PTD-FNK Alleviated LPS-Induced Oxidative Stress of Boar Testicular Sertoli Cells via Keap1-Nrf2 Pathway
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Testis Collection
2.2. Experimental Design
2.3. Oil Red O Stain Analysis and Identification
2.4. Cell Counting Kit-8 (CCK8)
2.5. Reactive Oxygen (ROS) Species Quantification in SCs
2.6. Measurement of Oxidant-Antioxidant Status in Boar SCs
2.7. Real-Time Quantitative PCR (qRT-PCR)
2.8. Enzyme-Linked Immunosorbent Analysis (ELISA)
2.9. Western Blot
2.10. His Pull-Down
2.11. Data Analysis
3. Result
3.1. Boar SC Isolation
3.2. The Establishment of an Oxidative Stress Model
3.3. Effect of PTD-FNK on the Activity of SCs
3.4. Effect of PTD-FNK on the Antioxidant Capacity of SCs
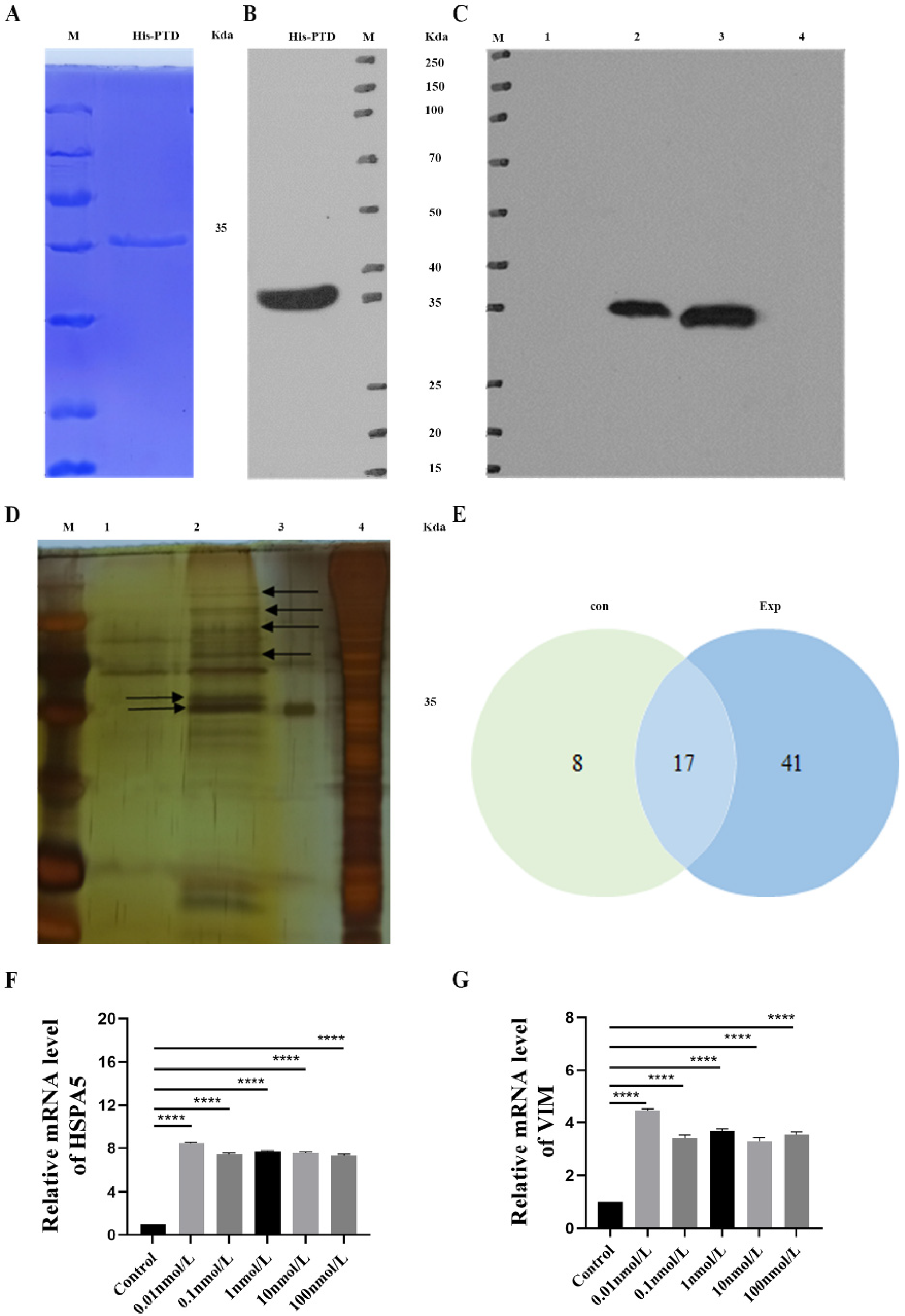
3.5. His Pull-Down Analysis of PTD-FNK Interaction Protein
3.6. PTD-FNK to Activate the Nrf2/Keap1 Pathway in LPS-Induced Boar SCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petricca, S.; Carnicelli, V.; Luzi, C.; Cinque, B.; Celenza, G.; Iorio, R. Oxidative Stress, Cytotoxic and Inflammatory Effects of Azoles Combinatorial Mixtures in Sertoli TM4 Cells. Antioxidants 2023, 12, 1142. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.C.; McBeath, E. Sertoli Cell-Germ Cell Interactions Within the Niche: Paracrine and Juxtacrine Molecular Communications. Front. Endocrinol. 2022, 13, 897062. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chen, C.; Zhang, J.; Gao, Y.; Zeng, X.; Zhang, X. Testicular aging, male fertility and beyond. Front. Endocrinol. 2022, 13, 1012119. [Google Scholar] [CrossRef]
- Liu, X.; Xi, H.; Han, S.; Zhang, H.; Hu, J. Zearalenone induces oxidative stress and autophagy in goat Sertoli cells. Ecotoxicol. Environ. Saf. 2023, 252, 114571. [Google Scholar] [CrossRef]
- Yang, X.; Liu, P.; Zhang, X.; Zhang, J.; Cui, Y.; Song, M.; Li, Y. T-2 toxin causes dysfunction of Sertoli cells by inducing oxidative stress. Ecotoxicol. Environ. Saf. 2021, 225, 112702. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.B.; Li, K.R.; Yi, N.; Li, X.M.; Wang, F.; Xue, B.; Pan, Y.S.; Yao, J.; Jiang, Q.; Wu, Z.F. miRNA-141 attenuates UV-induced oxidative stress via activating Keap1-Nrf2 signaling in human retinal pigment epithelium cells and retinal ganglion cells. Oncotarget 2017, 8, 13186–13194. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Q.; Chen, Z. The Nrf2 Pathway in Liver Diseases. Front. Cell Dev. Biol. 2022, 10, 826204. [Google Scholar] [CrossRef]
- Wu, K.C.; Liu, J.; Klaassen, C.D. Role of Nrf2 in preventing ethanol-induced oxidative stress and lipid accumulation. Toxicol. Appl. Pharmacol. 2012, 262, 321–329. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as Modulators of the Keap1/Nrf2/ARE Signaling Pathway. Oxid. Med. Cell Longev. 2020, 2020, 1675957. [Google Scholar] [CrossRef]
