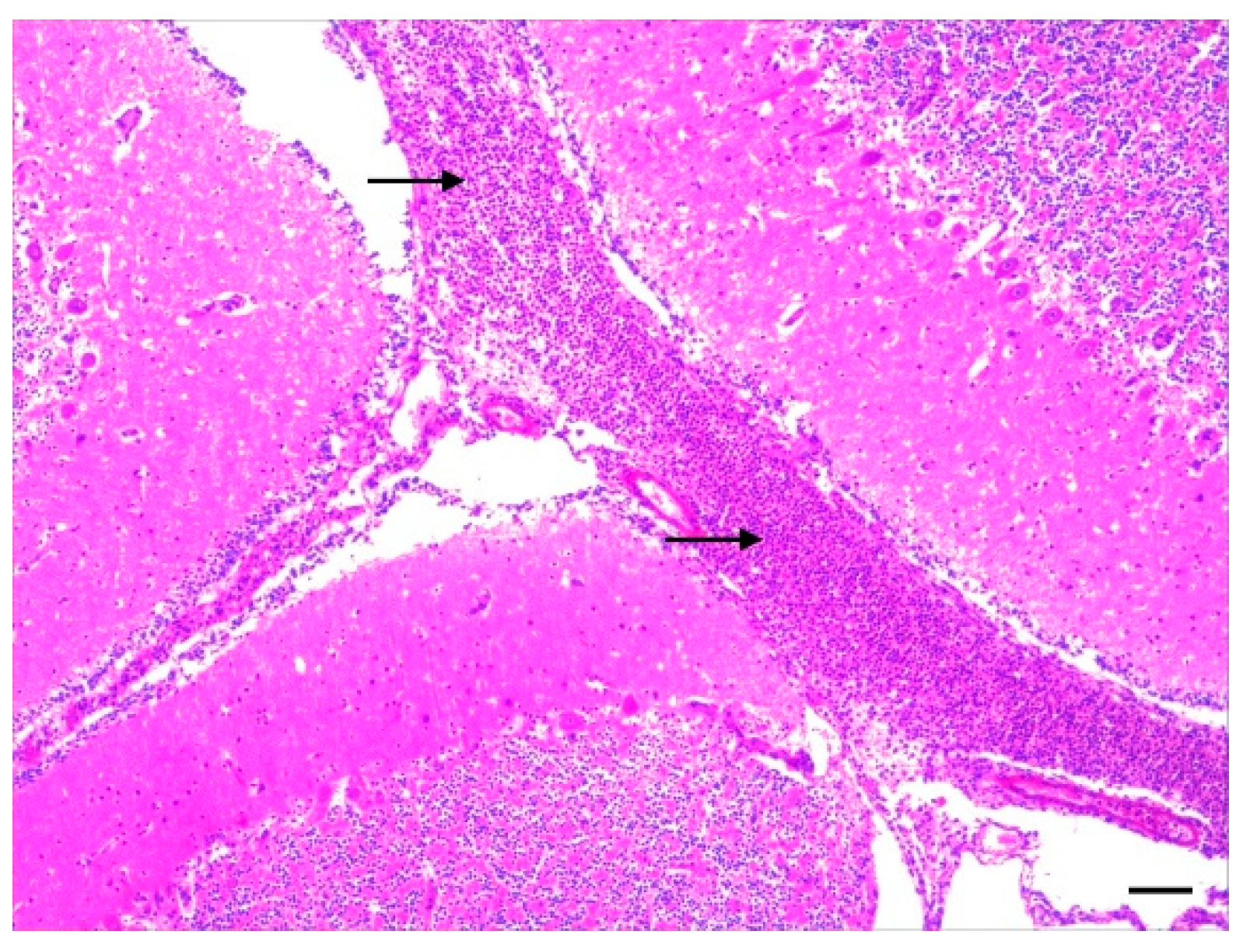
3.2. Histopathology
3.3. Bacteriology of Joint and Meningeal Swabs
3.4. Arthritis: Association between Detected Pathogens, Age Category, and Histopathological Lesions
3.5. CNS: Association between Detected Pathogens, Age Category, and Histopathological Lesions
3.6. S. suis cps Types Isolated
3.7. S. suis Positive Animals
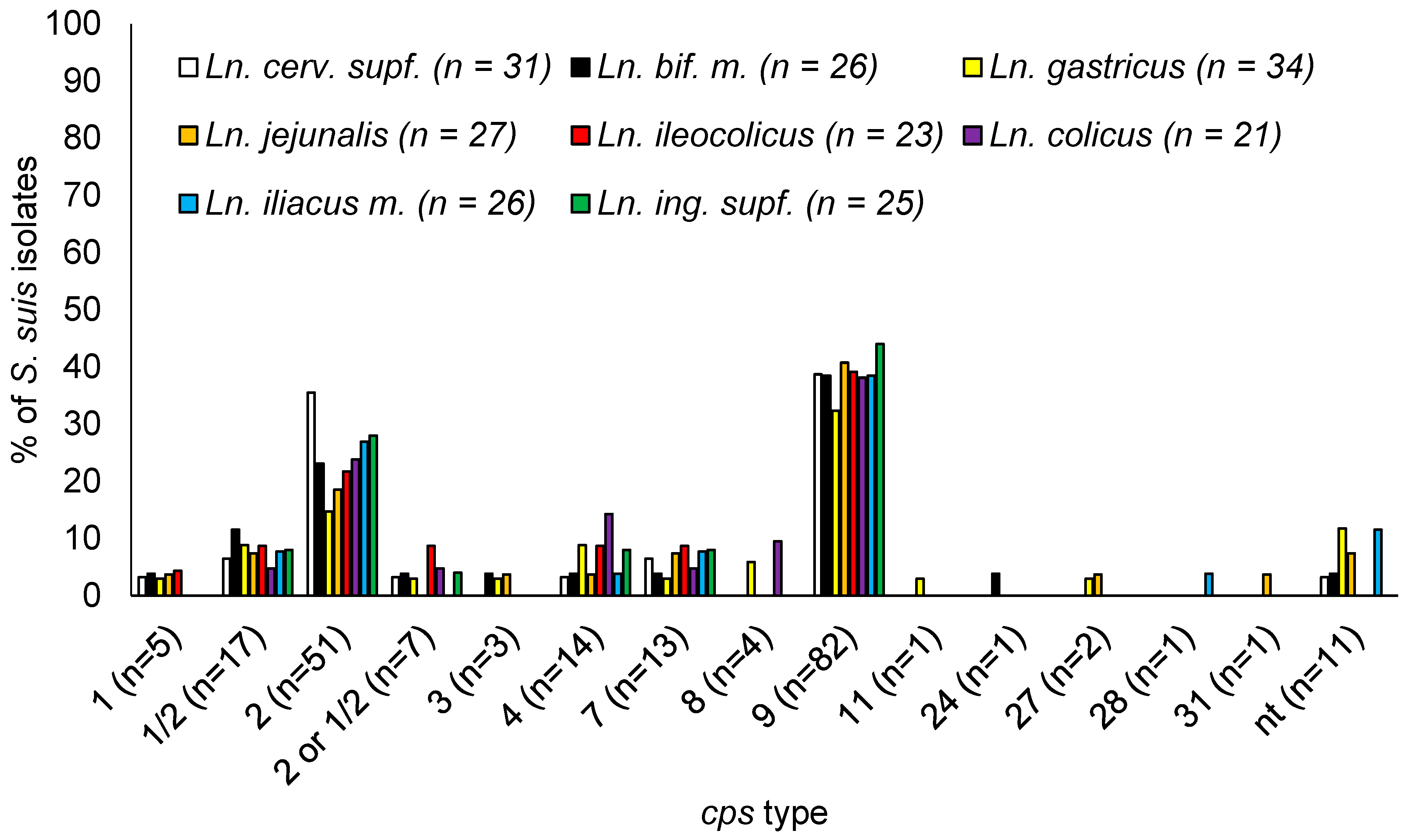
3.8. Occurrence of S. suis Genotypes per Site
4. Discussion
4.1. Arthritis
4.2. Meningitis
4.3. S. suis Isolates
4.4. Occurrence of S. suis by Animal and Age Category
4.5. Occurrence of S. suis in Lymph Nodes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madson, D.M.; Arruda, P.H.E.; Arruda, B.L. Nervous and Locomotor System. In Diseases of Swine; Zimmermann, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; Wiley-Blackwell: Chichester, UK, 2019; Volume 11, pp. 339–372. [Google Scholar]
- Neumann, E.J.; Ramirez, A.; Schwartz, K.J. Swine Disease Manual; America Association of Swine Veterinarians: Perry, KY, USA, 2009; Volume 4. [Google Scholar]
- Gottschalk, M.; Segura, M. Streptococcosis. In Diseases of Swine; Zimmermann, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; Wiley-Blackwell: Chichester, UK, 2019; Volume 11, pp. 934–950. [Google Scholar]
- Rieckmann, K.; Pendzialek, S.M.; Vahlenkamp, T.; Baums, C.G. A critical review speculating on the protective efficacies of autogenous Streptococcus suis bacterins as used in Europe. Porc. Health Manag. 2020, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Prufer, T.L.; Rohde, J.; Verspohl, J.; Rohde, M.; de Greeff, A.; Willenborg, J.; Valentin-Weigand, P. Molecular typing of Streptococcus suis strains isolated from diseased and healthy pigs between 1996–2016. PLoS ONE 2019, 14, e0210801. [Google Scholar] [CrossRef] [PubMed]
- MacInnes, J.I.; Gottschalk, M.; Lone, A.G.; Metcalf, D.S.; Ojha, S.; Rosendal, T.; Watson, S.B.; Friendship, R.M. Prevalence of Actinobacillus pleuropneumoniae, Actinobacillus suis, Haemophilus parasuis, Pasteurella multocida, and Streptococcus suis in representative Ontario swine herds. Can. J. Vet. Res. 2008, 72, 242–248. [Google Scholar] [PubMed]
- Staats, J.J.; Feder, I.; Okwumabua, O.; Chengappa, M.M. Streptococcus suis: Past and Present. Vet. Res. Commun. 1997, 21, 381–407. [Google Scholar] [CrossRef]
- Wisselink, H.J.; Smith, H.E.; Stockhofe-Zurwieden, N.; Peperkamp, K.; Vecht, U. Distribution of capsular types and production of muramidase-released protein (MRP) and extracellular factor (EF) of Streptococcus suis strains isolated from diseased pigs in seven European countries. Vet. Microbiol. 2000, 40, 2922–2929. [Google Scholar] [CrossRef] [PubMed]
