A Universal Multi-Epitope Vaccine Design Against Porcine Reproductive and Respiratory Syndrome Virus via Bioinformatics and Immunoinformatics Approaches
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition and Alignment of Representative Strain Sequences
2.2. Prediction and Selection of Antigenic Epitopes
2.3. Evaluation of Physicochemical and Biochemical Properties
2.4. Prediction, Refinement, and Validation of Protein Structure
2.5. Molecular Docking
2.6. Immune Response Simulation
2.7. Codon Optimization and In Silico Cloning
3. Results
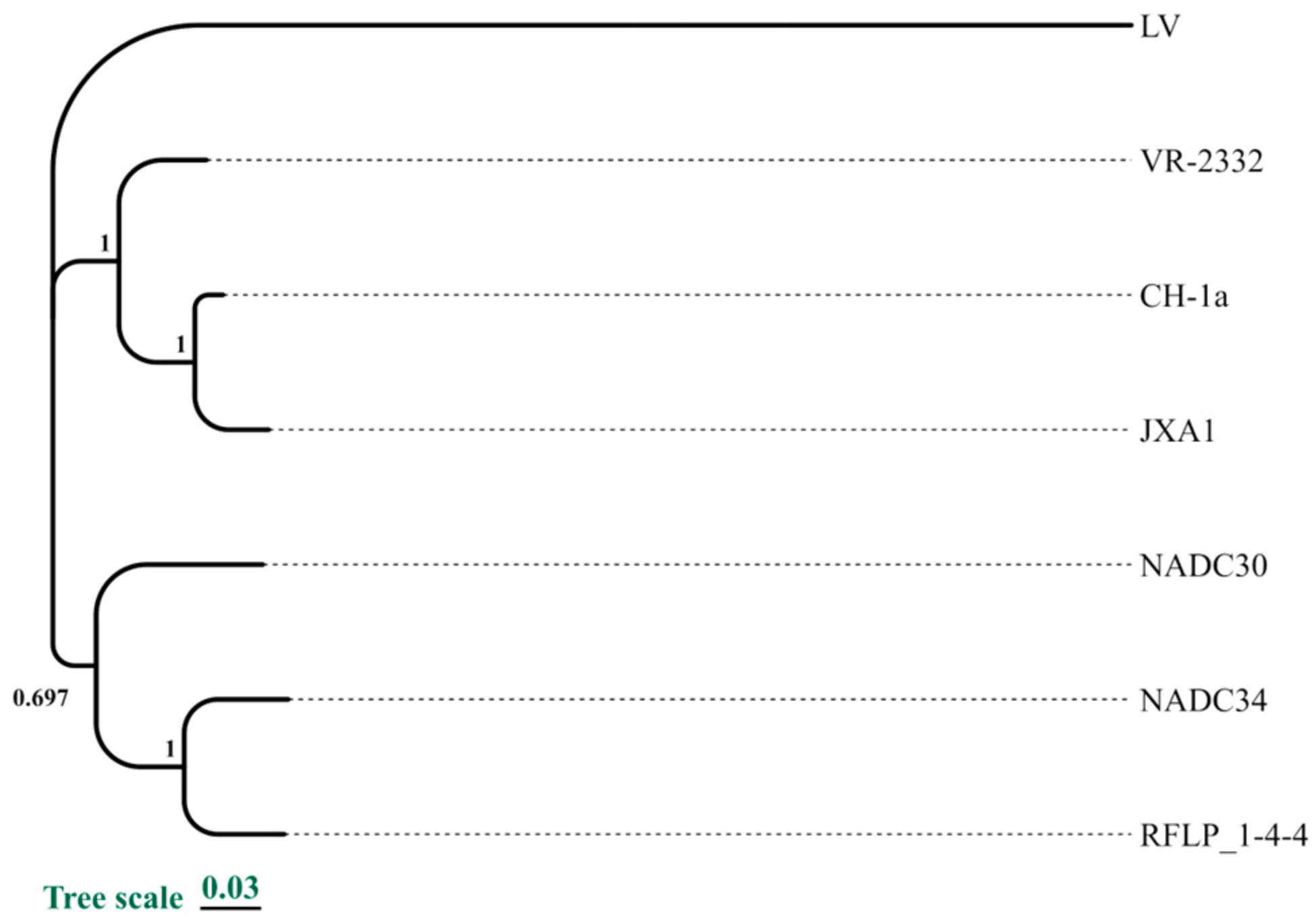
3.1. Selection of Reference Strains and Conserved Sequence Analysis
3.2. Prediction and Selection of Epitopes
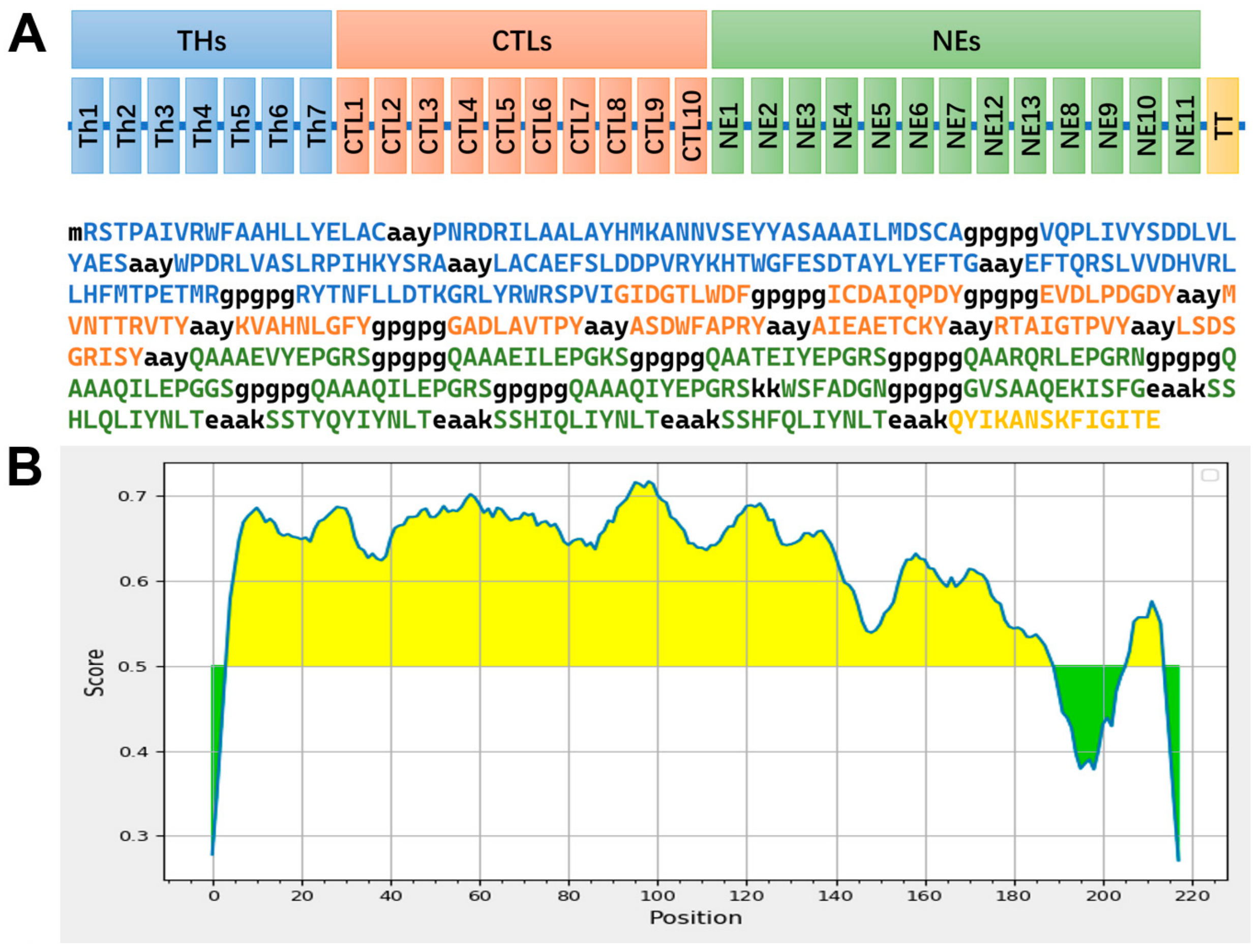
3.3. Construction of the Multi-Epitope Vaccine (THs-CTLs-NEs)
3.4. Physicochemical and Biochemical Properties of the Multi-Epitope Vaccine
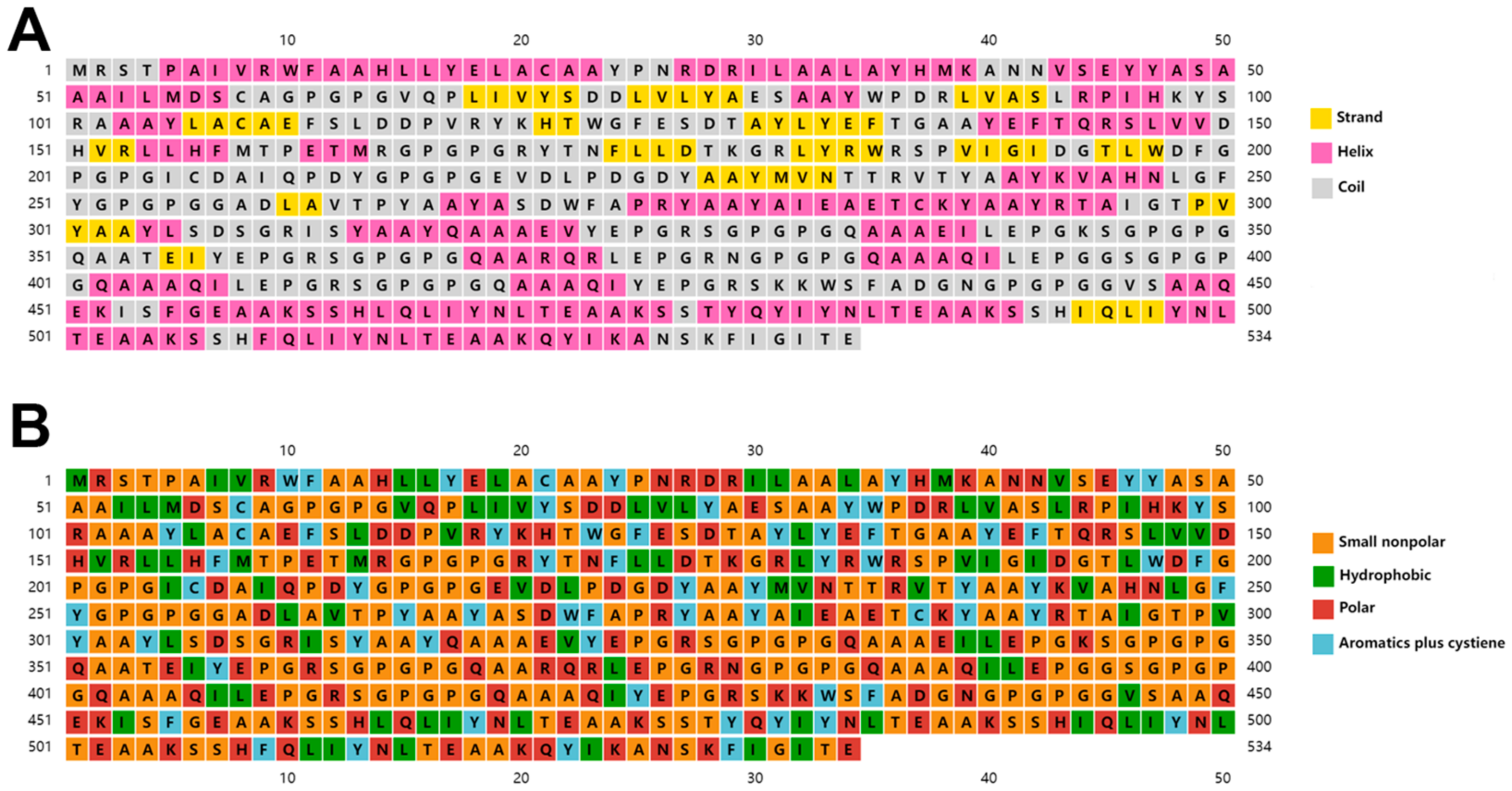
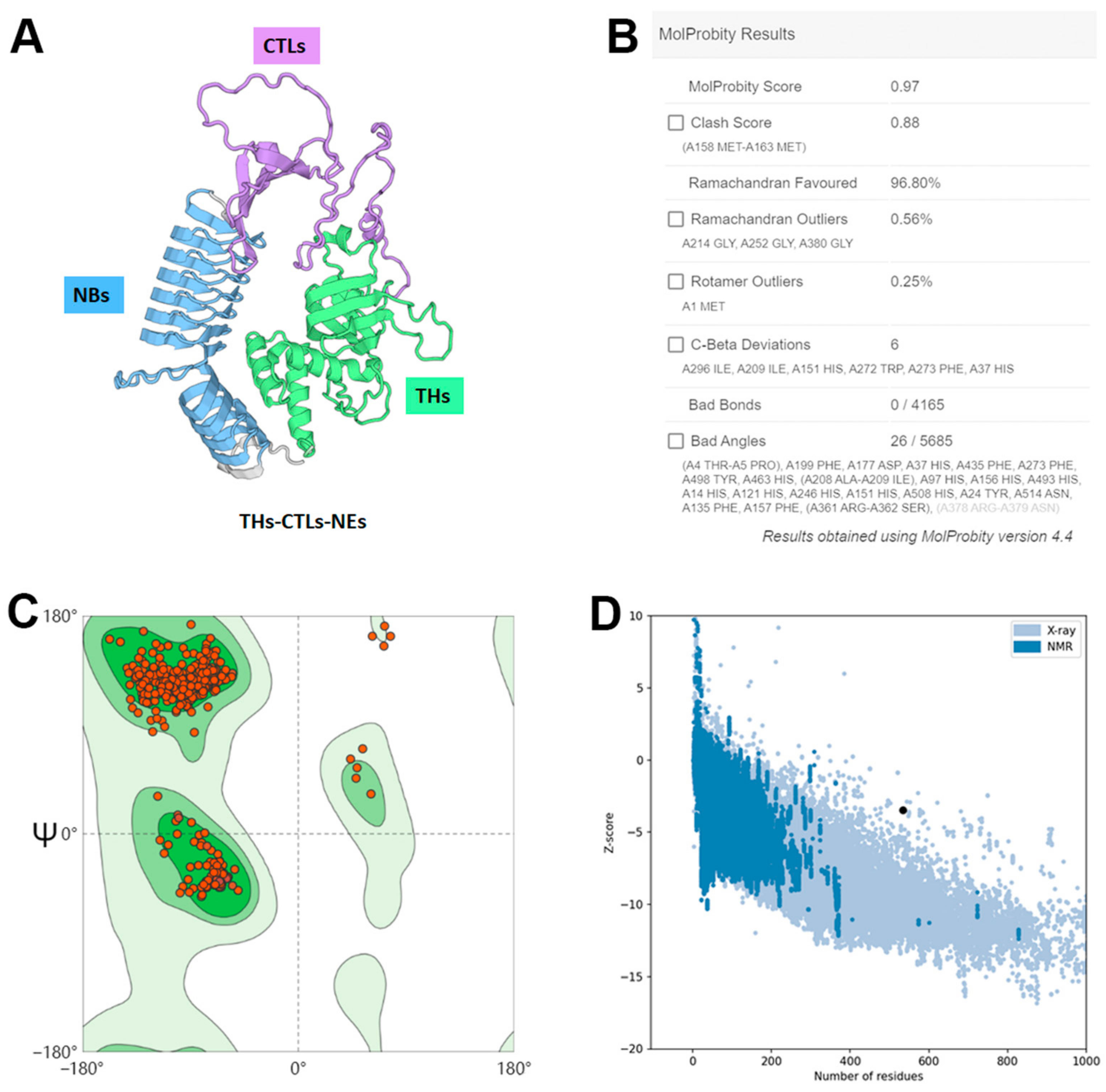
3.5. Secondary and Tertiary Structure Prediction of the Multi-Epitope Vaccine
3.6. Molecular Docking of the Constructed Vaccine with Porcine TLR2 and TLR4
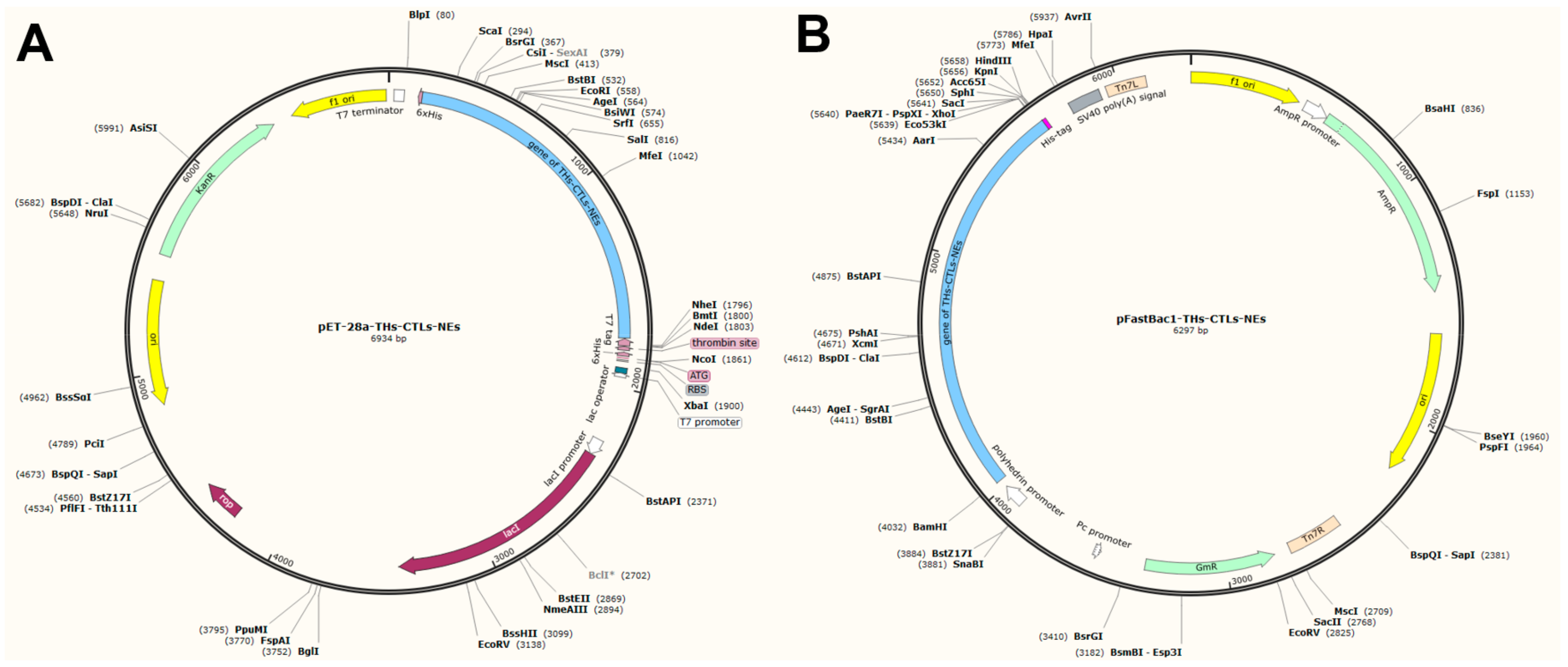
3.7. In Silico Cloning of the Vaccine Candidate
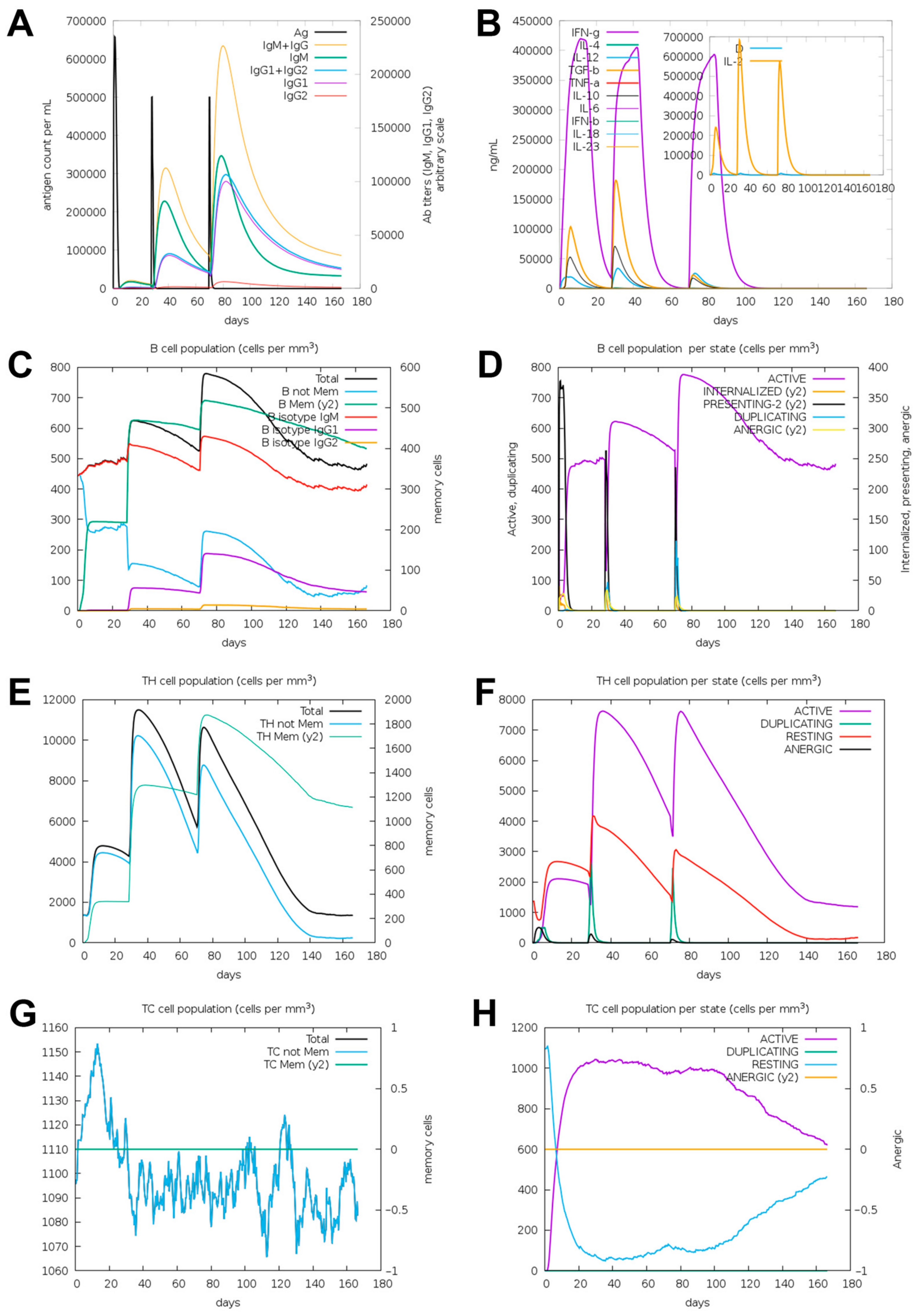
3.8. Immunogenicity Simulation of the Epitope-Based Vaccine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Z.; Wang, F.X.; Han, X.Y.; Guo, H.; Liu, C.Y.; Hou, L.N.; Wang, Y.X.; Zheng, H.; Wang, L.; Wen, Y.J. Recent advances in the study of NADC34-like porcine reproductive and respiratory syndrome virus in China. Front. Microbiol. 2022, 13, 950402. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Ban, J.; Wu, Z.; Cao, S.; Zhou, M.; Zhang, L.; Zhu, R.; Lu, H.; Zhu, S. Boosting PRRSV-Specific Cellular Immunity: The Immunological Profiling of an Fc-Fused Multi-CTL Epitope Vaccine in Mice. Vet. Sci. 2024, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Ruedas-Torres, I.; Rodríguez-Gómez, I.M.; Sánchez-Carvajal, J.M.; Larenas-Muñoz, F.; Pallarés, F.J.; Carrasco, L.; Gómez-Laguna, J. The jigsaw of PRRSV virulence. Vet. Microbiol. 