Comparison of Two Surgical Techniques Based on the Semitendinosus Myocutaneous Flap in Cats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
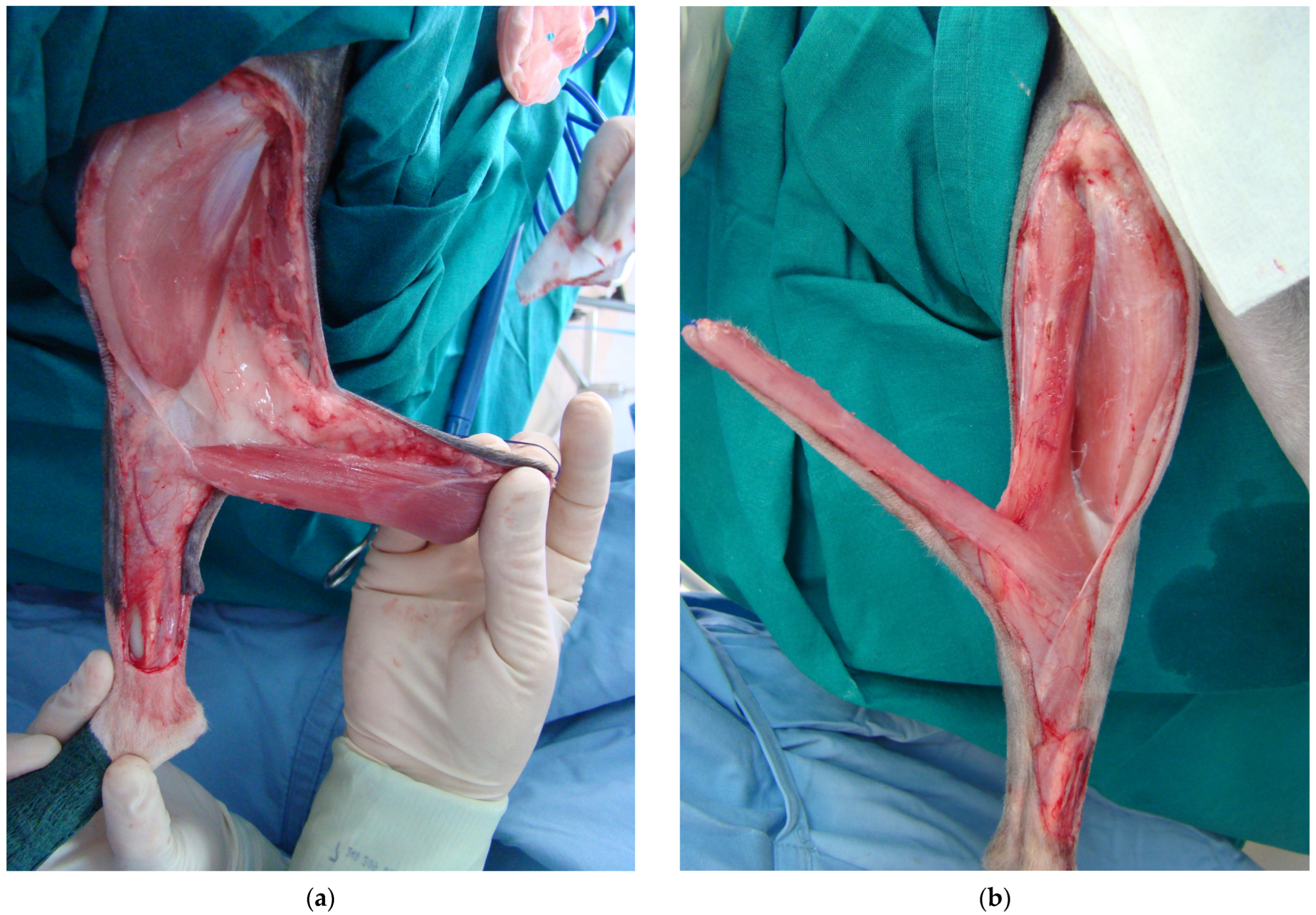
2.1. Cats
2.2. Study Design
2.3. Presurgical Management
2.4. Surgical Procedure
2.5. Postoperative Management
2.6. Clinical Assessment Scoring
2.7. Planimetry
2.8. CT-Angiography
2.9. Histological Evaluation of the ST and SST Myocutaneous Flaps
2.10. Statistical Analysis
3. Results
3.1. Clinical Assessment Scoring
3.2. Planimetry
3.3. CT-Angiography
3.4. Histological Evaluation of the ST and SST Myocutaneous Flaps
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCraw, J.B.; Dibbell, D.G.; Carraway, J.H. Clinical definition of independent myocutaneous vascular territories. Plast. Reconstr. Surg. 1977, 60, 341–352. [Google Scholar] [PubMed]
- Ariyan, S.; Cuono, C.B. Myocutaneous Flaps for Head and Neck Reconstruction. Head Neck Surg. 1980, 2, 321–345. [Google Scholar] [CrossRef] [PubMed]
- Mathes, S.J.; Nahai, F. Classification of the vascular anatomy of muscles: Experimental and clinical correlation. Plast. Reconstr. Surg. 1981, 67, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Pavletic, M.M.; Kostolich, M.; Koblik, P.; Engler, S. A comparison of the cutaneous trunci myocutaneous flap and latissimus dorsi myocutaneous flap in the dog. Vet. Surg. 1987, 16, 283–293. [Google Scholar] [CrossRef]
- Taylor, G.I.; Palmer, J.H. The vascular territories (angiosomes) of the body—Experimental study and clinical applications. Br. J. Plast. Surg. 1987, 40, 113–141. [Google Scholar] [CrossRef]
- Pavletic, M.M. Introduction to myocutaneous and muscle flaps. Vet. Clin. N. Am. Small Anim. Pract. 1990, 20, 127–146. [Google Scholar] [CrossRef]
- Taylor, G.I.; Minabe, T. The angiosomes of the mammals and other vertebrates. Plast. Reconstr. Surg. 1992, 89, 181–215. [Google Scholar] [CrossRef]
- De Mello-Filho, F.V.; Mamede, R.C.; Sader, A.A.; Velludo, M.A.; Vicente, W.V. Use of the platysma myocutaneous flap for cervical trachea reconstruction: An experimental study in dogs. Laryngoscope 1993, 103, 1161–1167. [Google Scholar] [CrossRef]
- Smith, Μ.; Shults, S.; Waldron, D.R.; Moon, M.L. Platysma myocutaneous flap for head and neck reconstruction in cats. Head Neck 1993, 15, 433–439. [Google Scholar] [CrossRef]
