Stress Biomarkers in Pigs: Current Insights and Clinical Application
Simple Summary
Abstract
1. Introduction
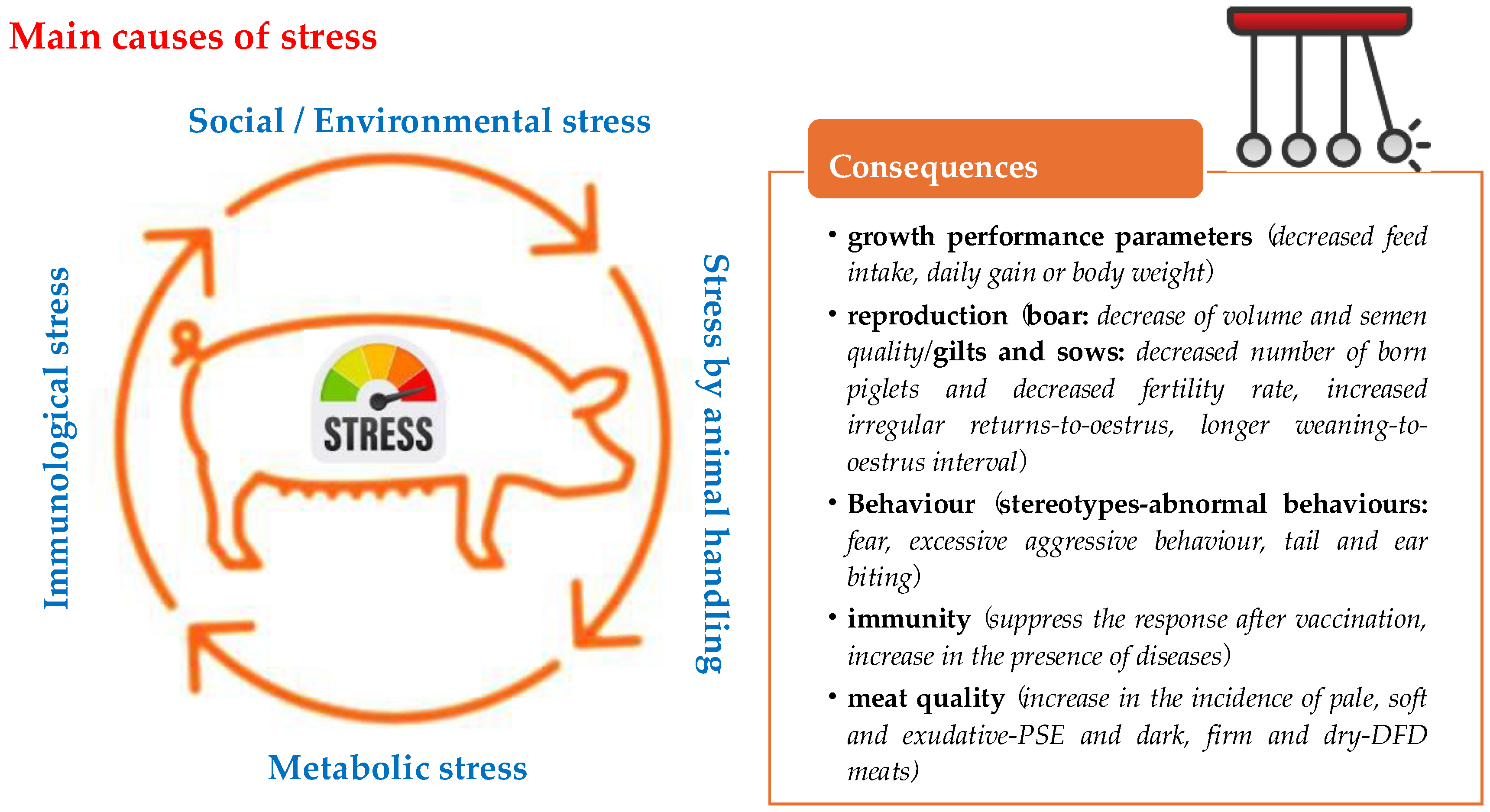
2. Stress Conditions in Intensive Pig Farming
3. Consequences of Stress for Pig Production
4. Stress Biomarkers in Pigs
4.1. Metabolic and Behavioral Stress Biomarkers
4.2. Immune Stress Biomarkers
4.3. Oxidative Stress Biomarkers
| Biomarker * | Physiological System | Biomatrices | Observations | References |
|---|---|---|---|---|
| SAA | Immune System | Saliva Blood (serum) |
| [40,77] |
| Hp | Immune System | Blood (serum) |
| [59] |
| Cortisol | HPA Axis and Endocrine System | Saliva Blood (serum) |
| [40] |
| Pig-MAP | Immune System | Blood (serum) |
| [58,78] |
| CRP | Immune System | Blood (serum) |
| [58] |
| CgA | Sympathetic-Adrenal-Medullary System | Saliva |
| [64,65] |
| TBARS | Oxidative Stress System | Blood (plasma) |
| [69,72] |
| CARBS | Oxidative Stress System | Blood (plasma) |
| [69] |
| IgA | Immune System | Saliva |
| [62,64] |
| IL-18 | Immune System | Saliva |
| [62] |
| Testosterone | HPA Axis and Endocrine System | Saliva |
| [64] |
| Serotonin | Oxidative Stress System | Blood (serum) |
| [77,79] |
5. Sampling for Stress Biomarkers in Clinical Practice
6. Clinical Practice: Behavioral or Health Disorders and Stress Biomarkers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, Y.; Xing, M.; Gu, X. Research Progress on Oxidative Stress and Its Nutritional Regulation Strategies in Pigs. Animals 2021, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Miro, S.; Tecles, F.; Ramon, M.; Escribano, D.; Hernandez, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, Consequences and Biomarkers of Stress in Swine: An Update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of Heat Stress on Animal Physiology, Metabolism, and Meat Quality: A Review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, N.; Sun, L.; Zhang, Y.; Wu, Y.; Wang, Y.; Liao, X.; Mi, J. Short-Term Cold Stress Can Reduce the Abundance of Antibiotic Resistance Genes in the Cecum and Feces in a Pig Model. J. Hazard. Mater. 2021, 416, 125868. [Google Scholar] [CrossRef]
- Ramirez, B.C.; Hayes, M.D.; Condotta, I.; Leonard, S.M. Impact of Housing Environment and Management on Pre-/Post-Weaning Piglet Productivity. J. Anim. Sci. 2022, 100, skac142. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Schmidt, C.G.; Michel, V.; et al. Welfare of Pigs during Transport. EFSA J. 2022, 20, e07445. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A Review of Recent Studies on Malondialdehyde as Toxic Molecule and Biological Marker of Oxidative Stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Pitts, A.D.; Weary, D.M.; Pajor, E.A.; Fraser, D. Mixing at Young Ages Reduces Fighting in Unacquainted Domestic Pigs. Appl. Anim. Behav. Sci. 2000, 68, 191–197. [Google Scholar] [CrossRef]
- Arnone, M.; Dantzer, R. Does Frustration Induce Aggression in Pigs? Appl. Anim. Ethol. 1980, 6, 351–362. [Google Scholar] [CrossRef]
- Coutellier, L.; Arnould, C.; Boissy, A.; Orgeur, P.; Prunier, A.; Veissier, I.; Meunier-Salaün, M.C. Pig’s Responses to Repeated Social Regrouping and Relocation during the Growing-Finishing Period. Appl. Anim. Behav. Sci. 2007, 105, 102–115. [Google Scholar] [CrossRef]
- Andersen, I.L.; Naevdal, E.; Bakken, M.; Boe, K.E. Aggression and Group Size in Domesticated Pigs, Sus scrofa: ‘When the Winner Takes It All and the Loser Is Standing Small’. Anim. Behav. 2004, 68, 965–975. [Google Scholar] [CrossRef]
