The Latent Threat in Wild Birds: Clostridium botulinum
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Clostridium botulinum General Characteristics
1.2. Hosts and Their Epizootic Potential
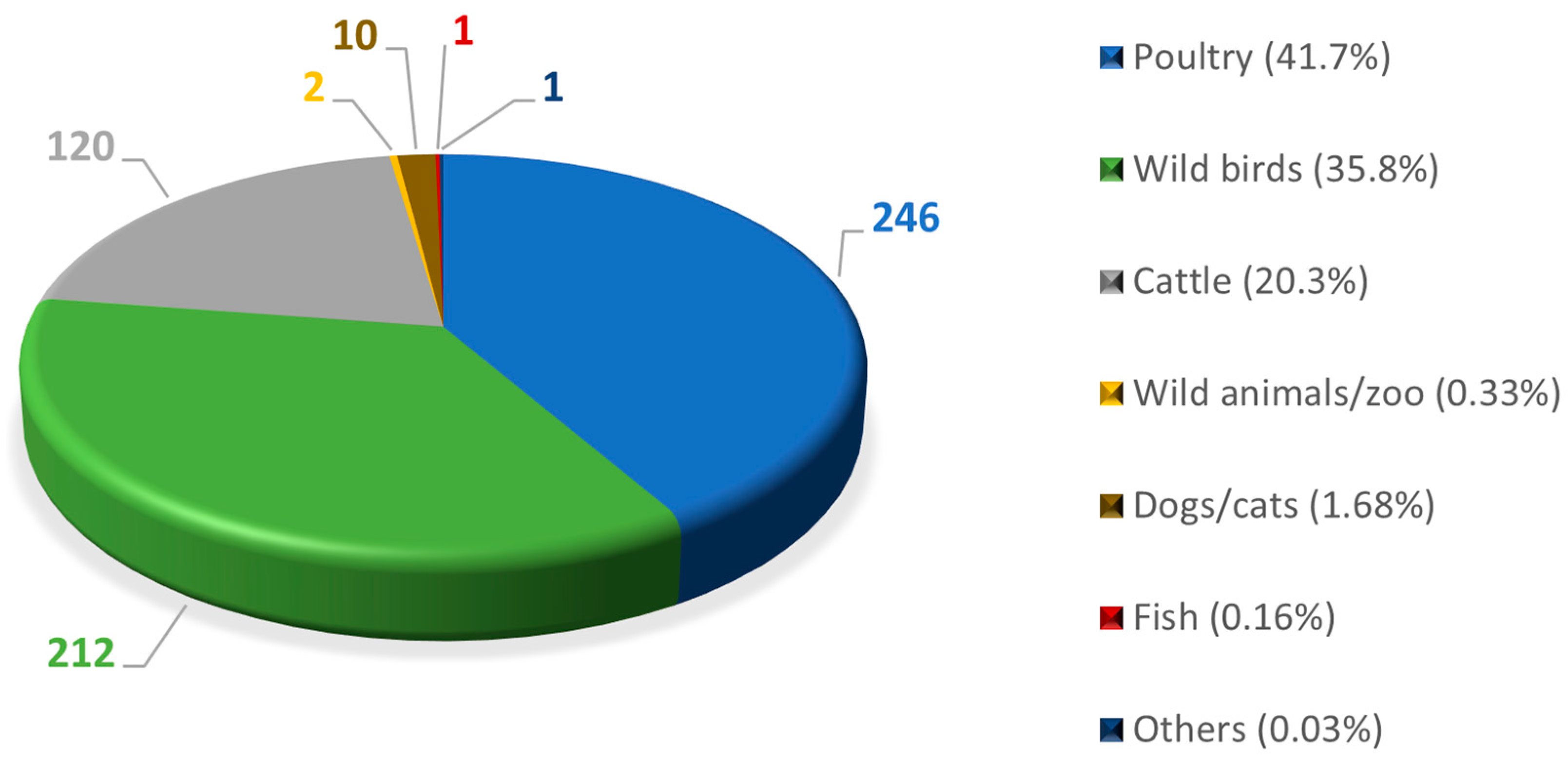
2. Relevance of Avian Botulism in Wild Birds
3. Situations That Favour Clostridium botulinum Transmission
4. ‘Carcass–Fly–Maggot’ Epidemiological Cycle
5. Wild Birds Most Susceptible to Intoxication
6. Pathogenicity Factors and the Effects of Their Neurotoxins
7. Clinical Manifestations and Diagnosis
8. Treatment of the Disease
9. Epidemiological Surveillance and Control Strategies
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Public Health Risk Associated with Botulism as Foodborne Zoonoses. Toxins 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Le Bouquin, S.; Lucas, C.; Souillard, R.; Le Maréchal, C.; Petit, K.; Kooh, P.; Jourdan-Da Silva, N.; Meurens, F.; Guillier, L.; Mazuet, C. Human and animal botulism surveillance in France from 2008 to 2019. Front. Public Health 2022, 10, 1003917. [Google Scholar] [CrossRef]
- Anza, I.; Skarin, H.; Vidal, D.; Lindberg, A.; Baverud, V.; Mateo, R. The same clade of Clostridium botulinum strains is causing avian botulism in southern and northern Europe. Anaerobe 2014, 26, 20–23. [Google Scholar] [CrossRef]
- Rocke, T.E. The Global Importance of Avian Botulism. In Waterbirds around the World; Boere, G.C., Galbraith, C.A., Stroud, D.A., Eds.; The Stationery Office: Edinburgh, UK, 2006; pp. 422–426. [Google Scholar]