- Reams, R.Y.; Glickman, L.T.; Harrington, D.D.; Bowersock, T.L.; Thacker, H.L. Streptococcus suis infection in swine: A retrospective study of 256 cases. Part I. Epidemiologic factors and antibiotic susceptibility patterns. J. Vet. Diagn. Investig. 1993, 5, 363–367. [Google Scholar] [CrossRef]
- Boetner, A.G.; Binder, M.; Bille-Hansen, V. Streptococcus suis infections in Danish pigs and experimental infection with Streptococcus suis serotype 7. Acta Pathol. Microbiol. Immunol. Scand. B 1987, 95, 233–239. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Jorsal, S.E.; Jensen, N.E. Serological characterization and antimicrobial susceptibility of Streptococcus suis isolates from diagnostic samples in Denmark during 1995 and 1996. Vet. Microbiol. 1998, 60, 59–66. [Google Scholar] [CrossRef]
- Rieckmann, K.; Seydel, A.; Szewczyk, K.; Klimke, K.; Rungelrath, V.; Baums, C.G. Streptococcus suis cps7: An emerging virulent sequence type (ST29) shows a distinct, IgM-determined pattern of bacterial survival in blood of piglets during the early adaptive immune response after weaning. Vet. Res. 2018, 49, 48. [Google Scholar] [CrossRef]
- Fittipaldi, N.; Segura, M.; Grenier, D.; Gottschalk, M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012, 7, 259–279. [Google Scholar] [CrossRef]
- Arends, J.P.; Hartwig, N.; Rudolphy, M.; Zanen, H.C. Carrier rate of Streptococcus suis capsular type 2 in palatine tonsils of slaughtered pigs. J. Clin. Microbiol. 1984, 20, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M.; Segura, M. The pathogenesis of the meningitis caused by Streptococcus suis: The unresolved questions. Vet. Microbiol. 2000, 76, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhu, X.; Jing, H.; Du, H.; Segura, M.; Zheng, H.; Kan, B.; Wang, L.; Bai, X.; Zhou, Y.; et al. Streptococcus suis sequence type 7 outbreak, Sichuan, China. EID J. 2006, 12, 1203–1258. [Google Scholar]
- Gottschalk, M.; Segura, M.; Xu, J. Streptococcus suis infections in humans: The Chinese experience and the situation in North America. Anim. Health Res. Rev. 2007, 8, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Swildens, B.; Stockhofe-Zurwieden, N.; Van Der Meulen, J.; Wisselink, H.; Nielen, M.; Niewold, T. Intestinal translocation of Streptococcus suis type 2 EF+ in pigs. Vet. Microbiol. 2004, 103, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, M.L.; Schultsz, C. A hypothetical model of host-pathogen interaction of Streptococcus suis in the gastro-intestinal tract. Gut Microbes 2016, 7, 154–162. [Google Scholar] [CrossRef]
- Warneboldt, F.; Sander, S.J.; Beineke, A.; Valentin-Weigand, P.; Kamphues, J.; Baums, C.G. Clearance of Streptococcus suis in Stomach Contents of Differently Fed Growing Pigs. Pathogens 2016, 5, 56. [Google Scholar] [CrossRef]