2021, 260, 109168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Liu, M.; Long, X.; Guo, C. Intervention strategies targeting virus and host factors against porcine reproductive and respiratory syndrome virus: A systematic review. Int. J. Biol. Macromol. 2024, 279, 135403. [Google Scholar] [CrossRef]
- Stadejek, T.; Stankevicius, A.; Murtaugh, M.P.; Oleksiewicz, M.B. Molecular evolution of PRRSV in Europe: Current state of play. Vet. Microbiol. 2013, 165, 21–28. [Google Scholar] [CrossRef]
- Benfield, D.A.; Nelson, E.; Collins, J.E.; Harris, L.; Goyal, S.M.; Robison, D.; Christianson, W.T.; Morrison, R.B.; Gorcyca, D.; Chladek, D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J. Vet. Diagn. Invest. 1992, 4, 127–133. [Google Scholar] [CrossRef]
- Zhou, L.; Han, J.; Yang, H. The evolution and diversity of porcine reproductive and respiratory syndrome virus in China. Vet. Microbiol. 2024, 298, 110252. [Google Scholar] [CrossRef]
- Sun, Y.F.; Liu, Y.; Yang, J.; Li, W.Z.; Yu, X.X.; Wang, S.Y.; Li, L.A.; Yu, H. Recombination between NADC34-like and QYYZ-like strain of porcine reproductive and respiratory syndrome virus with high pathogenicity for piglets in China. Transbound. Emerg. Dis. 2022, 69, e3202–e3207. [Google Scholar] [CrossRef]
- Jiao, S.; Zhang, J.; Wang, J.; Ma, X.; Li, G.; Li, J.; Cui, Z.; Li, D.; Li, P.; Zeng, Q.; et al. Whole-genome analysis of the recombination and evolution of newly identified NADC30-like porcine reproductive and respiratory syndrome virus strains circulated in Gansu province of China in 2023. Front. Vet. Sci. 2024, 11, 1372032. [Google Scholar] [CrossRef]