- Degner, D.A.; Walshaw, R.; Arnoczky, S.P.; Smith, R.J.; Patterson, J.S.; Degner, L.A.; Hamaide, A.; Rosenstein, D. Evaluation of the cranial rectus abdominus muscle pedicle flap as a blood supply for the caudal superficial epigastric skin flap in dogs. Vet. Surg. 1996, 25, 292–299. [Google Scholar] [CrossRef]
- Taylor, G.I. The angiosomes of the body and their supply to perforator flaps. Clin. Plast. Surg. 2003, 30, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Puerto, D.A.; Aronson, L.R. Use of a semitendinosus myocutaneous flap for soft-tissue reconstruction of a grade IIIB open tibial fracture in a dog. Vet. Surg. 2004, 33, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.P.; Degner, D.A. Evaluation of the Superior Labial Musculomucosal Flap in Dogs: An Angiographic Study and Case Report. Vet. Comp. Orthop. Traumatol. 2019, 32, 133–138. [Google Scholar] [PubMed]
- Chambers, J.N.; Rawlings, C.A. Applications of a semitendinosus muscle flap in two dogs. J. Am. Vet. Med. Assoc. 1991, 199, 84–86. [Google Scholar] [PubMed]
- Solano, M.; Purinton, P.T.; Chambers, J.N.; Munnell, J.F. Effects of vascular pedicle ligation on blood flow in canine semitendinosus muscle. Am. J. Vet. Res. 1995, 56, 731–735. [Google Scholar] [PubMed]
- Mortari, A.C.; Rahal, S.C.; Resende, L.A.; Dal-pai-silva, M.; Mamprim, M.J.; Corrêa, M.A.; Antunes, S.H. Electromyographical, ultrasonographical and morphological modifications in semitendinous muscle after transposition as ventral perineal muscle flap. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2005, 52, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Vnuk, D.; Babić, T.; Stejskal, M.; Capak, D.; Pirkić, B.; Harapin, I. Application of a semitendinosus muscle flap in the treatment of perineal hernia in a cat. Vet. Rec. 2005, 156, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Dermisiadou, E.; Panopoulos, I.; Psalla, D.; Georgiou, S.; Sideri, A.; Galatos, A.; Tsioli, V. Use of a semitendinosus myocutaneous flap for the coverage of hindlimb full-thickness skin defects in cats. J. Vet. Sci. 2023, 24, e14. [Google Scholar] [CrossRef]
- Baltzer, W.I.; Rist, P. Achilles tendon repair in dogs using the semitendinosus muscle: Surgical technique and short-term outcome in five dogs. Vet. Surg. 2009, 38, 770–779. [Google Scholar] [CrossRef]
- Morello, E.; Martano, M.; Zabarino, S.; Piras, L.A.; Nicoli, S.; Bussadori, R.; Buracco, P. Modified semitendinosus muscle transposition to repair ventral perineal hernia in 14 dogs. J. Small Anim. Pract. 2015, 56, 370–376. [Google Scholar] [CrossRef]