- Verdon, M.; Hansen, C.F.; Rault, J.L.; Jongman, E.; Hansen, L.U.; Plush, K.; Hemsworth, P.H. Effects of Group Housing on Sow Welfare: A Review. J. Anim. Sci. 2015, 93, 1999–2017. [Google Scholar] [CrossRef]
- Remience, V.; Wavreille, J.; Canart, B.; Meunier-Salau, M.C.; Prunier, A.; Bartiaux-Thill, N.; Nicks, B.; Vandenheede, M. Effects of Space Allowance on the Welfare of Dry Sows Kept in Dynamic Groups and Fed with an Electronic Sow Feeder. Appl. Anim. Behav. Sci. 2008, 112, 284–296. [Google Scholar] [CrossRef]
- Randolph, J.H.; Cromwell, G.L.; Stahly, T.S.; Kratzer, D.D. Effects of Group Size and Space Allowance on Performance and Behaviour of Swine. J. Anim. Sci. 1981, 53, 922–927. [Google Scholar] [CrossRef]
- Muráni, E.; Ponsuksii, S.; D’Eath, R.B.; Turner, S.P.; Kurt, E.; Evans, G.; Thölking, L.; Klont, R.; Foury, A.; Mormède, P.; et al. Association of HPA Axis Related Genetic Variation with Stress Reactivity and Aggressive Behaviour in Pigs. BMC Genet. 2010, 11, 74. [Google Scholar] [CrossRef]
- Squires, E.J. Effects on Animal Behaviour, Health and Welfare. In Applied Animal Endocrinology; Squires, E.J., Ed.; CABI Publishing: Cambridge, UK, 2003; pp. 192–225. [Google Scholar]
- Valros, A.; Munsterhjelm, C.; Puolanne, E.; Ruusunen, M.; Heinonen, M.; Peltoniemi, O.A.T.; Pösö, A.R. Physiological Indicators of Stress and Meat and Carcass Characteristics in Tail Bitten Slaughter Pigs. Acta Vet. Scand. 2013, 55, 75. [Google Scholar] [CrossRef]
- O’Connor, E.A.; Parker, M.O.; McLeman, M.A.; Demmers, T.G.; Lowe, J.C.; Cui, L.; Davey, E.L.; Owen, R.C.; Wathes, C.M.; Abeyesinghe, S.M. The Impact of Chronic Environmental Stressors on Growing Pigs, Sus scrofa (Part 1): Stress Physiology, Production and Play Behaviour. Animal 2010, 4, 1899–1909. [Google Scholar] [CrossRef]
- Pearce, S.C.; Gabler, N.K.; Ross, J.W.; Escobar, J.; Patience, J.F.; Rhoads, R.P.; Baumgard, L.H. The Effects of Heat Stress and Plane of Nutrition on Metabolism in Growing Pigs. J. Anim. Sci. 2013, 91, 2108–2118. [Google Scholar] [CrossRef]
- Sanz Fernandez, M.V.; Johnson, J.S.; Abuajamieh, M.; Stoakes, S.K.; Seibert, J.T.; Cox, L.; Kahl, S.; Elsasser, T.H.; Ross, J.W.; Isom, S.C. Effects of Heat Stress on Carbohydrate and Lipid Metabolism in Growing Pigs. Physiol. Rep. 2015, 3, e12315. [Google Scholar] [CrossRef]
- Beattie, V.E.; O’Connell, N.E.; Kilpatrick, D.J.; Moss, B.W. Influence of Environmental Enrichment on Welfare-Related Behavioural and Physiological Parameters in Growing Pigs. J. Anim. Sci. 2000, 70, 443–450. [Google Scholar] [CrossRef]
- Oczak, M.; Maschat, K.; Berckmans, D.; Vranken, E.; Baumgartner, J. Classification of Nest-Building Behaviour in Non-Crated Farrowing Sows on the Basis of Accelerometer Data. Biosyst. Eng. 2015, 140, 48–58. [Google Scholar] [CrossRef]
- Arellano, P.E.; Pijoan, C.; Jacobson, L.D.; Algers, B. Stereotyped Behaviour, Social Interactions and Suckling Pattern of Pigs Housed in Groups or in Single Crates. Appl. Anim. Behav. Sci. 1992, 2, 157–166. [Google Scholar] [CrossRef]
- Brouns, F.; Edwards, S.A. Social Rank and Feeding Behaviour of Group-Housed Sows Fed Competitively or Ad Libitum. Appl. Anim. Behav. Sci. 1994, 39, 225–235. [Google Scholar] [CrossRef]
- Song, C.; Jiang, J.; Han, X.; Yu, G.; Pang, Y. Effect of Immunological Stress to Neuroendocrine and Gene Expression in Different Swine Breeds. Mol. Biol. Rep. 2014, 41, 3569–3576. [Google Scholar] [CrossRef]
- Tuchscherer, A.; Kanitz, E.; Puppe, B.; Tuchscherer, A.; Viergutz, T. Changes in Endocrine and Immune Responses of Neonatal Pigs Exposed to a Psychosocial Stressor. Res. Vet. Sci. 2009, 87, 380–388. [Google Scholar] [CrossRef]