- Kikuti, M.; Paploski, I.A.D.; Pamornchainavakul, N.; Picasso-Risso, C.; Schwartz, M.; Yeske, P.; Leuwerke, B.; Bruner, L.; Murray, D.; Roggow, B.D.; et al. Emergence of a New Lineage 1C Variant of Porcine Reproductive and Respiratory Syndrome Virus 2 in the United States. Front. Vet. Sci. 2021, 8, 752938. [Google Scholar] [CrossRef] [PubMed]
- Renukaradhya, G.J.; Meng, X.J.; Calvert, J.G.; Roof, M.; Lager, K.M. Live porcine reproductive and respiratory syndrome virus vaccines: Current status and future direction. Vaccine 2015, 33, 4069–4080. [Google Scholar] [CrossRef] [PubMed]
- Renukaradhya, G.J.; Meng, X.J.; Calvert, J.G.; Roof, M.; Lager, K.M. Inactivated and subunit vaccines against porcine reproductive and respiratory syndrome: Current status and future direction. Vaccine 2015, 33, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef]
- Prakash, S.; Dhanushkodi, N.R.; Zayou, L.; Ibraim, I.C.; Quadiri, A.; Coulon, P.G.; Tifrea, D.F.; Suzer, B.; Shaik, A.M.; Chilukuri, A.; et al. Cross-protection induced by highly conserved human B, CD4+, and CD8+ T-cell epitopes-based vaccine against severe infection, disease, and death caused by multiple SARS-CoV-2 variants of concern. Front. Immunol. 2024, 15, 1328905. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Pu, W.; Pan, X.; Li, P.; Bai, Q.; Liang, S.; Li, C.; Yu, Y.; Yao, H.; et al. A multi-epitope subunit vaccine providing broad cross-protection against diverse serotypes of Streptococcus suis. NPJ Vaccines 2024, 9, 216. [Google Scholar] [CrossRef]
- Parvizpour, S.; Pourseif, M.M.; Razmara, J.; Rafi, M.A.; Omidi, Y. Epitope-based vaccine design: A comprehensive overview of bioinformatics approaches. Drug Discov. Today 2020, 25, 1034–1042. [Google Scholar] [CrossRef]
- Cao, Q.M.; Tian, D.; Heffron, C.L.; Subramaniam, S.; Opriessnig, T.; Foss, D.L.; Calvert, J.G.; Meng, X.-J. Cytotoxic T lymphocyte epitopes identified from a contemporary strain of porcine reproductive and respiratory syndrome virus enhance CD4+CD8+ T, CD8+ T, and γδ T cell responses. Virology 2019, 538, 35–44. [Google Scholar] [CrossRef]