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Percoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch. Dermatol. 1994, 130, 489–493. [Google Scholar] [CrossRef]
- Grey, J.E.; Enoch, S.; Harding, K.G. Wound assessment. BMJ 2006, 332, 285–288. [Google Scholar] [CrossRef]
- Ousey, K.; Cook, L. Wound Assessment: Made Easy. Wounds 2012, 8, 1–4. [Google Scholar]
- Epstein, M.E.; Rodan, I.; Griffenhagen, G.; Kadrlik, J.; Petty, M.; Robertson, S.; Simpson, W. 2015 AAHA/AAFP pain management guidelines for dogs and cats. J. Feline Med. Surg. 2015, 17, 251–272. [Google Scholar] [CrossRef]
- Mathews, K.; Kronen, P.W.; Lascelles, D.; Nolan, A.; Robertson, S.; Steagall, P.V.; Wright, B.; Yamashita, K. Guidelines for recognition, assessment and treatment of pain: WSAVA Global Pain Council members and co-authors of this document. J. Small Anim. Pract. 2014, 55, E10–E68. [Google Scholar] [CrossRef]
- Bohling, M.W.; Henderson, R.A.; Swaim, S.F.; Kincaid, S.A.; Wright, J.C. Cutaneous wound healing in the cat: A macroscopic description and comparison with cutaneous wound healing in the dog. Vet. Surg. 2004, 33, 579–587. [Google Scholar] [CrossRef]
- Bohling, M.W.; Henderson, R.A. Differences in cutaneous wound healing between dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 687–692. [Google Scholar] [CrossRef]
- Bohling, M.W.; Henderson, R.A.; Swaim, S.F.; Kincaid, S.A.; Wright, J.C. Comparison of the role of the subcutaneous tissues in cutaneous wound healing in the dog and cat. Vet. Surg. 2006, 35, 3–14. [Google Scholar] [CrossRef]
- Costa, W.; da Silva, A.L.; Costa, G.R.; Nunes, T.A. Histology of the rectus abdominis muscle in rats subjected to cranial and caudal devascularization. Acta Cirúrgica Bras. 2012, 27, 162–167. [Google Scholar] [CrossRef]
- Greaves, P.; Chouinard, L.; Ernst, H.; Mecklenburg, L.; Pruimboom-Brees, I.M.; Rinke, M.; Rittinghausen, S.; Thibault, S.; Von Erichsen, J.; Yoshida, T. Proliferative and Non-Proliferative Lesions of the Rat and Mouse Soft Tissue, Skeletal Muscle and Mesothelium. J. Toxicol. Pathol. 2013, 26, 1S–26S. [Google Scholar] [CrossRef]
- Siemionow, M.; Manikowski, W.; Gawronski, M. Histopathology of muscle flap microcirculation following prolonged ischemia. Microsurgery 1995, 16, 515–521. [Google Scholar] [CrossRef]