- Xie, Z.Y.; Xiao, Z.H.; Wang, F.F. Inhibition of autophagy reverses alcohol-induced hepatic stellate cells activation through activation of Nrf2-Keap1-ARE signaling pathway. Biochimie 2018, 147, 55–62. [Google Scholar] [CrossRef]
- Asoh, S.; Mori, T.; Nagai, S.; Yamagata, K.; Nishimaki, K.; Miyato, Y.; Shidara, Y.; Ohta, S. Zonal necrosis prevented by transduction of the artificial anti-death FNK protein. Cell Death Differ. 2005, 12, 384–394. [Google Scholar] [CrossRef]
- Katsura, K.; Takahashi, K.; Asoh, S.; Watanabe, M.; Sakurazawa, M.; Ohsawa, I.; Mori, T.; Igarashi, H.; Ohkubo, S.; Katayama, Y.; et al. Combination therapy with transductive anti-death FNK protein and FK506 ameliorates brain damage with focal transient ischemia in rat. J. Neurochem. 2008, 106, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Boisguérin, P.; Deshayes, S.; Gait, M.J.; O’Donovan, L.; Godfrey, C.; Betts, C.A.; Wood, M.J.; Lebleu, B. Delivery of therapeutic oligonucleotides with cell penetrating peptides. Adv. Drug Deliv. Rev. 2015, 87, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Asoh, S.; Ohsawa, I.; Ozaki, D.; Yamagata, K.; Ito, H.; Ohta, S. The anti-cell death FNK protein protects cells from death induced by freezing and thawing. Biochem. Biophys. Res. Commun. 2005, 330, 850–856. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, H.; Lin, S.; Qu, J.; Xiao, J.; Huang, Y.; Xiao, Y.; Fu, X.; Yang, Y.; Li, X. Cell-penetrating peptide TAT-mediated delivery of acidic FGF to retina and protection against ischemia-reperfusion injury in rats. J. Cell Mol. Med. 2010, 14, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Nawaz, M.S.; Kamal, M.A. In silico analysis of the amido phosphoribosyltransferase inhibition by PY873, PY899 and a derivative of isophthalic acid. Investig. New Drugs 2013, 31, 1355–1363. [Google Scholar] [CrossRef]
- Rapoport, M.; Lorberboum-Galski, H. TAT-based drug delivery system--new directions in protein delivery for new hopes? Expert. Opin. Drug Deliv. 2009, 6, 453–463. [Google Scholar] [CrossRef]
- Noguchi, T.; Kuo-Lan, F.; Lai, E.K.; Alexander, S.S.; King, M.M.; Olson, L.; Lee Poyer, J.; McCay, P.B. Specificity of a phenobarbital-induced cytochrome P-450 for metabolism of carbon tetrachloride to the trichloromethyl radical. Biochem. Pharmacol. 1982, 31, 615–624. [Google Scholar] [CrossRef]
- Shimokawa, K.; Oshiro, R.; Yamanaka, K.; Ashizawa, K.; Ohta, S.; Tatemoto, H. Improvement of the post-thaw qualities of Okinawan native Agu pig sperm frozen in an extender supplemented with antiapoptotic PTD-FNK protein. Theriogenology 2012, 78, 1446–1455. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Jin, Z.; Jin, E.; Fujiwara, M.; Ghazizadeh, M.; Asoh, S.; Ohta, S.; Kawanami, O. Anti-apoptotic PTD-FNK protein suppresses lipopolysaccharide-induced acute lung injury in rats. Exp. Mol. Pathol. 2007, 83, 377–384. [Google Scholar] [CrossRef]