- Wang, C.Y.; Peng, W.J.; Kuo, B.S.; Ho, Y.H.; Wang, M.S.; Yang, Y.T.; Chang, P.Y.; Shen, Y.H.; Hwang, K.P. Toward a pan-SARS-CoV-2 vaccine targeting conserved epitopes on spike and non-spike proteins for potent, broad and durable immune responses. PLoS Pathog. 2023, 19, e1010870. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Y. Epitope screening and vaccine molecule design of PRRSV GP3 and GP5 protein based on immunoinformatics. J. Cell Mol. Med. 2024, 28, e18103. [Google Scholar] [CrossRef]
- Gao, C.; He, X.; Quan, J.; Jiang, Q.; Lin, H.; Chen, H.; Qu, L. Specificity Characterization of SLA Class I Molecules Binding to Swine-Origin Viral Cytotoxic T Lymphocyte Epitope Peptides In Vitro. Front. Microbiol. 2017, 8, 2524. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.K.; Kim, H.R.; Jang, H.; Chang, K.S. Replacing the decoy epitope of PCV2 capsid protein with epitopes of GP3 and/or GP5 of PRRSV enhances the immunogenicity of bivalent vaccines in mice. J. Virol. Methods. 2020, 284, 113928. [Google Scholar] [CrossRef] [PubMed]
- Piñeyro, P.E.; Kenney, S.P.; Giménez-Lirola, L.G.; Heffron, C.L.; Matzinger, S.R.; Opriessnig, T.; Meng, X.J. Expression of antigenic epitopes of porcine reproductive and respiratory syndrome virus (PRRSV) in a modified live-attenuated porcine circovirus type 2 (PCV2) vaccine virus (PCV1-2a) as a potential bivalent vaccine against both PCV2 and PRRSV. Virus Res. 2015, 210, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, P.G. GP5 ectodomain epitope of porcine reproductive and respiratory syndrome virus, strain Lelystad virus. Virus Res. 2004, 102, 225–230. [Google Scholar] [CrossRef]
- Plagemann, P.G.; Rowland, R.R.; Faaberg, K.S. The primary neutralization epitope of porcine respiratory and reproductive syndrome virus strain VR-2332 is located in the middle of the GP5 ectodomain. Arch. Virol. 2002, 147, 2327–2347. [Google Scholar] [CrossRef]