- Pavletic, M.M. Vascular supply to the skin of the dog: A review. Vet. Surg. 1980, 9, 77–80. [Google Scholar] [CrossRef]
- Pavletic, M.M. Axial pattern flaps in small animal practice. Vet. Clin. N. Am. Small Anim. Pract. 1990, 20, 105–125. [Google Scholar] [CrossRef]
- Pavletic, M.M. Canine axial pattern flaps, using the omocervical, thoracodorsal, and deep circumflex iliac direct cutaneous arteries. Am. J. Vet. Res. 1981, 42, 391–406. [Google Scholar]
- Pang, C.Y. Ischemia-induced reperfusion injury in muscle flaps: Pathogenesis and major source of free radicals. J. Reconstr. Microsurg. 1990, 6, 77–83. [Google Scholar] [CrossRef]
- Kerrigan, C.L.; Stotland, M.A. Ischemia reperfusion injury: A review. Microsurgery 1993, 14, 165–175. [Google Scholar] [CrossRef]
- Spadola, F.; Neve, V.C.; Interlandi, C.D.; Spadaro, A.; Macrì, F.; Iannelli, N.M.; Costa, G.L. Hernioplasty with Peritoneal Flap for the Surgical Treatment of Umbilical Hernia in Swine. Animals 2022, 12, 3240. [Google Scholar] [CrossRef]
- Jackson, A.H.; Degner, D.A.; Jackson, I.T.; Miyawaki, T.; Silverberg, B.; Bradford, M.; Andrus, L. Deep circumflex iliac cutaneous free flap in cats. Vet. Surg. 2003, 32, 341–349. [Google Scholar] [CrossRef]
- Lanthier, T.; Miller, C.; McDonell, W.N.; Yager, J.A.; Roth, J.H. Use of laser Doppler flowmetry to determine blood flow in and viability of island axial pattern skin flaps in rabbits. Am. J. Vet. Res. 1990, 51, 1914–1921. [Google Scholar]
- Pratt, G.F.; Rozen, W.M.; Chubb, D.; Whitaker, I.S.; Grinsell, D.; Ashton, M.W.; Acosta, R. Modern adjuncts and technologies in microsurgery: An historical and evidence-based review. Microsurgery 2010, 30, 657–666. [Google Scholar] [CrossRef]
- Losken, A.; Styblo, T.M.; Schaefer, T.G.; Carlson, G.W. The use of fluorescein dye as a predictor of mastectomy skin flap viability following autologous tissue reconstruction. Ann. Plast. Surg. 2008, 61, 24–29. [Google Scholar] [CrossRef]
- Lee, J.H.; You, H.J.; Lee, T.Y.; Kang, H.J. Current Status of Experimental Animal Skin Flap Models: Ischemic Preconditioning and Molecular Factors. Int. J. Mol. Sci. 2022, 23, 5234. [Google Scholar] [CrossRef]





| Clinical Assessment Score | Day 7 | Day 14 | Day 21 | Day 30 |
|---|---|---|---|---|
| Group A | 8.5 (3–10) | 7.5 (0–27) | 7 (0–22) | 0 (0–19) |
| Group B | 10 (7–15) | 12.5 (8–18) | 11.5 (0–20) | 7 (0–18) |
| Planimetric Measurements | Group A | Group B |
|---|---|---|