- Jo, A.; Denduluri, S.; Zhang, B.; Wang, Z.; Yin, L.; Yan, Z.; Kang, R.; Shi, L.L.; Mok, J.; Lee, M.J.; et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014, 1, 149–161. [Google Scholar] [CrossRef]
- Suzuki, Y.J. Cell signaling pathways for the regulation of GATA4 transcription factor: Implications for cell growth and apoptosis. Cell Signal 2011, 23, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ishikado, A.; Nishida, N.; Ninomiya, K.; Fujiwara, H.; Kobayashi, Y.; Yoshikawa, M. Hepatoprotective, superoxide scavenging, and antioxidative activities of aromatic constituents from the bark of Betula platyphylla var. japonica. Bioorganic Med. Chem. Lett. 1998, 8, 2939–2944. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Asakawa, M.; Fujii, H.; Maki, A.; Amemiya, H.; Yamamoto, M.; Matsuda, M.; Matsumoto, Y. Edaravone, a novel free radical scavenger, prevents liver injury and mortality in rats administered endotoxin. J. Pharmacol. Exp. Ther. 2003, 307, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Asoh, S.; Ohsawa, I.; Mori, T.; Katsura, K.; Hiraide, T.; Katayama, Y.; Kimura, M.; Ozaki, D.; Yamagata, K.; Ohta, S. Protection against ischemic brain injury by protein therapeutics. Proc. Natl. Acad. Sci. USA 2002, 99, 17107–17112. [Google Scholar] [CrossRef]
- Wang, J.; Lee, J.; Liem, D.; Ping, P. HSPA5 Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene 2017, 618, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, L.M.; Ting, J.; Lee, A.S. Identity of the immunoglobulin heavy-chain-binding protein with the 78,000-dalton glucose-regulated protein and the role of posttranslational modifications in its binding function. Mol. Cell Biol. 1988, 8, 4250–4256. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Chang, Y.J.; Huang, Y.P.; Li, Z.L.; Chen, C.H. GRP78 knockdown enhances apoptosis via the down-regulation of oxidative stress and Akt pathway after epirubicin treatment in colon cancer DLD-1 cells. PLoS ONE 2012, 7, e35123. [Google Scholar] [CrossRef]
- Chen, N.; Wan, X.; Wang, M.; Li, Y.; Wang, X.; Zeng, L.; Zhou, J.; Zhang, Y.; Cheng, S.; Shen, Y. Cross-talk between Vimentin and autophagy regulates blood-testis barrier disruption induced by cadmium. Environ. Pollut. 2024, 346, 123625. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, T.M.; Burbach, B.J.; Shimizu, Y.; Ervasti, J.M. β-Actin specifically controls cell growth, migration, and the G-actin pool. Mol. Biol. Cell 2011, 22, 4047–4058. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Esmaeilniakooshkghazi, A.; Patnaik, S.; Wang, Y.; George, S.P.; Ahrorov, A.; Hou, J.K.; Herron, A.J.; Sesaki, H.; Khurana, S. Villin-1 and Gelsolin Regulate Changes in Actin Dynamics That Affect Cell Survival Signaling Pathways and Intestinal Inflammation. Gastroenterology 2018, 154, 1405–1420. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, J.; Jones, D.P. Mitochondrial control of apoptosis: The role of cytochrome c. Biochim. Biophys. Acta 1998, 1366, 139–149. [Google Scholar] [CrossRef]