- Merlot, E.; Mounier, A.M.; Prunier, A. Endocrine Response of Gilts to Various Common Stressors: A Comparison of Indicators and Methods of Analysis. Physiol. Behav. 2011, 102, 259–265. [Google Scholar] [CrossRef]
- Prunier, A.; Mounier, A.M.; Hay, M. Effects of Castration, Tooth Resection, or Tail Docking on Plasma Metabolites and Stress Hormones in Young Pigs. J. Anim. Sci. 2005, 83, 216–222. [Google Scholar] [CrossRef]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The Biological Stress of Early Weaned Piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19–23. [Google Scholar] [CrossRef]
- European Food Safety Authority. Opinion of the Scientific Panel on Animal Health and Welfare on a Request from the Commission Related to Welfare of Weaners and Rearing Pigs: Effects of Different Space Allowances and Floor Types. EFSA J. 2005, 268, 1–19. [Google Scholar] [CrossRef]
- Escribano, D.; Ko, H.-L.; Chong, Q.; Llonch, L.; Manteca, X.; Llonch, P. Salivary Biomarkers to Monitor Stress Due to Aggression after Weaning in Piglets. Res. Vet. Sci. 2019, 123, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Bench, C.; Rioja-Lang, F.; Hayne, S.; Gonyou, H. Group Gestation Sow Housing with Individual Feeding—II: How Space Allowance, Group Size and Composition, and Flooring Affect Sow Welfare. Livest. Sci. 2013, 152, 218–227. [Google Scholar] [CrossRef]
- Horback, K. 284 Prop 12 and Its Implications for Future on-Farm Animal Welfare in the United States. J. Anim. Sci. 2021, 99 (Suppl. 1), 8–9. [Google Scholar] [CrossRef]
- Einarsson, S.; Sjunnesson, Y.; Hulten, F.; Eliasson-Selling, L.; Dalin, A.M.; Lundeheim, N.; Magnusson, U. A 25 years experience of group-housed sows-reproduction in animal welfare-friendly systems. Acta Vet. Scand. 2014, 56, 37. [Google Scholar] [CrossRef]
- Otten, W.; Puppe, B.; Stabenow, B.; Kanitz, E.; Schön, P.; Brüssow, K.; Nürnberg, G. Agonistic interactions and physiological reactions of top- and bottom-ranking pigs confronted with a familiar and an unfamiliar group: Preliminary results. Appl. Anim. Behav. Sci. 1997, 55, 79–90. [Google Scholar] [CrossRef]
- Merlot, E.; Meunier-Salaün, M.-C.; Prunier, A. Behavioural, endocrine and immune consequences of mixing in weaned piglets. Appl. Anim. Behav. Sci. 2004, 85, 247–257. [Google Scholar] [CrossRef]
- Ruis, M.A.W.; Brake, J.H.A.; Engel, B.; Ekkel, E.D.; Buist, W.G.; Blokhuis, H.J.; Koolhaas, J.M. The circadian rhythm of salivary cortisol in growing pigs: Effects of age, gender, and stress. Physiol. Behav. 1997, 62, 623–630. [Google Scholar] [CrossRef]
- Soler, L.; Gutiérrez, A.; Escribano, D.; Fuentes, M.; Cerón, J.J. Response of salivary haptoglobin and serum amyloid A to social isolation and short road transport stress in pigs. Res. Vet. Sci. 2013, 95, 298–302. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z.; Liu, W.; Yin, C.; Ci, L.; Zhao, R.; Yang, X. Salivary haptoglobin and chromogranin A as non-invasive markers during restraint stress in pigs. Res. Vet. Sci. 2017, 114, 27–30. [Google Scholar] [CrossRef]
- Brown, S.N.; Knowles, T.G.; Wilkins, L.J.; Chadd, S.A.; Warriss, P.D. The response of pigs to being loaded or unloaded onto commercial animal transporters using three systems. Vet. J. 2005, 170, 91–100. [Google Scholar] [CrossRef]
- Council Regulation (EC) No 1/2005 of 22 December 2004 on the protection of animals during transport and related operations and amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/97. Off. J. Eur. Union 2005, 5, 1–44.