- Anza, I.; Vidal, D.; Feliu, J.; Crespo, E.; Mateo, R. Differences in the Vulnerability of Waterbird Species to Botulism outbreaks in Mediterranean Wetlands: An Assessment of Ecological and Physiological Factors. Appl. Environ. Microbiol. 2016, 82, 3092–3099. [Google Scholar] [CrossRef] [PubMed]
- Cromie, R.L.; Lee, R.; Delahay, R.J.; Newth, J.L.; O’Brien, M.F.; Fairlamb, H.A.; Reeves, J.P.; Stroud, D.A. Ramsar Wetland Disease Manual: Guidelines for Assessment, Monitoring and Management of Animal Disease in Wetlands; Ramsar Technical Report No 7. Ramsar Convention Secretariat: Gland, Switzerland, 2012; Available online: https://www.ramsar.org/sites/default/files/documents/library/rtr7-disease.pdf (accessed on 5 May 2023).
- Meurens, F.; Carlin, F.; Federighi, M.; Filippitzi, M.E.; Fournier, M.; Fravalo, P.; Ganière, J.P.; Grisot, L.; Guillier, L.; Hilaire, D.; et al. Clostridium botulinum type C, D, C/D, and D/C: An update. Front. Microbiol. 2023, 13, 1099184. [Google Scholar] [CrossRef] [PubMed]
- Rawson, M.A.; Dempster, A.W.; Humphreys, C.M.; Minton, N.P. Pathogenicity and virulence of Clostridium botulinum. Virulence 2023, 14, 2205251. [Google Scholar] [CrossRef]
- Carter, A.T.; Paul, C.J.; Mason, D.R.; Twine, S.M.; Alston, M.J.; Logan, S.M.; Austin, J.W.; Peck, M.W. Independent evolution of neurotoxin and flagellar genetic loci in proteolytic Clostridium botulinum. BioMed. Cent. Genom. 2009, 10, 115. [Google Scholar] [CrossRef]
- Skarin, H.; Ljung, L. Clostridium botulinum type C/D [Photography]. VetBact. 2013. Available online: www.vetbact.org/popup/image.php?imgtable=vetbact_images&imgid=439 (accessed on 12 April 2023).