- Silva, L.M.G.; Baums, C.G.; Rehm, T.; Wisselink, H.J.; Goethe, R.; Valentin-Weigand, P. Virulence-associated gene profiling of Streptococcus suis isolates by PCR. Vet. Microbiol. 2006, 115, 117–127. [Google Scholar] [CrossRef]
- de Greeff, A.; Wisselink, H.J.; de Bree, F.M.; Schultsz, C.; Baums, C.G.; Thi, H.N.; Stockhofe-Zurwieden, N.; Smith, H.E. Genetic diversity of Streptococcus suis isolates as determined by comparative genome hybridization. BMC Microbiol. 2011, 11, 161. [Google Scholar] [CrossRef]
- Kerdsin, A.; Dejsirilert, S.; Akeda, Y.; Sekizaki, T.; Hamada, S.; Gottschalk, M.; Oishi, K. Fifteen Streptococcus suis serotypes identified by multiplex PCR. J. Med. Microbiol. 2012, 61, 1669–1672. [Google Scholar] [CrossRef]
- Okura, M.; Lachance, C.; Osaki, M.; Sekizaki, T.; Maruyama, F.; Nozawa, T.; Nakagawa, I.; Hamada, S.; Rossignol, C.; Gottschalk, M.; et al. Development of a two-step multiplex PCR assay for typing of capsular polysaccharide synthesis gene clusters of Streptococcus suis. J. Clin. Microbiol. 2014, 52, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, S.; Okura, M.; Takamatsu, D.; Corsaut, L.; Gottschalk, M. Development of a mismatch amplification mutation assay to correctly serotype isolates of Streptococcus suis serotypes 1, 2, 1/2, and 14. J. Vet. Diagn. Investig. 2020, 32, 490–494. [Google Scholar] [CrossRef]
- Athey, T.B.; Teatero, S.; Lacouture, S.; Takamatsu, D.; Gottschalk, M.; Fittipaldi, N. Determining Streptococcus suis serotype from short-read whole-genome sequencing data. BMC Microbiol. 2016, 16, 162. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.; Bornemann, N.; Lehnert, S.; de Greeff, A.; Strutzberg-Minder, K.; Rieckmann, K.; Baums, C.G. Survival patterns of Streptococcus suis serotypes 1 and 14 in porcine blood indicate cross-reactive bactericidal antibodies in naturally infected pigs. Vet. Microbiol. 2021, 260, 109183. [Google Scholar] [CrossRef]
- Hill, B.; Corney, B.; Wagner, T. Importance of Staphylococcus hyicus ssp. hyicus as a cause of arthritis in pigs up to 12 weeks of age. Aust. Vet. J. 1996, 73, 179–181. [Google Scholar]
- Nielsen, N.C.; Bille, N.; Larsen, J.L.; Svendsen, J. Preweaning mortality in pigs. Nord. Vet. Med. 1975, 27, 529–543. [Google Scholar]
- Smith, W.J.; Mitchell, C.D. Observations on injuries to suckled pigs confined on perforated floors with special reference to expanded metal. Pig. J. 1976, 1, 91–104. [Google Scholar]
- Cloutier, G.; D’Allaire, S.; Martinez, G.; Surprenant, C.; Lacouture, S.; Gottschalk, M. Epidemiology of Streptococcus suis serotype 5 infection in a pig herd with and without clinical disease. Vet. Microbiol. 2003, 97, 135–151. [Google Scholar] [CrossRef]