- Smulders, D.; Verbeke, G.; Mormède, P.; Geers, R. Validation of a behavioural observation tool to assess pig welfare. Physiol. Behav. 2006, 89, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G.P. Biological response to stress: Implications for animal welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 1–22. [Google Scholar]
- Lee, C.; Giles, L.R.; Bryden, W.L.; Downing, J.L.; Owens, P.C.; Kirby, A.C.; Wynn, P.C. Performance and endocrine responses of group housed weaner pigs exposed to the air quality of a commercial environment. Livest. Prod. Sci. 2005, 93, 255–262. [Google Scholar] [CrossRef]
- Einarsson, S.; Brandt, Y.; Lundeheim, N.; Madej, A. Stress and its influence on reproduction in pigs: A review. Acta Vet. Scand. 2008, 50, 48. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, K.; Habing, G. Social stress as a cause of diseases in farm animals: Current knowledge and future directions. Vet. J. 2015, 206, 15–21. [Google Scholar] [CrossRef]
- Escribano, D.; Gutiérrez, A.M.; Tecles, F.; Cerón, J.J. Changes in saliva biomarkers of stress and immunity in domestic pigs exposed to a psychosocial stressor. Res. Vet. Sci. 2015, 102, 38–44. [Google Scholar] [CrossRef]
- Rubio, C.P.; Mainau, E.; Cerón, J.J.; Contreras-Aguilar, M.D.; Martínez-Subiela, S.; Navarro, E.; Tecles, F.; Manteca, X.; Escribano, D. Biomarkers of oxidative stress in saliva in pigs: Analytical validation and changes in lactation. BMC Vet. Res. 2019, 15, 144. [Google Scholar] [CrossRef]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; Van Reenen, C.G.; et al. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Cray, C.; Zaias, J.; Altman, N.H. Acute phase response in animals: A review. Comp. Med. 2009, 59, 517–526. [Google Scholar]
- de Leeuw, J.A.; Ekkel, E.D. Effects of feeding level and the presence of a foraging substrate on the behaviour and stress physiological response of individually housed gilts. Appl. Anim. Behav. Sci. 2004, 86, 15–25. [Google Scholar] [CrossRef]
- Escribano, D.; Fuentes-Rubio, M.; Cerón, J.J. Validation of an automated chemiluminescent immunoassay for salivary cortisol measurements in pigs. J. Vet. Diagn. Investig. 2012, 24, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Subiela, S.; Martínez-Miró, S.; Cerón, J.J.; Tecles, F. Changes in alpha-amylase activity, concentration and isoforms in pigs after an experimental acute stress model: An exploratory study. BMC Vet. Res. 2018, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Giergiel, M.; Olejnik, M.; Jabłoński, A.; Posyniak, A. The markers of stress in swine oral fluid. J. Vet. Res. 2021, 65, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Hosseindoust, A.; Ha, S.; Moturi, J.; Mun, J.; Tajudeen, H.; Kim, J. Dietary fiber for gestating sows during heat stress: Effects on reproductive performance and stress level. 2021; preprint. [Google Scholar] [CrossRef]
- Mohan, N.H.; Nath, A.; Thomas, R.; Kumar, S.; Banik, S.; Das, A.K.; Das, R.K.; Sarma, D.K. Relationship between plasma, saliva, urinary and faecal cortisol levels in pigs. Indian J. Anim. Sci. 2020, 90, 768–772. [Google Scholar] [CrossRef]
- Piñeiro, C.; Piñeiro, M.; Morales, J.; Andrés, M.; Lorenzo, E.; Pozo, M.D.; Álava, M.A.; Lampreave, F. Pig-MAP and haptoglobin concentration reference values in swine from commercial farms. Vet. J. 2009, 179, 78–84. [Google Scholar] [CrossRef]
- García-Celdrán, M.; Ramis, G.; Quereda, J.J.; Armero, E. Reduction of transport-induced stress on finishing pigs by increasing lairage time at the slaughter house. J. Swine Health Prod. 2012, 20, 118–122. [Google Scholar] [CrossRef]
- Escribano, D.; Fuentes-Rubio, M.; Cerón, J.J. Salivary testosterone measurements in growing pigs: Validation of an automated chemiluminescent immunoassay and its possible use as an acute stress marker. Res. Vet. Sci. 2014, 97, 20–25. [Google Scholar] [CrossRef]
- Bilandzic, N.; Simic, B.; Kmetic, I. Effect of three-day ACTH administration on concentrations of cholesterol, cortisol, progesterone, testosterone and LH in the boars. Slov. Vet. Res. 2012, 49, 123–132. [Google Scholar]
- Muneta, Y.; Minagawa, Y.; Nakane, T.; Shibahara, T.; Yoshikawa, T.; Omata, Y. Interleukin-18 expression in pig salivary glands and salivary content changes during acute immobilization stress. Stress 2011, 14, 549–556. [Google Scholar] [CrossRef]
- Escribano, D.; Campos, P.H.R.F.; Gutiérrez, A.M.; Le Floc, N.; Cerón, J.J.; Merlot, E. Effect of repeated administration of lipopolysaccharide on inflammatory and stress markers in saliva of growing pigs. Vet. J. 2014, 200, 393–397. [Google Scholar] [CrossRef]