- Espelund, M.; Klaveness, D. Botulism outbreaks in natural environment—An update. Front. Microbiol. 2014, 5, 287. [Google Scholar] [CrossRef]
- Souillard, R.; Le Marechal, C.; Balaine, L.; Rouxel, S.; Poezevara, T.; Ballan, V.; Chemaly, M.; Le Bouquin, S. Manure contamination with Clostridium botulinum after avian botulism outbreaks: Management and potential risk of dissemination. VetRecord 2020, 187, 233. [Google Scholar] [CrossRef]
- Shen, A.; Edwards, A.N.; Sarker, M.R.; Paredes-Sabja, D. Sporulation and Germination in Clostridial Pathogens. Microbiol. Spectr. 2019, 7, GPP3-0017-2018. [Google Scholar] [CrossRef]
- Le Gratiet, T.; Poezevara, T.; Rouxel, S.; Houard, E.; Mazuet, C.; Chemaly, M.; Le Maréchal, C. Development of An Innovative and Quick Method for the Isolation of Clostridium botulinum Strains Involved in Avian Botulism Outbreaks. Toxins 2020, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.W. Clostridium botulinum and the safety of minimally heated, chilled foods: An emerging issue? J. Appl. Microbiol. 2006, 101, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, F.C. Botulismo. Cienc. Vet. 2016, 18, 34–53. [Google Scholar] [CrossRef][Green Version]
- Circella, E.; Camarda, A.; Bano, L.; Marzano, G.; Lombardi, R.; D’Onghia, F.; Greco, G. Botulism in Wild Birds and Changes in Environmental Habitat: A Relationship to be Considered. Animals 2019, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Vidal, D.; Anza, I.; Taggart, M.A.; Pérez-Ramírez, E.; Crespo, E.; Hofle, U.; Mateo, R. Environmental Factors Influencing the Prevalence of a Clostridium botulinum Type C/D Mosaic Strain in Nonpermanent Mediterranean Wetlands. Appl. Environ. Microbiol. 2013, 79, 4264–4271. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. Microbes help vultures eat rotting meat. Nature 2014. [Google Scholar] [CrossRef]
- Work, T.M.; Klavitter, J.L.; Reynolds, M.H.; Blehert, D. Avian botulism: A case study in translocated endangered Laysan ducks (Anas laysanensis) on Midway Atoll. J. Wildl. Dis. 2010, 46, 499–506. [Google Scholar] [CrossRef]
- Chou, S.J.; Shieh, Y.C.; Yu, C.Y. Hematologic and biochemistry values for black-faced spoonbills (Platalea minor) with and recovering from botulism. J. Wildl. Dis. 2008, 44, 781–784. [Google Scholar] [CrossRef][Green Version]
- Europa Press. El Ministerio de Agricultura Confirma el Botulismo Aviar como Causa de la Muerte de 446 aves Acuáticas en Álava. El Economista. 2014. Available online: https://ecodiario.eleconomista.es/espana/noticias/6023330/08/14/El-Ministerio-de-Agricultura-confirma-el-botulismo-aviar-como-causa-de-la-muerte-de-446-aves-acuaticas-en-Alava.html (accessed on 5 May 2023).
- Breederland, M. Avian Botulism and the Great Lakes. MSU Extension. 2014. Available online: https://www.canr.msu.edu/news/avian_botulism_and_the_great_lakes (accessed on 5 May 2023).
- Robinson, A. Leicester Park Warns over Feeding Ducks Bread after Disease Outbreak. BBC NEWS. 2014. Available online: https://www.bbc.com/news/uk-england-leicestershire-28755625 (accessed on 5 May 2023).
- Moros, M. El Piles Suma 80 Muertes de Aves, que los Ecologistas Achacan al Botulismo. El Comercio. 2014. Available online: https://www.elcomercio.es/gijon/201409/28/piles-suma-muertes-aves-20140928013948-v.html (accessed on 5 May 2023).
- Europa Press. Un Brote de Botulismo Aviar en la Balsa de Arkante Mata 32 aves Acuáticas. Iagua. 2015. Available online: https://www.iagua.es/noticias/espana/ep/15/07/16/brote-botulismo-aviar-balsa-arkaute-mata-32-aves-acuaticas (accessed on 5 May 2023).