- Benarroch, E.E. Nrf2, cellular redox regulation, and neurologic implications. Neurology 2017, 88, 1942–1950. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Tang, H.; Pan, R.; Wang, H.; Jin, F.; Yan, X.; Xing, Y.; Chen, G.; Fu, Y.; et al. Madecassoside ameliorates lipopolysaccharide-induced neurotoxicity in rats by activating the Nrf2-HO-1 pathway. Neurosci. Lett. 2019, 709, 134386. [Google Scholar] [CrossRef]
- Lv, H.; Zhu, C.; Wei, W.; Lv, X.; Yu, Q.; Deng, X.; Ci, X. Enhanced Keap1-Nrf2/Trx-1 axis by daphnetin protects against oxidative stress-driven hepatotoxicity via inhibiting ASK1/JNK and Txnip/NLRP3 inflammasome activation. Phytomedicine 2020, 71, 153241. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hua, C.; Yang, X.; Fan, X.; Song, H.; Peng, L.; Ci, X. Pterostilbene prevents LPS-induced early pulmonary fibrosis by suppressing oxidative stress, inflammation and apoptosis in vivo. Food Funct. 2020, 11, 4471–4484. [Google Scholar] [CrossRef]
- Wolfson, M.L.; Correa, F.; Leishman, E.; Vercelli, C.; Cymeryng, C.; Blanco, J.; Bradshaw, H.B.; Franchi, A.M. Lipopolysaccharide-induced murine embryonic resorption involves changes in endocannabinoid profiling and alters progesterone secretion and inflammatory response by a CB1-mediated fashion. Mol. Cell Endocrinol. 2015, 411, 214–222. [Google Scholar] [CrossRef]
- Ying, S.; Qin, J.; Dai, Z.; An, H.; Zhu, H.; Chen, R.; Yang, X.; Wu, W.; Shi, Z. Effects of LPS on the Secretion of Gonadotrophin Hormones and Expression of Genes in the Hypothalamus-Pituitary-Ovary (HPG) Axis in Laying Yangzhou Geese. Animals 2020, 10, 2259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.J.; Pang, Y.W.; Zhao, X.M.; Du, W.H.; Hao, H.S.; Zhu, H.B. Effects of lipopolysaccharide on maturation of bovine oocyte in vitro and its possible mechanisms. Oncotarget 2017, 8, 4656–4667. [Google Scholar] [CrossRef] [PubMed]
- Shepel, E.; Grushka, N.; Makogon, N.; Sribna, V.; Pavlovych, S.; Yanchii, R. Changes in DNA integrity and gene expression in ovarian follicular cells of lipopolysaccharide-treated female mice. Pharmacol. Rep. 2018, 70, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, D.F.; Bowen, J.M.; Krasa, H.B.; Thrun, L.A.; Viguié, C.; Karsch, F.J. Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: A simultaneous view from hypophyseal portal and peripheral blood. Endocrinology 1997, 138, 4273–4281. [Google Scholar] [CrossRef]



| Primer Name | Base Sequence(5′ to 3′) | Length | Accession Number |
|---|---|---|---|
| GATA4 | F: 5′-TCTCGATATGTTTGATGAACTTCTC-3′ R: 5′-GTCTTCGATTTGTTA AGGTTCTTG-3′ | 378 | XM_013990299.2 |