- Escribano, D.; Tvarijonaviciute, A.; Tecles, F.; Cerón, J.J. Serum paraoxonase type-1 activity in pigs: Assay validation and evolution after an induced experimental inflammation. Vet. Immunol. Immunopathol. 2015, 163, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Escribano, D.; Soler, L.; Gutiérrez, A.M.; Martínez-Subiela, S.; Cerón, J.J. Measurement of chromogranin A in porcine saliva: Validation of a time-resolved immunofluorometric assay and evaluation of its application as a marker of acute stress. Animal 2013, 7, 640–647. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Petrotos, K.; Tsatsakis, A.M.; Kouretas, D.; Goutzourelas, N. Feed supplemented with polyphenolic byproduct from olive mill wastewater processing improves the redox status in blood and tissues of piglets. Food Chem. Toxicol. 2015, 86, 319–327. [Google Scholar] [CrossRef]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal 2018, 12, 246–255. [Google Scholar] [CrossRef]
- Papatsiros, V.G.; Eliopoulos, C.; Voulgarakis, N.; Arapoglou, D.; Riahi, I.; Sadurní, M.; Papakonstantinou, G.I. Effects of a Multi-Component Mycotoxin-Detoxifying Agent on Oxidative Stress, Health and Performance of Sows. Toxins 2023, 15, 580. [Google Scholar] [CrossRef]
- Papatsiros, V.G.; Katsogiannou, E.G.; Papakonstantinou, G.I.; Michel, A.; Petrotos, K.; Athanasiou, L.V. Effects of Phenolic Phytogenic Feed Additives on Certain Oxidative Damage Biomarkers and the Performance of Primiparous Sows Exposed to Heat Stress under Field Conditions. Antioxidants 2022, 11, 593. [Google Scholar] [CrossRef]
- Papatsiros, V.G.; Papakonstantinou, G.I.; Voulgarakis, N.; Eliopoulos, C.; Marouda, C.; Meletis, E.; Valasi, I.; Kostoulas, P.; Arapoglou, D.; Riahi, I.; et al. Effects of a Curcumin/Silymarin/Yeast-Based Mycotoxin Detoxifier on Redox Status and Growth Performance of Weaned Piglets under Field Conditions. Toxins 2024, 16, 168. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, Y.F.; Zou, Y.; Hu, X.M.; Zheng, L.F.; Wei, H.K.; Peng, J. Effects of dietary oregano essential oil supplementation on the stress response, antioxidative capacity, and HSPs mRNA expression of transported pigs. Livest. Sci. 2015, 180, 143–149. [Google Scholar] [CrossRef]
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S.; Peng, J. Effects of Dietary Supplementation of Oregano Essential Oil to Sows on Oxidative Stress Status, Lactation Feed Intake of Sows, and Piglet Performance. BioMed Res. Int. 2015, 2015, 525218. [Google Scholar] [CrossRef]
- Schuh, S.; Muller, L.K.F.; Campos, L.P.; Moresco, R.N.; Baldissera, M.D.; de Oliveira, S.C.; Campigotto, G.; Da Silva, A.S.; Paiano, D. Effect of supplementation of newborn piglets with spray dry blood plasma on weight gain and serum biochemical variables. Comp. Clin. Pathol. 2016, 25, 1029–1033. [Google Scholar] [CrossRef]
- Sauerwein, H.; Schmitz, S.; Hiss, S. The acute phase protein haptoglobin and its relation to oxidative status in piglets undergoing weaning-induced stress. Redox Rep. 2005, 10, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Bruschetta, G.; Leonardi, F.; Licata, P.; Iannelli, N.M.; Fernàndez-Parra, R.; Bruno, F.; Messina, L.; Costa, G.L. Oxidative Stress in Relation to Serotonin under General Anaesthesia in Dogs Undergoing Ovariectomy. Vet. Q. 2024, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bruschetta, G.; D’Ascola, A.; Medica, P.; Ferlazzo, A.M. Physical Exercise Affects Serotoninergic System in Horse Leukocytes. J. Equine Vet. Sci. 2020, 88, 102969. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, W.; Yin, C.; Zhao, R.; Yang, X. Response to lipopolysaccharide in salivary components and the submandibular gland of pigs. Livest. Sci. 2014, 167, 323–330. [Google Scholar] [CrossRef]
- Saco, Y.; Martínez-Lobo, F.; Cortey, M.; Pato, R.; Peña, R.; Segalés, J.; Prieto, C.; Bassols, A. C-reactive protein, haptoglobin and pig-major acute phase protein profiles of pigs infected experimentally by different isolates of porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 2016, 183, 9–15. [Google Scholar] [CrossRef]
- Bruschetta, G.; Zanghì, G.; Giunta, R.P.; Ferlazzo, A.M.; Satué, K.; D’Ascola, A.; Fazio, E. Short Road Transport and Slaughter Stress Affects the Expression Profile of Serotonin Receptors, Adrenocortical, and Hematochemical Responses in Horses. Vet. Sci. 2024, 11, 113. [Google Scholar] [CrossRef]
- López-Martínez, M.J.; Escribano, D.; Ortín-Bustillo, A.; Franco-Martínez, L.; González-Arostegui, L.G.; Cerón, J.J.; Rubio, C.P. Changes in Biomarkers of Redox Status in Saliva of Pigs after an Experimental Sepsis Induction. Antioxidants 2022, 11, 1380. [Google Scholar] [CrossRef]
- Cerón, J.J.; Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Miró, S.; López-Martínez, M.J.; Ortín-Bustillo, A.; Franco-Martínez, L.; Rubio, C.P.; Muñoz-Prieto, A.; Tvarijonaviciute, A.; et al. Basics for the potential use of saliva to evaluate stress, inflammation, immune system, and redox homeostasis in pigs. BMC Vet. Res. 2022, 18, 81. [Google Scholar] [CrossRef]
- Ortín-Bustillo, A.; Escribano, D.; López-Arjona, M.; Botia, M.; Fuentes, P.; Martínez-Miró, S.; Rubio, C.P.; García-Manzanilla, E.; Franco-Martínez, L.; Pardo-Marín, L.; et al. Changes in a Comprehensive Profile of Saliva Analytes in Fattening Pigs during a Complete Productive Cycle: A Longitudinal Study. Animals 2022, 12, 1865. [Google Scholar] [CrossRef]