- Jackson, C.; Maud, P. Avian Botulism Confirmed as Cause of Death of Ducks at Civic Reserve, Mornington. Herald Sun. 2017. Available online: https://www.heraldsun.com.au/leader/south-east/avian-botulism-confirmed-as-cause-of-death-of-ducks-at-civic-reserve-mornington/news-story/b136628db4298f208e0683c37b2e1f46 (accessed on 5 May 2023).
- Harris, D. Outbreak Kills 1000 Waterbrids at Canterbury Wastewater Plants. Stuff. 2018. Available online: https://www.stuff.co.nz/environment/101747128/outbreak-kills-1000-water-birds-at-canterbury-wastewater-plants (accessed on 5 May 2023).
- Marshall, D. Avian Botulism Kills 30 Swans in South Yorkshire. BBC NEWS. 2018. Available online: https://www.bbc.com/news/uk-england-south-yorkshire-45330710 (accessed on 5 May 2023).
- Presència, A. Erradican el Botulismo Aviar en el Tancat de la Pipa Tras Matar a 659 Aves. Levante. 2019. Available online: https://www.levante-emv.com/comunitat-valenciana/2019/08/20/erradican-botulismo-aviar-tancat-pipa-14016417.html (accessed on 5 May 2023).
- ACN. Un Brote de Botulismo Aviar Acaba con Cincuenta Patos en los Ríos Mèder y Gurri de Vic. La Vanguardia. 2019. Available online: https://www.lavanguardia.com/local/catalunya/20190827/464273321006/brote-brotulismo-aviar-cincuenta-patos-rios-meder-gurri-vic.html (accessed on 5 May 2023).
- Ruiz, M.A. Un Brote de Botulismo Amenaza a las Aves Acuáticas de Campotéjar. La Verdad. 2019. Available online: https://www.laverdad.es/lospiesenlatierra/noticias/brote-botulismo-amenaza-20190627004227-ntvo.html (accessed on 5 May 2023).
- Matheny, K. 20 Dead Canada Geese Pulled out of Pond in Madison Heights; Botulism Suspected. Detroit free Press. 2020. Available online: https://eu.freep.com/story/news/local/michigan/oakland/2020/07/06/20-dead-canada-geese-pulled-out-madison-heights/5366269002/ (accessed on 5 May 2023).
- Crónica. La Mortandad de Numerosas aves en la Playa de KM 8 fue por Botulismo Aviar. Crónica. 2021. Available online: https://www.diariocronica.com.ar/noticias/2021/01/16/44595-la-mortandad-de-numerosas-aves-en-la-playa-de-km-8-fue-por-botulismo-aviar (accessed on 5 May 2023).
- Rosciano, N.G.; Cossa, N.A.; Farace, M.I.; Ojeda, V.; Seijas, S. Brote de botulismo tipo C en aves acuáticas del lago Nahuel Huapi y área del Parque Nacional, Argentina. El Hornero 2021, 36, 83–88. [Google Scholar] [CrossRef]
- Almeida, R. Birds killed by avian botulism spread through hot Auckland summer. RNZ. 2022. Available online: https://www.rnz.co.nz/news/national/465357/birds-killed-by-avian-botulism-spread-through-hot-auckland-summer (accessed on 5 May 2023).
- The Gympie Times. Worst We’ve Seen: 38 Animals Dead in Botulism Outbreak. The Courier Mail. 2022. Available online: https://www.couriermail.com.au/subscribe/news/1/?sourceCode=CMWEB_WRE170_a_GGL&dest=https%3A%2F%2Fwww.couriermail.com.au%2Fnews%2Fqueensland%2Fgympie%2Ftwinnies-rescue-warn-of-lake-alford-toxic-outbreak-as-38-birds-die%2Fnews-story%2Ff6bac0b9c8cc9a8902685a1f94abd4cc&memtype=anonymous&mode=premium&v21=dynamic-groupa-test-noscore&V21spcbehaviour=append (accessed on 5 May 2023).