- Baums, C.G.; Bruggemann, C.; Kock, C.; Beineke, A.; Waldmann, K.H.; Valentin-Weigand, P. Immunogenicity of an autogenous Streptococcus suis bacterin in preparturient sows and their piglets in relation to protection after weaning. Clin. Vaccine Immunol. 2010, 17, 1589–1597. [Google Scholar] [CrossRef]
- Corsaut, L.; Misener, M.; Canning, P.; Beauchamp, G.; Gottschalk, M.; Segura, M. Field Study on the Immunological Response and Protective Effect of a Licensed Autogenous Vaccine to Control Streptococcus suis Infections in Post-Weaned Piglets. Vaccines 2020, 8, 384. [Google Scholar] [CrossRef]
- Corsaut, L.; Martelet, L.; Goyette-Desjardins, G.; Beauchamp, G.; Denicourt, M.; Gottschalk, M.; Segura, M. Immunogenicity study of a Streptococcus suis autogenous vaccine in preparturient sows and evaluation of passive maternal immunity in piglets. BMC Vet. Res. 2021, 17, 72. [Google Scholar] [CrossRef]
- McGavin, M.D.; Zachary, J.F. Pathologic Basis of Veterinary Disease, 4th ed.; Mosby: Maryland Heights, MO, USA, 2006; p. 1488. [Google Scholar]
- Beineke, A.; Bennecke, K.; Neis, C.; Schroder, C.; Waldmann, K.H.; Baumgartner, W.; Valentin-Weigand, P.; Baums, C.G. Comparative evaluation of virulence and pathology of Streptococcus suis serotypes 2 and 9 in experimentally infected growers. Vet. Microbiol. 2008, 128, 423–430. [Google Scholar] [CrossRef]
- Okwumabua, O.; O’Connor, M.; Shull, E. A polymerase chain reaction (PCR) assay specific for Streptococcus suis based on the gene encoding the glutamate dehydrogenase. FEMS Microbiol. Lett. 2003, 218, 79–84. [Google Scholar] [CrossRef]
- Tien, L.H.T.; Sugiyama, N.; Duangsonk, K.; Tharavichitkul, P.; Osawa, R. Phenotypic and PCR-based identification of bacterial strains isolated from patients with suspected streptococcus suis infection in northern Thailand. Jpn. J. Infect. Dis. 2012, 65, 171–174. [Google Scholar] [CrossRef]
- Ishida, S.; le Tien, H.T.; Osawa, R.; Tohya, M.; Nomoto, R.; Kawamura, Y.; Takahashi, T.; Kikuchi, N.; Kikuchi, K.; Sekizaki, T. Development of an appropriate PCR system for the reclassification of Streptococcus suis. J. Microbiol. Methods 2014, 107, 66–70. [Google Scholar] [CrossRef]
- Okura, M.; Osaki, M.; Nomoto, R.; Sakura, A.; Osawa, R.; Sekizaki, T.; Takamatsu, D. Current Taxonomical Situation of Streptococcus suis. Pathogens 2016, 5, 45. [Google Scholar] [CrossRef]
- Luque, I.; Tarradas, C.; Astorga, A.; Perea, A.; Wisselink, H.J.; Vecht, U. The presence of muramidase released protein and extracellular factor protein in various serotypes of Streptococcus suis isolated from diseased and healthy pigs in Spain. Res. Vet. Sci. 1999, 66, 69–72. [Google Scholar] [CrossRef]