- Olsen, C.; Karriker, L.; Wang, C.; Binjawadagi, B.; Renukaradhya, G.; Kittawornrat, A.; Lizano, S.; Coetzee, J.; Main, R.; Meiszberg, A.; et al. Effect of collection material and sample processing on pig oral fluid testing results. Vet. J. 2013, 198, 158–163. [Google Scholar] [CrossRef]
- Franco-Martínez, L.; Ortín-Bustillo, A.; Rubio, C.P.; Escribano, D.; López-Arjona, M.; García-Manzanilla, E.; Cerón, J.J.; Martínez-Subiela, S.; Tvarijonaviciute, A.; Tecles, F. Effects of pen faeces and feed contamination in biomarkers determination in oral fluid of pigs. Res. Vet. Sci. 2022, 152, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, M.A.S.; López-Martínez, M.J.; Franco-Martínez, L.; Cerón, J.J.; Ortín-Bustillo, A.; Rubio, C.P.; Manzanilla, E.G. Analysing biomarkers in oral fluid from pigs: Influence of collection strategy and age of the pig. Porc. Health Manag. 2023, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Nemeckova, M.; Medkova, D.; Sardi, L.; Hodkovicova, N. Non-invasive methods for analysing pig welfare biomarkers. Vet. Med. 2024, 69, 137–155. [Google Scholar] [CrossRef]
- Kick, A.R.; Tompkins, M.B.; Almond, G.W. Stress and Immunity in the Pig. CABI Rev. 2011, 6, 51–67. [Google Scholar] [CrossRef]
- Gimsa, U.; Tuchscherer, M.; Kanitz, E. Psychosocial Stress and Immunity—What Can We Learn From Pig Studies? Front. Behav. Neurosci. 2018, 12, 64. [Google Scholar] [CrossRef]
- Dhabhar, F.S.; McEwen, B.S. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain Behav. Immun. 1997, 11, 286–306. [Google Scholar] [CrossRef]
- Kanitz, E.; Tuchscherer, M.; Puppe, B.; Tuchscherer, A.; Stabenow, B. Consequences of repeated early isolation in domestic piglets (Sus scrofa) on their behavioural, neuroendocrine, and immunological responses. Brain Behav. Immun. 2004, 18, 35–45. [Google Scholar] [CrossRef]
- Aschbacher, K.; O’Donovan, A.; Wolkowitz, O.M.; Dhabhar, F.S.; Su, Y.; Epel, E. Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 2013, 38, 1698–1708. [Google Scholar] [CrossRef]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Delgado-Ortega, M.; Marc, D.; Dupont, J.; Trapp, S.; Berri, M.; Meurens, F. SOCS Proteins in infectious diseases of mammals. Vet. Immunol. Immunopathol. 2013, 151, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Salak-Johnson, J.L.; McGlone, J.J. Making sense of apparently conflicting data: Stress and immunity in swine and cattle. J. Anim. Sci. 2007, 85, E81–E88. [Google Scholar] [CrossRef]
- Sánchez, J.; Matas, M.; Ibáñez-López, F.J.; Hernández, I.; Sotillo, J.; Gutiérrez, A.M. The Connection Between Stress and Immune Status in Pigs: A First Salivary Analytical Panel for Disease Differentiation. Front. Vet. Sci. 2022, 9, 881435. [Google Scholar] [CrossRef] [PubMed]
- Buchet, A.; Belloc, C.; Leblanc-Maridor, M.; Merlot, E. Effects of age and weaning conditions on blood indicators of oxidative status in pigs. PLoS ONE 2017, 12, e0178487. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.; Fellström, C.; Lindberg, R.; Wallgren, P.; Jensen-Waern, M. Experimental swine dysentery: Comparison between infection models. J. Med. Microbiol. 2004, 53, 273–280. [Google Scholar] [CrossRef]
- Heegaard, P.M.H.; Klausen, J.; Nielsen, J.P.; González-Ramón, N.; Piñeiro, M.; Lampreave, F.; Alava, M.A. The porcine acute phase response to infection with Actinobacillus pleuropneumoniae. Haptoglobin, C-reactive protein, major acute phase protein and serum amyloid A protein are sensitive indicators of infection. Comp. Biochem. Physiol. B 1998, 119, 365–373. [Google Scholar] [CrossRef]
- Sjölund, M.; Fossum, C.; de la Fuente, A.J.M.; Alava, M.; Juul-Madsen, H.R.; Lampreave, F.; Wallgren, P. Effects of different antimicrobial treatments on serum acute phase responses and leucocyte counts in pigs after a primary and a secondary challenge infection with Actinobacillus pleuropneumoniae. Vet. Rec. 2011, 169, 70. [Google Scholar] [CrossRef]
- López-Martínez, M.J.; Ornelas, M.A.S.; Amarie, R.E.; Manzanilla, E.G.; Martínez-Subiela, S.; Tecles, F.; Tvarijonaviciute, A.; Escribano, D.; González-Bulnes, A.; Cerón, J.J.; et al. Changes in salivary biomarkers of stress, inflammation, redox status, and muscle damage due to Streptococcus suis infection in pigs. BMC Vet. Res. 2023, 19, 100. [Google Scholar] [CrossRef]
- Sørensen, N.S.; Tegtmeier, C.; Andresen, L.O.; Piñeiro, M.; Toussaint, M.; Campbell, F.; Lampreave, F.; Heegaard, P. The porcine acute phase protein response to acute clinical and subclinical experimental infection with Streptococcus suis. Vet. Immunol. Immunopathol. 2006, 113, 157–168. [Google Scholar] [CrossRef]
- Pomorska-Mol, M.; Markowska-Daniel, I.; Kwit, K.; Stepniewska, K.; Pejsak, Z. Kinetics of the response of four positive acute phase proteins in pigs experimentally infected with toxigenic Pasteurella multocida. Vet. Microbiol. 2011, 152, 429–435. [Google Scholar] [CrossRef]