- Philips, A. Tiny Resident Raises Concerns after Finding 46 Dead Ducks. Orilliamatters.com. 2022. Available online: https://www.orilliamatters.com/local-news/tiny-resident-raises-concerns-after-finding-46-dead-ducks-6014142 (accessed on 5 May 2023).
- Barbanza, O. Adega Sospecha de la Existencia de un Brote de Botulismo que Afecta a aves Acuáticas en Ribeira. Diario de Arousa. 2022. Available online: https://www.diariodearousa.com/articulo/o-barbanza/adega-sospecha-existencia-brote-botulismo-afecta-aves-acuaticas-ribeira-3893161 (accessed on 5 May 2023).
- Dahlstrom, M. 640 birds die in Aussie reserve ‘Labelled potential health risk’. yahoo! News. 2023. Available online: https://au.news.yahoo.com/640-birds-die-in-aussie-reserve-labelled-potential-health-risk-ducks-victoria-025015018.html?guccounter=1&guce_referrer=aHR0cHM6Ly93d3cuZ29vZ2xlLmNvbS8&guce_referrer_sig=AQAAAFp4NYllVAjkI26Byc9VGyctCPgnhoLdBygnuK4oqrXnImPMSc1zdt2y0272BsyzohwMeE32RUwYt1pYnuOo97vq20k7esYQENglzccozAL6sUe1zll5IG6aWlTkPVWcLchYa_ePoYfOySp4nKKs_e8DTAZErCAvNFIKfioNlgk1 (accessed on 5 May 2023).
- Palmer, J.S.; Hough, R.L.; West, H.M.; Avery, L.M. A review of the abundance, behaviour and detection of clostridial pathogens in agricultural soils. Eur. J. Soil Sci. 2019, 70, 911–929. [Google Scholar] [CrossRef]
- Friend, M.; Franson, J.C. (Eds.) Field Manual of Wildlife Diseases; General Field Procedures and Diseases of Birds; Geological Survey: Madison, WI, USA, 1999; ISBN 0-607-88096-1. [Google Scholar]
- Pérez-Fuentetaja, A.; Clapsadl, M.D.; Getchell, R.G.; Bowser, P.R.; Lee, W.T. Clostridium botulinum type E in Lake Erie: Interannual differences and role of benthic invertebrates. J. Great Lakes Res. 2011, 37, 238–244. [Google Scholar] [CrossRef]
- Russo, S.E.; Legge, R.; Weber, K.A.; Brodie, E.L.; Goldfarb, K.C.; Benson, A.K.; Tan, S. Bacterial community structure of contrasting soils underlying Bornean rain forest: Inferences from microarray and next-generation sequencing methods. Soil Biol. Biochem. 2012, 55, 48–59. [Google Scholar] [CrossRef]
- Girardin, H.; Morris, C.E.; Albagnac, C.; Dreux, N.; Glaux, C.; Nguyen-The, C. Behaviour of the pathogen surrogates Listeria innocua and Clostridium sporogenes during production of parsley in fields fertilized with contaminated amendments. Animals 2005, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.H.; Johnson, K.N.; Schvaneveldt, E.R.; Dewey, D.L.; Uyehara, K.J.; Hess, S.C. Efficacy of detection canines for avian botulism surveillance and mitigation. Conserv. Sci. Pract. 2020, 3, e397. [Google Scholar] [CrossRef]
- Dos Santos, I.R.; Raiter, J.; Brunner, C.B.; Molossi, F.A.; Henker, L.C.; Pont, T.P.D.; de Camargo, L.J.; Alves, R.S.; Canal, C.W.; da Silva Martins, A.; et al. An outbreak of type C botulism in free-ranging Southern lapwing (Vanellus chilensis). Vet. Res. Commun. 2023. [Google Scholar] [CrossRef] [PubMed]
- Evelsizer, D.D.; Clark, R.G.; Bollinger, T.K. Relationships between local carcass density and risk of mortality in molting mallards during avian botulism outbreaks. J. Wildl. Dis. 2010, 46, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef]
- Soos, C.; Wobeser, G. Identification of Primary Substrate in the Initiation of Avian Botulism Outbreaks. J. Wildl. Manag. 2006, 70, 43–53. [Google Scholar] [CrossRef]