- Fittipaldi, N.; Fuller, T.; Teel, J.; Wilson, T.; Wolfram, T.; Lowery, D.; Gottschalk, M. Serotype distribution and production of muramidase-released protein, extracellular factor and suilysin by field strains of Streptococcus suis isolated in the United States. Vet. Microbiol. 2009, 139, 310–317. [Google Scholar] [CrossRef]
- Baums, C.G.; Verkuhlen, G.J.; Rehm, T.; Silva, L.M.; Beyerbach, M.; Pohlmeyer, K.; Valentin-Weigand, P. Prevalence of Streptococcus suis genotypes in wild boars of Northwestern Germany. Appl. Environ. Microbiol. 2007, 73, 711–717. [Google Scholar] [CrossRef]
- Blume, V.; Luque, I.; Vela, A.I.; Borge, C.; Maldonado, A.; Domínguez, L.; Tarradas, C.; Fernández-Garayzábal, J.F. Genetic and virulence-phenotype characterization of serotypes 2 and 9 of Streptococcus suis swine isolates Int. Microbiol. 2009, 12, 161–166. [Google Scholar] [CrossRef]
- Allgaier, A.; Goethe, R.; Wisselink, H.J.; Smith, H.E.; Valentin-Weigand, P. Relatedness of Streptococcus suis isolates of various serotypes and clinical backgrounds as evaluated by macrorestriction analysis and expression of potential virulence traits. J. Clin. Microbiol. 2001, 39, 445–453. [Google Scholar] [CrossRef]
- Wisselink, H.J.; Joosten, J.J.; Smith, H.E. Multiplex PCR assays for simultaneous detection of six major serotypes and two virulence-associated phenotypes of Streptococcus suis in tonsillar specimens from pigs. J. Clin. Microbiol. 2002, 40, 2922–2929. [Google Scholar] [CrossRef]
- Vecht, U.; Wisselink, H.J.; van Dijk, J.E.; Smith, H.E. Virulence of Streptococcus suis type 2 strains in newborn germfree pigs depends on phenotype. Infect. Immun. 1992, 60, 550–556. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, H.; Gottschalk, M.; Bai, X.; Lan, R.; Ji, S.; Liu, H.; Xu, J. Development of multiplex PCR assays for the identification of the 33 serotypes of Streptococcus suis. PLoS ONE 2013, 8, e72070. [Google Scholar] [CrossRef]
- Kerdsin, A.; Akeda, Y.; Hatrongjit, R.; Detchawna, U.; Sekizaki, T.; Hamada, S.; Gottschalk, M.; Oishi, K. Streptococcus suis serotyping by a new multiplex PCR. J. Med. Microbiol. 2014, 63, 824–830. [Google Scholar] [CrossRef]
- Roy, D.; Athey, T.B.T.; Auger, J.-P.; Goyette-Desjardins, G.; van Calsteren, M.-R.; Takamatsu, D.; Okura, M.; Teatero, S.; Alcorlo, M.; Hermoso, J.A.; et al. A single amino acid polymorphism in the glycosyltransferase Cps K defines four Streptococcus suis serotypes. Sci. Rep. 2017, 7, 4066. [Google Scholar] [CrossRef]
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef]