- Grau-Roma, L.; Heegaard, P.M.; Hjulsager, C.K.; Sibila, M.; Kristensen, C.; Allepuz, A.; Piñeiro, M.; Larsen, L.; Segalés, J.; Fraile, L. Pig-major acute phase protein and haptoglobin serum concentrations correlate with PCV2 viremia and the clinical course of postweaning multisystemic wasting syndrome. Vet. Microbiol. 2009, 138, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Piñeiro, C.; Lampreave, F.; Nofrarías, M.; Mateu, E.; Calsamiglia, M.; Andrés, M.; Morales, J.; Piñeiro, M.; Domingo, M. Haptoglobin and pig-major acute phase protein are increased in pigs with postweaning multisystemic wasting syndrome (PMWS). Vet. Res. 2004, 35, 275–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stevenson, L.S.; McCullough, K.; Vincent, I.; Gilpin, D.F.; Summerfield, A.; Nielsen, J.; McNeilly, F.; Adair, B.M.; Allan, G.M. Cytokine and C-reactive protein profiles induced by porcine circovirus type 2 experimental infection in 3-week-old piglets. Viral Immunol. 2006, 19, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.M.; Martínez-Subiela, S.; Soler, L.; Pallarés, F.J.; Cerón, J.J. Use of saliva for haptoglobin and C-reactive protein quantifications in porcine respiratory and reproductive syndrome affected pigs in field conditions. Vet. Immunol. Immunopathol. 2009, 132, 218–223. [Google Scholar] [CrossRef]
- Soler, L.; Gutiérrez, A.; Cerón, J.J. Serum amyloid A measurements in saliva and serum in growing pigs affected by porcine respiratory and reproductive syndrome in field conditions. Res. Vet. Sci. 2012, 93, 1266–1270. [Google Scholar] [CrossRef]
- Štukelj, M.; Toplak, I.; Nemec Svete, A. Blood antioxidant enzymes (SOD, GPX), biochemical and haematological parameters in pigs naturally infected with porcine reproductive and respiratory syndrome virus. Pol. J. Vet. Sci. 2013, 16, 369–376. [Google Scholar] [CrossRef]
- Gómez-Laguna, J.; Salguero, F.J.; Pallares, F.J.; de Marco, M.F.; Barranco, I.; Cerón, J.; Martínez-Subiela, S.; Van Reeth, K.; Carrasco, L. Acute phase response in porcine reproductive and respiratory syndrome virus infection. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, e51–e58. [Google Scholar] [CrossRef]
- Asai, T.; Mori, M.; Okada, M.; Uruno, K.; Yazawa, S.; Shibata, I. Elevated serum haptoglobin in pigs infected with porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 1999, 70, 143–148. [Google Scholar] [CrossRef]
- Barbé, F.; Atanasova, K.; Van Reeth, K. Cytokines and acute phase proteins associated with acute swine influenza infection in pigs. Vet. J. 2011, 187, 48–53. [Google Scholar] [CrossRef]
- Pomorska-Mól, M.; Markowska-Daniel, I.; Pejsak, Z. Acute phase protein response during subclinical infection of pigs with H1N1 swine influenza virus. Vet. Microbiol. 2012, 159, 499–503. [Google Scholar] [CrossRef]
- Sanchez-Cordon, P.J.; Cerón, J.J.; Nunez, A.; Martínez-Subiela, S.; Pedrera, M.; Romero-Trevejo, J.L.; Garrido, M.R.; Gómez-Villamandos, J.C. Serum concentrations of C-reactive protein, serum amyloid A, and haptoglobin in pigs inoculated with African swine fever or classical swine fever viruses. Am. J. Vet. Res. 2007, 68, 772–777. [Google Scholar] [CrossRef]
- Deblanc, C.; Robert, F.; Pinard, T.; Gorin, S.; Quéguiner, S.; Gautier-Bouchardon, A.V.; Ferré, S.; Garraud, J.; Cariolet, R.; Brack, M.; et al. Pre-infection of pigs with Mycoplasma hyopneumoniae induces oxidative stress that influences outcomes of a subsequent infection with a swine influenza virus of H1N1 subtype. Vet. Microbiol. 2013, 162, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Pomorska-Mól, M.; Dors, A.; Kwit, K.; Czyżewska-Dors, E.; Pejsak, Z. Coinfection modulates inflammatory responses, clinical outcome and pathogen load of H1N1 swine influenza virus and Haemophilus parasuis infections in pigs. BMC Vet. Res. 2017, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Pomorska-Mól, M.; Markowska-Daniel, I.; Kwit, K.; Stepniewska, K.; Pejsak, Z. C-reactive protein, haptoglobin, serum amyloid A and pig major acute phase protein response in pigs simultaneously infected with H1N1 swine influenza virus and Pasteurella multocida. BMC Vet. Res. 2013, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Pomorska-Mól, M.; Dors, A.; Kwit, K.; Kowalczyk, A.; Stasiak, E.; Pejsak, Z. Kinetics of single and dual infection of pigs with swine influenza virus and Actinobacillus pleuropneumoniae. Vet. Microbiol. 2017, 201, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.D.; Fuentes, P.; Tecles, F.; Martínez-Subiela, S.; Martínez, J.S.; Muñoz, A.; Cerón, J.J. Porcine acute phase protein concentrations in different diseases in field conditions. J. Vet. Med. Infect. Dis. Vet. Public Health 2006, 53, 488–493. [Google Scholar] [CrossRef]
- Carpintero, R.; Alonso, C.; Piñeiro, M.; Iturralde, M.; Andrés, M.; Le Potier, M.-F.; Madec, F.; Álava, M.; Piñeiro, A.; Lampreave, F. Pig major acute-phase protein and apolipoprotein A-I responses correlate with the clinical course of experimentally induced African swine fever and Aujeszky’s disease. Vet. Res. 2007, 38, 741–753. [Google Scholar] [CrossRef]
- Quereda, J.J.; Gómez, S.; Seva, J.; Ramis, G.; Cerón, J.J.; Muñoz, A.; Pallarés, F.J.; Dvm, J.J.Q.; Dvm, S.G.; Dvm, F.J.P. Acute phase proteins as a tool for differential diagnosis of wasting diseases in growing pigs. Vet. Rec. 2012, 170, 21. [Google Scholar] [CrossRef]