- Ostrowski, M.; Galeota, J.A.; Jar, A.M.; Platt, K.B.; Osorio, F.A.; Lopez, O.J. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J. Virol. 2002, 76, 4241–4250. [Google Scholar] [CrossRef]
- Wissink, E.H.J.; van Wijk, H.A.R.; Kroese, M.V.; Weiland, E.; Meulenberg, J.J.M.; Rottier, P.J.M.; van Rijn, P.A. The major envelope protein, GP5, of a European porcine reproductive and respiratory syndrome virus contains a neutralization epitope in its N-terminal ectodomain. J. Gen. Virol. 2003, 84, 1535–1543. [Google Scholar] [CrossRef]
- Costers, S.; Lefebvre, D.J.; Van Doorsselaere, J.; Vanhee, M.; Delputte, P.L.; Nauwynck, H.J. GP4 of porcine reproductive and respiratory syndrome virus contains a neutralizing epitope that is susceptible to immunoselection in vitro. Arch. Virol. 2010, 155, 371–378. [Google Scholar] [CrossRef]
- Vanhee, M.; Costers, S.; Van Breedam, W.; Geldhof, M.F.; Van Doorsselaere, J.; Nauwynck, H.J. A variable region in GP4 of European-type porcine reproductive and respiratory syndrome virus induces neutralizing antibodies against homologous but not heterologous virus strains. Viral Immunol. 2010, 23, 403–413. [Google Scholar] [CrossRef]
- Van Breedam, W.; Costers, S.; Vanhee, M.; Gagnon, C.A.; Rodríguez-Gómez, I.M.; Geldhof, M.; Verbeeck, M.; Van Doorsselaere, J.; Karniychuk, U.; Nauwynck, H.J. Porcine reproductive and respiratory syndrome virus (PRRSV)-specific mAbs: Supporting diagnostics and providing new insights into the antigenic properties of the virus. Vet. Immunol. Immunopathol. 2011, 141, 246–257. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, M.; Wang, N.; Yang, Y.; Wang, D. Designing a Candidate Multi-Epitope Vaccine against Transmissible Gastroenteritis Virus Based on Immunoinformatic and Molecular Dynamics. Int. J. Mol. Sci. 