| Flap width (cm) | 1.64 ± 0.30 | 1.49 ± 0.23 |
| Flap length (cm) | 7.27 ± 0.46 | 6.95 ± 0.42 |
| Initial total flap area (cm2) | 11.79 ± 1.44 | 9.06 ± 1.47 |
| % final total flap area | 68.36 ± 27.18 | 51.83 ± 22.48 |
| % flap necrosis on day 7 | 12.39 ± 27.03 | 12.13 ± 22.39 |
| % flap necrosis on day 14 | 28 ± 26 | 34.83 ± 17.23 |
| % flap necrosis on day 21 | 21.07 ± 28.23 | 16.57 ± 24.04 |
| % flap necrosis on day 30 | 8.52 ± 16.33 | 0.63 ± 2.34 |
| Parameters | Day 0 | Day10 | Day 30 | |
|---|---|---|---|---|
| Muscle length (cm) | group A | 10.76 ± 0.97 | NA | NA |
| group B | 11.03 ± 0.3 | NA | NA | |
| Muscle width day 0 (cm) | group A | 2.11 ± 0.19 | 2.25 ± 0.26 | 2.16 ± 0.2 |
| group B | 1.97 ± 0.11 | NA | NA | |
| Proximal gluteal artery caliber (mm) | group A | 0.85 ± 0.02 | NA | NA |
| group B | 0.84 ± 0.02 | 1.02 ± 0.05 | 0.86 ± 0.02 | |
| Proximal gluteal vein caliber (mm) | group A | 1.25 ± 0.05 | NA | NA |
| group B | 1.25 ± 0.02 | 1.41 ± 0.13 | 1.28 ± 0.02 | |
| Distal caudal femoral artery caliber (mm) | group A | 0.86 ± 0.05 | 1.03 ± 0.04 | 0.86 ± 0.03 |
| group B | 0.86 ± 0.02 | 1.02 ± 0.04 | 0.89 ± 0.04 | |
| Distal caudal femoral vein caliber (mm) | group A | 1.26 ± 0.03 | 1.50 ± 0.04 | 1.26 ± 0.19 |
| group B | 1.25 ± 0.02 | 1.40 ± 0.05 | 1.27 ± 0.02 | |
| Muscle density-proximal point when no contrast agent given (dP) (HU) | group A | 52.49 ± 3.73 | 57.89 ± 3.3 | 55.98 ± 3.24 |
| group B | 52.38 ± 2.34 | 56.00 ± 2.54 | 54.46 ± 2.47 | |
| Muscle density-medial point when no contrast agent given (dM) (HU) | group A | 54.05 ± 3.72 | 55.02 ± 3.3 | 54.39 ± 3.26 |
| group B | 54.35 ± 1.85 | 55.05 ± 2.16 | 53.36 ± 2.17 | |
| Muscle density-distal point when no contrast agent given (dD) (HU) | group A | 52.94 ± 3.71 | 54.17 ± 3.16 | 51.85 ± 3.77 |
| group B | 53.16 ± 2.45 | 53.45 ± 2.2 | 52.13 ± 1.99 | |
| Muscle density on the proximal point—arterial phase (dPa) (HU) | group A | 67.49 ± 5.52 | 87.63 ± 4.93 | 81.87 ± 3.28 |
| group B | 65.08 ± 3.65 | 85.39 ± 4.09 | 80.46 ± 2.72 | |
| Muscle density on the medial point—arterial phase (dMa) (HU) | group A | 71.92 ± 5.05 | 79.50 ± 2.70 | 74.31 ± 2.6 |
| group B | 69.52 ± 3.87 | 79.07 ± 2.03 | 74.67 ± 1.94 | |
| Muscle density on the distal point—arterial phase (dDa) (HU) | group A | 69.3 ± 5.36 | 71.88 ± 1.76 | 67.87 ± 2.12 |
| group B | 66.98 ± 3.54 | 66.77 ± 8.82 | 63.42 ± 8.15 | |