- Vela, A.I.; Goyache, J.; Tarradas, C.; Luque, I.; Mateos, A.; Moreno, M.A.; Borge, C.; Perea, J.A.; Dominguez, L.; Fernandez-Garayzabal, J.F. Analysis of genetic diversity of Streptococcus suis clinical isolates from pigs in Spain by pulsed-field gel electrophoresis. J. Clin. Microbiol. 2003, 41, 2498–2502. [Google Scholar] [CrossRef]
- Tarradas, C.; Perea, A.; Vela, A.; Goyache, J.; Dominguez, L.; Fernández-Garaizabal, J.; Borge, C.; Huerta, B.; Luque, I. Distribution of serotypes of Streptococcus suis isolated from diseased pigs in Spain. Vet. Rec. 2004, 154, 665–666. [Google Scholar] [CrossRef]
- Luque, I.; Blume, V.; Borge, C.; Vela, A.I.; Perea, J.A.; Márquez, J.M.; Fernández-Garayzábal, J.F.; Tarradas, C. Genetic analysis of Streptococcus suis isolates recovered from diseased and healthy carrier pigs at different stages of production on a pig farm. Vet. J. 2010, 186, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Schultsz, C.; Jansen, E.; Keijzers, W.; Rothkamp, A.; Duim, B.; Wagenaar, J.A.; van der Ende, A. Differences in the population structure of invasive Streptococcus suis strains isolated from pigs and from humans in The Netherlands. PLoS ONE 2012, 7, e33854. [Google Scholar] [CrossRef]
- Scherrer, S.; Rosato, G.; Spoerry Serrano, N.; Stevens, M.J.A.; Rademacher, F.; Schrenzel, J.; Gottschalk, M.; Stephan, R.; Peterhans, S. Population structure, genetic diversity and pathotypes of Streptococcus suis isolated during the last 13 years from diseased pigs in Switzerland. Vet. Res. 2020, 51, 85. [Google Scholar] [CrossRef]
- Oehlmann, S.; Krieger, A.-K.; Gisch, N.; Meurer, M.; de Buhr, N.; von Köckritz-Blickwede, M.; Schütze, N.; Baums, C.G. D-Alanylation of Lipoteichoic Acids in Streptococcus suis Reduces Association With Leukocytes in Porcine Blood. Front. Microbiol. 2022, 13, 822369. [Google Scholar]
- Bennel, M.A.; Husband, A.J. Route of lymphocyte migration in pigs. I. Lymphocyte circulation in gut-associated lymphoid tissue. Immunology 1981, 42, 469–474. [Google Scholar]
- Su, Y.; Yao, W.; Perez-Gutierrez, O.N.; Smidt, H.; Zhu, W.Y. Changes in abundance of Lactobacillus spp. and Streptococcus suis in the stomach, jejunum and ileum of piglets after weaning. FEMS Microbiol. Ecol. 2008, 66, 546–555. [Google Scholar] [CrossRef]
- Baele, M.; Chiers, K.; Devriese, L.A.; Smith, H.E.; Wisselink, H.J.; Vaneechoutte, M.; Haesebrouck, F. The Gram-positive tonsillar and nasal flora of piglets before and after weaning. J. Appl. Microbiol. 2001, 91, 997–1003. [Google Scholar] [CrossRef]
- Segura, M.; Calzas, C.; Grenier, D.; Gottschalk, M. Initial steps of the pathogenesis of the infection caused by Streptococcus suis: Fighting against nonspecific defenses. FEBS Lett. 2016, 590, 3772–3799. [Google Scholar] [CrossRef] [PubMed]