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, G.; Xiong, Y.; Li, F.; Chen, Y.; Cheng, Y.; Ma, J.; Wang, H.; Yan, Y.; Wang, Z.; et al. Development of a Universal Multi-Epitope Vaccine Candidate against Streptococcus suis Infections Using Immunoinformatics Approaches. Vet. Sci. 2023, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, S.; Amirzakaria, J.Z.; Ghasemian, A. In silico design and validation of a novel multi-epitope vaccine candidate against structural proteins of Chikungunya virus using comprehensive immunoinformatics analyses. PLoS ONE 2023, 18, e0285177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Tan, C.; Li, C.; Ma, S.; Wen, H.; Yang, H.; Rao, M.; Zhang, P.; Peng, W.; Cui, Y.; et al. Design of a multi-epitope vaccine against six Nocardia species based on reverse vaccinology combined with immunoinformatics. Front. Immunol. 2023, 14, 1100188. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.; Li, J.; Dai, Z.; Huang, J.; Deng, F.; Wang, X.; Yue, X.; Hu, X.; Li, Y.; et al. Designing a multi-epitope vaccine against coxsackievirus B based on immunoinformatics approaches. Front. Immunol. 2022, 13, 933594. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, X.; Noor, A.U.; Zhang, X.; Song, C.; Sun, H. Enhancing half-life and cytotoxicity of porcine respiratory and reproductive syndrome virus soluble receptors by taming their Fc domains. Vet. Microbiol. 2022, 273, 109526. [Google Scholar] [CrossRef]
- Rath, T.; Baker, K.; Dumont, J.A.; Peters, R.T.; Jiang, H.; Qiao, S.W.; Lencer, W.I.; Pierce, G.F.; Blumberg, R.S. Fc-fusion proteins and FcRn: Structural insights for longer-lasting and more effective therapeutics. Crit. Rev. Biotechnol. 2015, 35, 235–254. [Google Scholar] [CrossRef]
- Melgoza-González, E.A.; Bustamante-Córdova, L.; Hernández, J. Recent advances in antigen targeting to antigen-presenting cells in veterinary medicine. Front. Immunol. 2023, 14, 1080238. [Google Scholar] [CrossRef]
- Danazumi, A.U.; Iliyasu Gital, S.; Idris, S.; Bs Dibba, L.; Balogun, E.O.; Górna, M.W. Immunoinformatic design of a putative multi-epitope vaccine candidate against Trypanosoma brucei gambiense. Comput. Struct. Biotechnol. J. 2022, 20, 5574–5585. [Google Scholar] [CrossRef]