| Muscle density on the proximal point—venous phase (dPv) (HU) | group A | 73.65 ± 5.47 | 93.88 ± 5.7 | 87.40 ± 3.59 |
| group B | 71.20 ± 4.59 | 92.69 ± 4.83 | 86.89 ± 2.88 | |
| Muscle density on the medial point—venous phase (dMv) (HU) | group A | 78.21 ± 6.73 | 84.25 ± 2.92 | 78.11 ± 2.53 |
| group B | 74.19 ± 3.44 | 86.50 ± 4.12 | 79.42 ± 2.18 | |
| Muscle density on the distal point—venous phase (dDv) (HU) | group A | 75.23 ± 5.15 | 78.92 ± 2.43 | 73.22 ± 1.47 |
| group B | 72.62 ± 4 | 76.52 ± 4.38 | 70.63 ± 5.83 |
| Histological Parameters | Day 0 (n = 12) | Day 14 (n = 12) | 6 Months (n = 6) | 12 Months (n = 6) | |
|---|---|---|---|---|---|
| Inflammation | group A | 0 (0) | 2 (0–2) | 0 (0–1) | 0 (0–2) |
| group B | 0 (0) | 2 (0–3) | 0 (0–1) | 0 (0–2) | |
| Degeneration/Necrosis | group A | 2 (1–2) | 3 (0–3) | 2 (1–2) | 1 (0–3) |
| group B | 2 (1–3) | 3 (1–3) | 1 (0–2) | 1 (0–2) | |
| Neovascularization | group A | 0 (0) | 0,5 (0–2) | 0 (0) | 0 (0–1) |
| group B | 0 (0) | 1 (0–2) | 0 (0) | 0 (0–1) | |
| Fibrosis | group A | 0 (0) | 1 (0–3) | 0 (0–1) | 0 (0–1) |
| group B | 0 (0) | 0 (0–3) | 1 (0–1) | 1 (0–1) | |
| Edema | group A | 1 (0–1) | 1 (1–2) | 0 (0–1) | 1 (0–2) |
| group B | 1 (0–1) | 1 (0–3) | 0 (0–1) | 1 (0–1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dermisiadou, E.; Panopoulos, I.; Psalla, D.; Georgiou, S.; Sideri, A.; Galatos, A.; Tsioli, V. Comparison of Two Surgical Techniques Based on the Semitendinosus Myocutaneous Flap in Cats. Vet. Sci. 2024, 11, 6. https://doi.org/10.3390/vetsci11010006
Dermisiadou E, Panopoulos I, Psalla D, Georgiou S, Sideri A, Galatos A, Tsioli V. Comparison of Two Surgical Techniques Based on the Semitendinosus Myocutaneous Flap in Cats. Veterinary Sciences. 2024; 11(1):6. https://doi.org/10.3390/vetsci11010006
Chicago/Turabian StyleDermisiadou, Eleftheria, Ioannis Panopoulos, Dimitra Psalla, Stefanos Georgiou, Aikaterini Sideri, Apostolos Galatos, and Vassiliki Tsioli. 2024. "Comparison of Two Surgical Techniques Based on the Semitendinosus Myocutaneous Flap in Cats" Veterinary Sciences 11, no. 1: 6. https://doi.org/10.3390/vetsci11010006
APA StyleDermisiadou, E., Panopoulos, I., Psalla, D., Georgiou, S., Sideri, A., Galatos, A., & Tsioli, V. (2024). Comparison of Two Surgical Techniques Based on the Semitendinosus Myocutaneous Flap in Cats. Veterinary Sciences, 11(1), 6. https://doi.org/10.3390/vetsci11010006