| Target Gen | GenBank Accession Number | Amplicon [bp] | Reference |
|---|---|---|---|
| gdh | CP000408 | 954 | IVD GmbH (Validation 2012) |
| epf | X71881 | 708 | IVD GmbH (Validation 2012) |
| cps 1 or 14 | AF155804 | 555 | IVD GmbH (Validation 2012) |
| cps 2 or 1/2 | AF118389 | 443 | based on [21] |
| cps 7 | AF164515 | 379 | [21] |
| cps 9 | AF155805 | 303 | [21] |
| sly | Z36907 | 248 | [21] |
| mrp | X64450 | 188 | [21] |
| srtD | AB066354 | 139 | based on [22] |
| Result of Bacteriological Culture of Joint Swab (%) | Age Category (Number of Examined Joint Swabs) | |||
|---|---|---|---|---|
| Suckling Piglets (n = 25 *) | Weaning Piglets (n = 77) | Fattening Pigs (n = 18) | Total (n = 120) | |
| Escherichia coli | 3 (12.0) | 0 | 0 | 3 (2.5) |
| Escherichia coli and Staphylococcus hyicus | 1 (4.0) | 0 | 0 | 1 (0.8) |
| Escherichia coli and Streptococcus suis | 1 (4.0) | 0 | 0 | 1 (0.8) |
| Glaesserella parasuis | 0 | 3 (3.9) | 1 (6.0) | 4 (3.3) |
| Helcococcus spp. | 1 (4.0) | 0 | 0 | 1 (0.8) |
| Mycoplasma hyorhinis | 0 | 5 (6.5) | 0 | 5 (4.2) |
| Mycoplasma hyosynoviae | 0 | 0 | 1 (6.0) | 1 (0.8) |
| Proteus spp. | 0 | 1 (1.3) | 0 | 1 (0.8) |
| Salmonella spp. | 0 | 1 (1.3) | 0 | 1 (0.8) |
| Staphylococcus aureus | 3 (12.0) | 1 (1.3) | 0 | 4 (3.3) |
| Staphylococcus hyicus | 2 (8.0) | 0 | 0 | 2 (1.6) |
| Streptococcus dysgalactiae | 2 (8.0) | 2 (2.6) | 0 | 4 (3.3) |
| Streptococcus suis | 5 (20.0) | 39 (50.6) | 1 (6.0) | 45 (37.5) |
| Trueperella pyogenes | 0 | 2 (2.6) | 1 (6.0) | 3 (2.5) |
| negative | 7 (28.0) | 23 (29.9) | 14 (78.0) | 44 (36.7) |
| total | 25 (100.0) | 77 (100.0) | 18 (100.0) | 120 (100.0) |
| Diagnosis of Pathohistological Examination | Result of Bacteriological Culture of Meningeal Swab | Age Category (Number of Examined Meningeal Swabs) | |||
|---|---|---|---|---|---|
| Suckling Piglets (n = 7) | Weaning Piglets (n = 109) | Fattening Pigs (n = 17) | Total (n = 133) | ||
| Leptomeningitis | Eschericia coli | 1 | 0 | 0 | 1 |
| Glaesserella parasuis | 0 | 3 | 0 | 3 | |
| Streptococcus suis (S. suis) | 2 | 44 * | 0 | 46 | |
| Staphylococcus aureus (S. aureus) | 0 | 2 | 0 | 2 | |
| Trueperella pyogenes | 2 | 1 | 0 | 3 | |
| negative | 1 | 4 | 1 | 6 | |
| total | 6 | 54 | 1 | 61 | |
| Meningoencephalitis | S. suis | 0 | 13 | 2 | 15 |
| Streptococcus dysgalactiae | 0 | 0 | 1 | 1 | |
| negative | 1 | 6 | 4 | 11 | |
| total | 1 | 19 | 7 | 27 | |
| Cerebrospinal angiopathy | S. suis | 0 | 1 | 0 | 1 |
| S. suis and S. aureus | 0 | 1 | 0 | 1 | |
| Actinobacillus minor | 0 | 1 | 0 | 1 | |
| Enterococcus spp. | 0 | 1 | 0 | 1 | |
| negative | 0 | 19 | 5 | 24 | |
| total | 0 | 23 | 5 | 28 | |
| Hyperaemia and without lesions | S. suis | 0 | 1 | 0 | 1 |