- Andongma, B.T.; Huang, Y.; Chen, F.; Tang, Q.; Yang, M.; Chou, S.H.; Li, X.; He, J. In silico design of a promiscuous chimeric multi-epitope vaccine against Mycobacterium tuberculosis. Comput. Struct. Biotechnol. J. 2023, 21, 991–1004. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, J.; McGovern, B.L.; Ranzenigo, A.; Huang, W.; Sang, Z.; Shen, J.; Diaz-Tapia, R.; Pham, N.D.; Teunissen, A.J.P.; et al. Adaptive multi-epitope targeting and avidity-enhanced nanobody platform for ultrapotent, durable antiviral therapy. Cell 2024, 187, 6966–6980.e23. [Google Scholar] [CrossRef] [PubMed]

| Epitope | Protein | Sequence | Length | Core Sequence | Epitope Conservation | High Binders |
|---|---|---|---|---|---|---|
| Th1 | Nsp9 | RSTPAIVRWFAAHLLYELAC (H/N) | 20 | VRWFAAHLL | 6/7 | 12 |
| Th2 | Nsp9 | PNRDRILAALAYHMKANNVSEYYASAAAILMDSCA (N/S) | 35 | ILAALAYHM YYASAAAIL | 6/7 | 28 |
| Th3 | Nsp9 | VQPLIVYSDDLVLYAES | 17 | VYSDDLVLY | 6/7 | 9 |
| Th4 | Nsp11 | WPDRLVASLRPIHKYSRA (A/T) | 18 | VTSLRPIHK | 6/7 | 12 |
| Th5 | Nsp12 | LACAEFSLDDPVRYKHTWGFESDTAYLYEFTG (R/K) | 32 | FESDTAYLY FSLDDPVRY | 6/7 | 22 |
| Th6 | GP4 | EFTQRSLVVDHVRLLHFMTPETMR | 24 | LVVDHVRLL LLHFMTPET | 5/7 | 9 |
| Th7 | GP5 | RYTNFLLDTKGRLYRWRSPVI (R/K) | 21 | FLLDTKGRL | 5/7 | 8 |
| Epitope | Protein | Sequence | Length | Epitope Conservation |
|---|---|---|---|---|
| CTL1 | nsp9 | GIDGTLWDF | 9 | 5/7 |
| CTL2 | nsp10 | ICDAIQPDY | 9 | 6/7 |
| CTL3 | nsp10 | EVDLPDGDY | 9 | 5/7 |
| CTL4 | nsp10 | MVNTTRVTY | 9 | 5/7 |
| CTL5 | nsp11 | KVAHNLGFY | 9 | 6/7 |
| CTL6 | nsp12 | GADLAVTPY | 9 | 6/7 |
| CTL7 | GP2a | ASDWFAPRY | 9 | 6/7 |
| CTL8 | GP2a | AIEAETCKY | 9 | 6/7 |
| CTL9 | GP4 | RTAIGTPVY | 9 | 6/7 |
| CTL10 | N | LSDSGRISY | 9 | 6/7 |
| Epitope | Protein | Sequence | Length | Strain | References |
|---|---|---|---|---|---|