| SOX9 | F:5′- CCGGCTCCTACTACAGCCAC-3′ R:5′- GTGGCCAGGCCACTCTTGCT-3′ | 383 | NM_213843.2 |
| Primer Name | Base Sequence (5′ to 3′) | Length | Accession Number |
|---|---|---|---|
| β-actin | F:5′-CTAGTTACACACACGCGGCTCT-3′ R:5′-CATGAATACCCTGCACAGATCG-3′ | 127 | XM_003357 |
| SOD2 | F: 5′-CTGGCCAAGGGAGATGTTAC-3′ R: 5′-AAAGACCCAAAGTCACGCTT-3′ | 167 | NM_001322819.2 |
| GSH-px | F:5′-CTCATGACCGACCCCAAGTT-3′ R:5′-GTCAGAAAGCGACGGCTGTA-3′ | 128 | NM_214201.1 |
| CAT | F: 5′-CCTGCAACGTTCTGTAAGGC-3′ R: 5′-ATATCAGGTTTCTGCGCGGC-3′ | 109 | NM_214301.2 |
| Nrf2 | F: 5′-GACTCCAAGGGGTTGCGAAGG-3′ R: 5′-CCCAAACCCCAATCCCGTAG-3′ | 80 | XM_005671981.3 |
| Keap1 | F: 5′-CATCGGCATCGCCAACTTC-3′ R: 5′-ACCAGTTGGCAGTGGGACAG-3′ | 135 | NM_012289.4 |
| NQO1 | F:5′- ATGTATGACAAAGGACCCTTCC -3′ R:5′ - TCCCTTGCAGAGAGTACATGG -3′ | 88 | NM_001159613.1 |
| HSPA5 | F: 5′-CATCACGCCGTCCTATGTCG-3′ R: 5′-CGTCAAAGACCGTTCTCG -3′ | 104 | NM_005347.5 |
| VIM | F:5′-CAGTATGAAAGCGTGGCTGC-3′ R:5′-AGGGACTCGTTAGTGCCTTT-3′ | 197 | NM_003380.5 |
| HO-1 | F: 5′-TGTACCGCTCCCGAATGAAC-3′ R: 5′-TGGTCCTTAGTGTCCTGGGT-3′ | 142 | NM_001004027.1 |
| Accession | Name | Gene | Coverage | Unique Peptides |
|---|---|---|---|---|
| A0A4X1UFV5 | GRP78 | HSPA5 | 51 | 4 |
| A0A4X1UCE3 | Vimentin | VIM | 31 | 15 |
| A0A4X1TQB9 | Actin | ACTB | 37 | 14 |
| F1SRY3 | Villin-1 | VIL1 | 8 | 6 |
| F1SGG3 | Cytokeratin-1 | KRT1 | 8 | 5 |
| A0A5G2QGK | Tubulin beta chain | TUBB4B | 14 | 1 |
| A0A4X1TY33 | Tropomyosin alpha-1 chain | TPM1 | 12 | 4 |
| A5A759 | Keratin 2A | KRT2A | 5 | 2 |
| A0A4X1VYY1 | Prelamin-A/C | LMNA | 7 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, W.; Huang, Q.; Ma, Q.; Song, X.; Zhang, X.; Li, X.; Wang, X.; Wang, S.; Wang, Y.; Xiao, Z.; et al. PTD-FNK Alleviated LPS-Induced Oxidative Stress of Boar Testicular Sertoli Cells via Keap1-Nrf2 Pathway. Vet. Sci. 2024, 11, 543. https://doi.org/10.3390/vetsci11110543
Ji W, Huang Q, Ma Q, Song X, Zhang X, Li X, Wang X, Wang S, Wang Y, Xiao Z, et al. PTD-FNK Alleviated LPS-Induced Oxidative Stress of Boar Testicular Sertoli Cells via Keap1-Nrf2 Pathway. Veterinary Sciences. 2024; 11(11):543. https://doi.org/10.3390/vetsci11110543
Chicago/Turabian StyleJi, Weixia, Qiuyan Huang, Qiqi Ma, Xingxing Song, Xin Zhang, Xun Li, Xiaoye Wang, Sutian Wang, Yanling Wang, Zhengzhong Xiao, and et al. 2024. "PTD-FNK Alleviated LPS-Induced Oxidative Stress of Boar Testicular Sertoli Cells via Keap1-Nrf2 Pathway" Veterinary Sciences 11, no. 11: 543. https://doi.org/10.3390/vetsci11110543
APA StyleJi, W., Huang, Q., Ma, Q., Song, X., Zhang, X., Li, X., Wang, X., Wang, S., Wang, Y., Xiao, Z., & Hu, C. (2024). PTD-FNK Alleviated LPS-Induced Oxidative Stress of Boar Testicular Sertoli Cells via Keap1-Nrf2 Pathway. Veterinary Sciences, 11(11), 543. https://doi.org/10.3390/vetsci11110543