| negative | 0 | 12 | 4 | 16 | |
| total | 0 | 13 | 4 | 17 | |
| Suckling Piglet | Weaning Piglet | Fattening Pig | Total | ||
|---|---|---|---|---|---|
| pigs examined | n | 27 | 144 | 30 | 201 |
| % | 13.4 | 71.6 | 14.9 | 100.0 | |
| pigs with cultural detection of S. suis | n | 16 | 93 | 15 | 124 |
| % | 12.9 | 75.0 | 12.1 | 100.0 | |
| A | % | 59.3 | 64.6 | 50.0 | 61.7 |
| Lymph Node (Number of Examined Lymph Nodes) | No. of S. suis Detections per Age Category | Total No. of S. suis Detections | |||
|---|---|---|---|---|---|
| Suckling Piglets | Weaning Piglets | Fattening Pigs | No. | (%) | |
| Ln. cervicalis superficialis dorsalis dexter (n = 201) | 3 | 36 | 2 | 41 | 20.4 |
| Ln. bifurcationis medius (n = 201) | 1 | 30 | 4 | 35 | 17.4 |
| Ln. gastricus (n = 201) | 1 | 32 * | 4 | 37 | 18.4 |
| Ln. jejunalis (n = 201) | 2 | 28 | 1 | 31 | 15.4 |
| Ln. ileocolicus (n = 201) | 1 | 27 | 0 | 28 | 13.9 |
| Ln. colicus (n = 162) | 3 | 21 | 0 | 24 | 14.8 |
| Ln. iliacus medialis dexter (n = 201) | 1 | 31 | 2 | 34 | 16.9 |
| Ln. inguinalis superficialis dexter (n = 201) | 2 | 35 * | 1 | 38 | 18.9 |
| total (n = 1569) | 14 | 240 | 14 | 268 | 17.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bornemann, N.N.; Mayer, L.; Lacouture, S.; Gottschalk, M.; Baums, C.G.; Strutzberg-Minder, K. Invasive Bacterial Infections of the Musculoskeletal and Central Nervous System during Pig Rearing: Detection Frequencies of Different Pathogens and Specific Streptococcus suis Genotypes. Vet. Sci. 2024, 11, 17. https://doi.org/10.3390/vetsci11010017
Bornemann NN, Mayer L, Lacouture S, Gottschalk M, Baums CG, Strutzberg-Minder K. Invasive Bacterial Infections of the Musculoskeletal and Central Nervous System during Pig Rearing: Detection Frequencies of Different Pathogens and Specific Streptococcus suis Genotypes. Veterinary Sciences. 2024; 11(1):17. https://doi.org/10.3390/vetsci11010017
Chicago/Turabian StyleBornemann, Ninette Natascha, Leonie Mayer, Sonia Lacouture, Marcelo Gottschalk, Christoph Georg Baums, and Katrin Strutzberg-Minder. 2024. "Invasive Bacterial Infections of the Musculoskeletal and Central Nervous System during Pig Rearing: Detection Frequencies of Different Pathogens and Specific Streptococcus suis Genotypes" Veterinary Sciences 11, no. 1: 17. https://doi.org/10.3390/vetsci11010017
APA StyleBornemann, N. N., Mayer, L., Lacouture, S., Gottschalk, M., Baums, C. G., & Strutzberg-Minder, K. (2024). Invasive Bacterial Infections of the Musculoskeletal and Central Nervous System during Pig Rearing: Detection Frequencies of Different Pathogens and Specific Streptococcus suis Genotypes. Veterinary Sciences, 11(1), 17. https://doi.org/10.3390/vetsci11010017