| NE1 | GP3 | QAAAEVYEPGRS | 12 | CH-1a | [22,23] |
| NE2 | GP3 | QAAAEILEPGKS | 12 | JXA1 | N/A |
| NE3 | GP3 | QAATEIYEPGRS | 12 | VR-2332 | N/A |
| NE4 | GP3 | QAARQRLEPGRN | 12 | LV | N/A |
| NE5 | GP3 | QAAAQILEPGGS | 12 | NADC30 | N/A |
| NE6 | GP3 | QAAAQILEPGRS | 12 | RFLP_1-4-4 | N/A |
| NE7 | GP3 | QAAAQIYEPGRS | 12 | NADC34 | N/A |
| NE8 | GP5 | SSHLQLIYNLT | 11 | VR-2332 RFLP_1-4-4 NADC30 NADC34 | [24,25] |
| NE9 | GP5 | SSTYQYIYNLT | 11 | LV | [26] |
| NE10 | GP5 | SSHIQLIYNLT | 11 | JXA1 | N/A |
| NE11 | GP5 | SSHFQLIYNLT | 11 | CH-1a | N/A |
| NE12 | GP5 | WSFADGN | 7 | LV | [27] |
| NE13 | GP4 | GVSAAQEKISFG | 12 | LV | [28,29,30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, X.; Wu, Z.; Feng, Q.; Jia, W.; Xie, J.; Zhou, Q.; Ban, J.; Zhu, S. A Universal Multi-Epitope Vaccine Design Against Porcine Reproductive and Respiratory Syndrome Virus via Bioinformatics and Immunoinformatics Approaches. Vet. Sci. 2024, 11, 659. https://doi.org/10.3390/vetsci11120659
Lei X, Wu Z, Feng Q, Jia W, Xie J, Zhou Q, Ban J, Zhu S. A Universal Multi-Epitope Vaccine Design Against Porcine Reproductive and Respiratory Syndrome Virus via Bioinformatics and Immunoinformatics Approaches. Veterinary Sciences. 2024; 11(12):659. https://doi.org/10.3390/vetsci11120659
Chicago/Turabian StyleLei, Xinnuo, Zhi Wu, Qi Feng, Wenfeng Jia, Jun Xie, Qingkang Zhou, Jinzhao Ban, and Shanyuan Zhu. 2024. "A Universal Multi-Epitope Vaccine Design Against Porcine Reproductive and Respiratory Syndrome Virus via Bioinformatics and Immunoinformatics Approaches" Veterinary Sciences 11, no. 12: 659. https://doi.org/10.3390/vetsci11120659
APA StyleLei, X., Wu, Z., Feng, Q., Jia, W., Xie, J., Zhou, Q., Ban, J., & Zhu, S. (2024). A Universal Multi-Epitope Vaccine Design Against Porcine Reproductive and Respiratory Syndrome Virus via Bioinformatics and Immunoinformatics Approaches. Veterinary Sciences, 11(12), 659. https://doi.org/10.3390/vetsci11120659