- Anza, I.; Vidal, D.; Mateo, R. New insight in the epidemiology of avian botulism outbreaks: Necrophagous flies as vectors of Clostridium botulinum type C/D. Environ. Microbiol. Rep. 2014, 6, 738–743. [Google Scholar] [CrossRef]
- Laird, J.; Andrews, D.W.; Chip, D.V.; Moore, D.J.; Hebert, C.E.; Douglas, G.; Williams, K. The importance of island surveys in documenting disease-related mortality and Botulism E in Great Lakes colonial waterbirds. J. Great Lakes Res. 2014, 40, 58–63. [Google Scholar] [CrossRef]
- Hidalgo, H.; Montecino, D.F. Botulismo en aves acuáticas silvestres. TecnoVet 2008, 14, 16–21. [Google Scholar]
- Zepeda, M.L.; Roggenbuck, M.; Manzano, K.; Hestbjerg, L.; Brunak, S.; Gilbert, M.T.P.; Sicheritz-Pontén, T. Protective role of the vulture facial skin and gut microbiomes aid adaptation to scavenging. Acta Vet. Scand. 2018, 60, 61. [Google Scholar] [CrossRef] [PubMed]
- Meloni, E.; Le Maréchal, C.; Millot, F.; Payne, A.; Calenge, C.; Mazuet, C.; Chemaly, M.; Rouxel, S.; Poezevara, T.; Avouac, A.; et al. Exposure of waterfowl to Clostridium botulinum in France. Front. Conserv. Sci. 2023, 4, 1011555. [Google Scholar] [CrossRef]
- Holmes, P. Avian botulism—A recurring paralytic disease of wild UK waterbirds. Vet. Rec. 2019, 185, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L. The life history of a botulinum toxin molecule. Toxicon 2013, 68, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Restani, L.; Giribaldi, F.; Manich, M.; Bercsenyi, K.; Menendez, G.; Rossetto, O.; Caleo, M.; Schiavo, G. Botulinum Neurotoxins A and E Undergo Retrograde Axonal Transport in Primary Motor Neurons. PLoS Pathog. 2012, 8, e1003087. [Google Scholar] [CrossRef] [PubMed]
- Anniballi, F.; Fiore, A.; Löfström, C.; Skarin, H.; Auricchio, B.; Woudstra, L.B.; Koene, M.; Baverud, V.; Hansen, T.; Fach, P.; et al. Management of Animal Botulism Outbreaks: From Clinical Suspicion to Practical Countermeasures to Prevent or Minimize Outbreaks. Biosecur. Bioterror. Biodef. Strateg. Pract. Sci. 2013, 11, 191–199. [Google Scholar] [CrossRef]
- Hedeland, M.; Moura, H.; Baverud, V.; Woolfitt, A.R.; Bondesson, U.; Barr, J.R. Confirmation of botulism in birds and cattle by the mouse bioassay and Endopep-MS. J. Med. Microbiol. 2011, 60, 1299–1305. [Google Scholar] [CrossRef]
- Masters, A.M.; Palmer, D.G. Confirmation on botulism diagnosis in Australian bird samples by ELISA and RT rtPCR. J. Vet. Diagn. Investig. 2021, 33, 684–694. [Google Scholar] [CrossRef]
- Heredia, A.M. ¿Cómo hacer frente a la Neurotoxina Botulínica en Aves Silvestres? Psychol. Lat. 2018, Especial, 205–207. [Google Scholar]
- Silva, R.O.S.; Gómez, S.Y.M.; Medeiros, L.B.; Marques, M.V.R.; Silva, A.S.G.; Mureb, E.N.; Oliveira, C.A.; Favoretto, S.M.; Lobato, F.C.F.; Martins, R.S. Antitoxin therapy of natural avian botulism outbreaks occurred in Brazil. Anaerobe 2017, 48, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Mahesh, D.; Singh, J.; Suri, M. Bird-Area Water-Bodies Dataset (BAWD) and Predictive AI Model for Avian Botulism Outbreak (AVI-BoT). q-bio.QM 2022. submitted. [Google Scholar] [CrossRef]
- Instituto de Investigación de Recursos Cinegéticos (IREC). Guía de Vigilancia Sanitaria Fauna Silvestre; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2019. [Google Scholar]