- Royer, E.; Barbé, F.; Guillou, D.; Rousselière, Y.; Chevaux, E. Development of an oxidative stress model in weaned pigs highlighting plasma biomarkers’ specificity to stress inducers. J. Anim. Sci. 2016, 94 (Suppl. 3), 48–53. [Google Scholar] [CrossRef]
- Dimri, U.; Bandyopadhyay, S.; Singh, S.K.; Ranjan, R.; Mukherjee, R.; Yatoo, M.I.; Patra, P.; De, U.; Dar, A. Assay of alterations in oxidative stress markers in pigs naturally infested with Sarcoptes scabiei var suis. Vet. Parasitol. 2014, 205, 295–299. [Google Scholar] [CrossRef]
- Wierzchosławski, K.; Kwit, K.; Pejsak, Z.; Pomorska-Mól, M. Selected serum acute-phase proteins in peripartum sows and evaluation of their diagnostic usefulness. Anim. Reprod. Sci. 2018, 191, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, F.; Becker, S.; Kuehling, J.; Schrade, H.; Lechner, M.; Ringseis, R.; Eder, K.; Moritz, A.; Reiner, G. Inflammation and necrosis syndrome is associated with alterations in blood and metabolism in pigs. BMC Vet. Res. 2022, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Marco-Ramell, A.; Pato, R.; Peña, R.; Saco, Y.; Manteca, X.; de la Torre, J.R.; Bassols, A. Identification of serum stress biomarkers in pigs housed at different stocking densities. Vet. J. 2011, 190, e66–e71. [Google Scholar] [CrossRef] [PubMed]
- Valros, A.; López-Martínez, M.J.; Munsterhjelm, C.; López-Arjona, M.; Cerón, J.J. Novel saliva biomarkers for stress and infection in pigs: Changes in oxytocin and procalcitonin in pigs with tail-biting lesions. Res. Vet. Sci. 2022, 153, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wymore Brand, M.; Souza, C.K.; Gauger, P.; Arruda, B.; Vincent Baker, A.L. Biomarkers associated with vaccine-associated enhanced respiratory disease following influenza A virus infection in swine. Vet. Immunol. Immunopathol. 2024, 273, 110787. [Google Scholar] [CrossRef]
| Health–Welfare Disorders or Pathogens * | Stress Biomarker ** | Age | Biomatrices | References |
|---|---|---|---|---|
| Swine dysentery (Brachyspira hyodysenteriae) |
| Weaners Growers Finishers | Serum | [98] |
| Porcine pleuropneumonia (App) |
| Growers | Serum | [99] |
| Growers | Serum | [100] | |
| S. suis |
| Growers | Saliva | [101] |
| Weaners | Serum | [102] | |
| P. multocida |
| Weaners | Serum | [103] |
| PCV2 (PMWS) |
| Weaners Growers | Serum | [104,105] |
| Weaners | Serum | [106] | |
| PRRSV |
| Weaners | Serum | [80] |
| Weaners Growers Finishers | Saliva Serum | [107] | |
| Growers | Saliva Serum | [108] | |
| Weaners Growers Finishers | Serum | [109] | |
| Weaners | Serum | [110] | |
| Weaners | Serum | [111] | |
| SIV |
| Weaners | Serum | [112] |
| Weaners | Serum | [113] | |
| ASFV or CSFV |
| Growers Finishers | Serum | [114] |
| Co-infection with M. hyo and SIV (H1N1) |
| Weaners | Serum | [115] |
| Co-infection with swine influenza virus (H1N1) and Haemophilus parasuis |
| Weaners | Serum | [116] |
| Co-infection with SIV (H1N1) and P. multocida |
| Weaners | Serum | [117] |
| Co-infection with SIV (H1N1) and App |
| Weaners | Serum | [118] |
| Co-infection with PRRSV, ADV, PCV2 and M. hyo |
| Weaners | Serum | [119] |
| Co-infection with ASFV and ADV |
| Growers Finishers | Serum | [120] |
| Porcine respiratory disease complex (PRDC) |
| Serum | [121] | |
| Vaccination at weaning against PCV2 and SIV, under heat stress |
| Weaners | Blood | [122] |
| Sarcoptes scabiei var. suis |
| 1–2 years of age | Blood Skin | [123] |
| Post-partum dysgalactia syndrome (PDS) |
| Sows | Serum | [124] |
| Swine inflammation and necrosis syndrome (SINS) |
| Suckling Piglets Weaners | Serum | [125] |
| Stocking density |
| Growers | Serum | [126] |
| Tail biting |
| Weaners | Saliva | [127] |
| Endotoxemia, administration of LPS |
| Growers | Saliva | [63] |
| Vaccine-associated enhanced respiratory disease following SIV infection |
| Weaners | Serum | [128] |
| Heat stress |
| Sows | Plasma | [68] |
| Mycotoxicosis (aflatoxin B1, fumonisins: fumonisin B1 and fumonisin B2) |
| Sows | Plasma | [69] |
| Mycotoxicosis (fumonisins) |
| Weaners | Plasma | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papatsiros, V.G.; Maragkakis, G.; Papakonstantinou, G.I. Stress Biomarkers in Pigs: Current Insights and Clinical Application. Vet. Sci. 2024, 11, 640. https://doi.org/10.3390/vetsci11120640
Papatsiros VG, Maragkakis G, Papakonstantinou GI. Stress Biomarkers in Pigs: Current Insights and Clinical Application. Veterinary Sciences. 2024; 11(12):640. https://doi.org/10.3390/vetsci11120640
Chicago/Turabian StylePapatsiros, Vasileios G., Georgios Maragkakis, and Georgios I. Papakonstantinou. 2024. "Stress Biomarkers in Pigs: Current Insights and Clinical Application" Veterinary Sciences 11, no. 12: 640. https://doi.org/10.3390/vetsci11120640
APA StylePapatsiros, V. G., Maragkakis, G., & Papakonstantinou, G. I. (2024). Stress Biomarkers in Pigs: Current Insights and Clinical Application. Veterinary Sciences, 11(12), 640. https://doi.org/10.3390/vetsci11120640