| Diagnosis Method | Description | Advantages (A) or Disadvantages (D) |
|---|---|---|
| Mouse bioassay | Intraperitoneal inoculation with a suspicious sample in mouse 1, and neutralisation with polyvalent antitoxin or boiling in mouse 2. If (+), mouse 1 shows clinical signs (ruffled fur, posterior third paralysis, wasp waist, death, etc.). Mouse 2 shows no signs. If (−), both mice survive. Types the BoNT of the outbreak if a neutralisation test is done with specific antitoxin. | (A): Detection of toxin in serum, biological samples (stool, food and gastric contents), environmental samples (sediments) and crops. (A): High sensitivity and specificity. (D): Detection takes one to several days. (D): Ethical issues. (D): Difficult interpretation of results due to nonspecific signs in mice or prior death. |
| Cultivation and isolation | 1º. Cultivation in liquid medium “chopped-meat-glucose-starch”. 2º. PCR confirmation of Clostridium Botulinum. 3º. If (+), solid medium culture, “blood agar”. | (D): Non-selective culture media. (D): Some strains lose the “bot” gene phage that encodes toxins, preventing their characterisation in mouse bioassays or PCR. |
| ELISA | It is carried out using polyclonal antibodies against a semi-purified toxic complex of BoNT. | (A): Analyses greater nº of samples than bioassay. (D): Less sensitive and specific than the bioassay. (D): False + due to the detection of inactive toxins that cross-react with other toxins. (D): False − due to the genetic variability of toxins. |
| Real-time PCR | 1º. Sample enrichment in a culture broth to germinate the spores, increase the number of microorganisms and dilute inhibitors. 2º. Performance of PCR where millions of copies of specific target genes of the microorganism are produced. | (A): Sensitive and specific. Faster than culture and bioassay; allows analysis of more samples. (A): Enables ecological and epidemiological studies. (A): Detects BoNT C, D in environment and tissues. (D): Only reverse transcriptase PCR detects gene and toxin activity. (D): False + due to the detection of dead cells. (D): False − due to loss of the “bot” gene. |
| Mass spectrometry | In vitro detection of the peptides that form botulinum toxins after cleaving SNARE proteins at specific points. | (A): Detects biologically active toxin. (A): High sensitivity and specificity. (D): Only commercially available for type A toxins. (D): Expensive equipment & specialised personnel. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Arnal, J.; Marín, C. The Latent Threat in Wild Birds: Clostridium botulinum. Vet. Sci. 2024, 11, 36. https://doi.org/10.3390/vetsci11010036
Gutiérrez-Arnal J, Marín C. The Latent Threat in Wild Birds: Clostridium botulinum. Veterinary Sciences. 2024; 11(1):36. https://doi.org/10.3390/vetsci11010036
Chicago/Turabian StyleGutiérrez-Arnal, Josep, and Clara Marín. 2024. "The Latent Threat in Wild Birds: Clostridium botulinum" Veterinary Sciences 11, no. 1: 36. https://doi.org/10.3390/vetsci11010036
APA StyleGutiérrez-Arnal, J., & Marín, C. (2024). The Latent Threat in Wild Birds: Clostridium botulinum. Veterinary Sciences, 11(1), 36. https://doi.org/10.3390/vetsci11010036







