Selenium Enhances the Growth of Bovine Endometrial Stromal Cells by PI3K/AKT/GSK-3β and Wnt/β-Catenin Pathways
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Isolation and Culture of Primary BESCs
2.3. Experimental Design
2.4. Cell Viability Assay
2.5. Western Blot Analysis
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Cell Cycle Analysis
2.8. Apoptosis Analysis
2.9. Statistical Analysis
3. Results
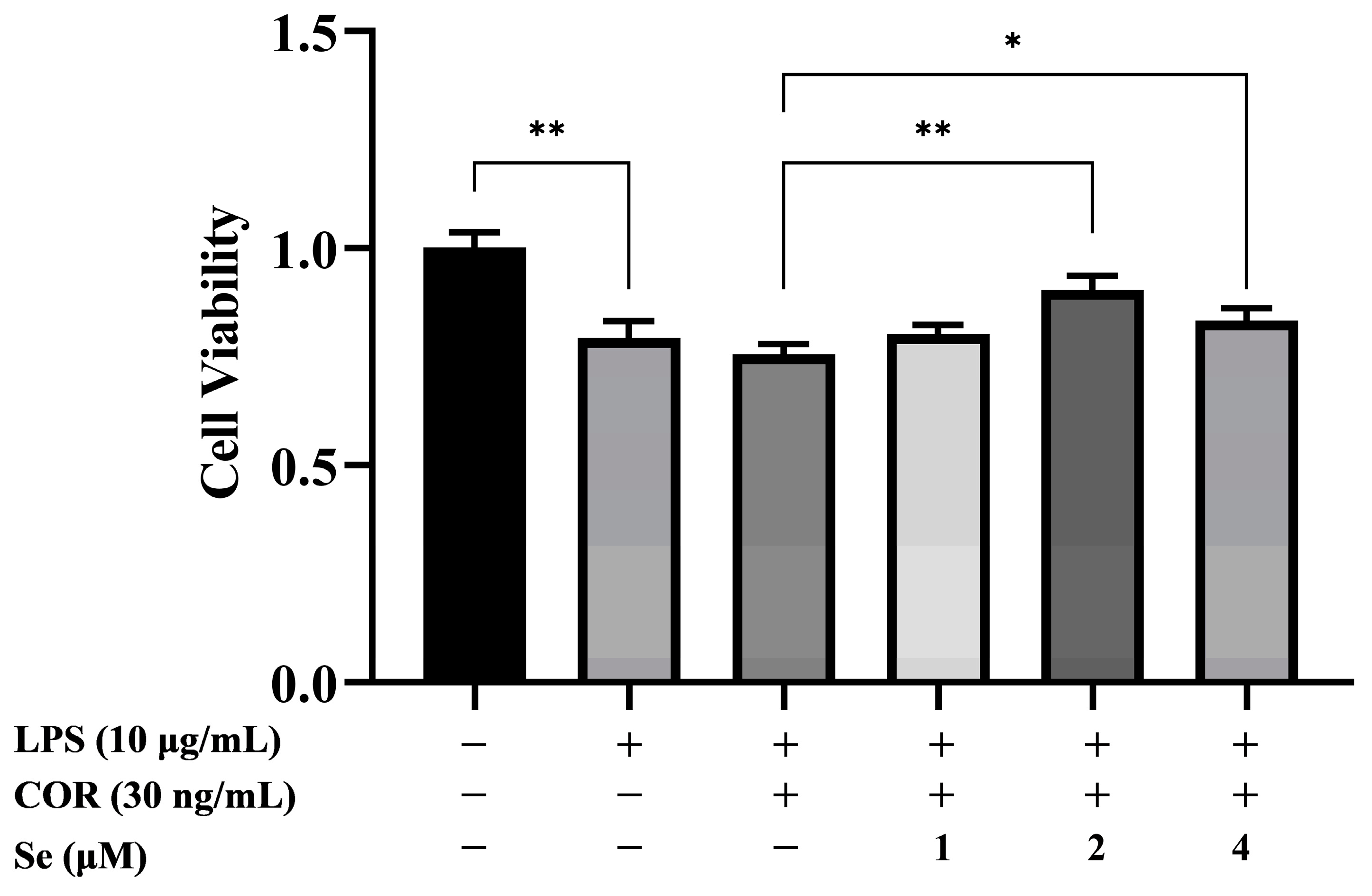
3.1. Se Promoted the Viability of BESCs
3.2. Se Increased the mRNA Levels of Growth Factors in BESCs
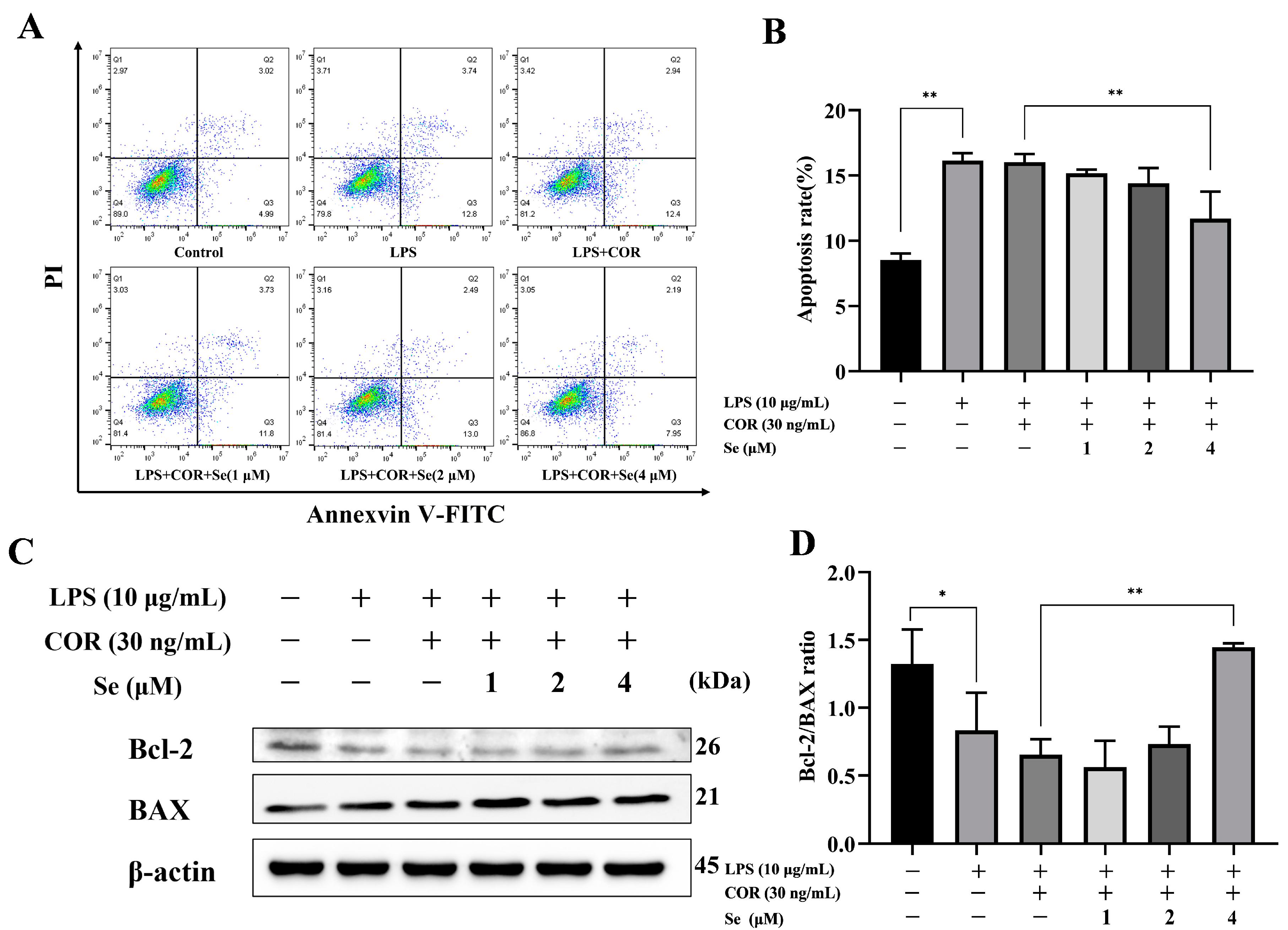
3.3. Se-Attenuated Apoptosis of BESCs
3.4. Se Promoted the Cell Cycle of BESCs
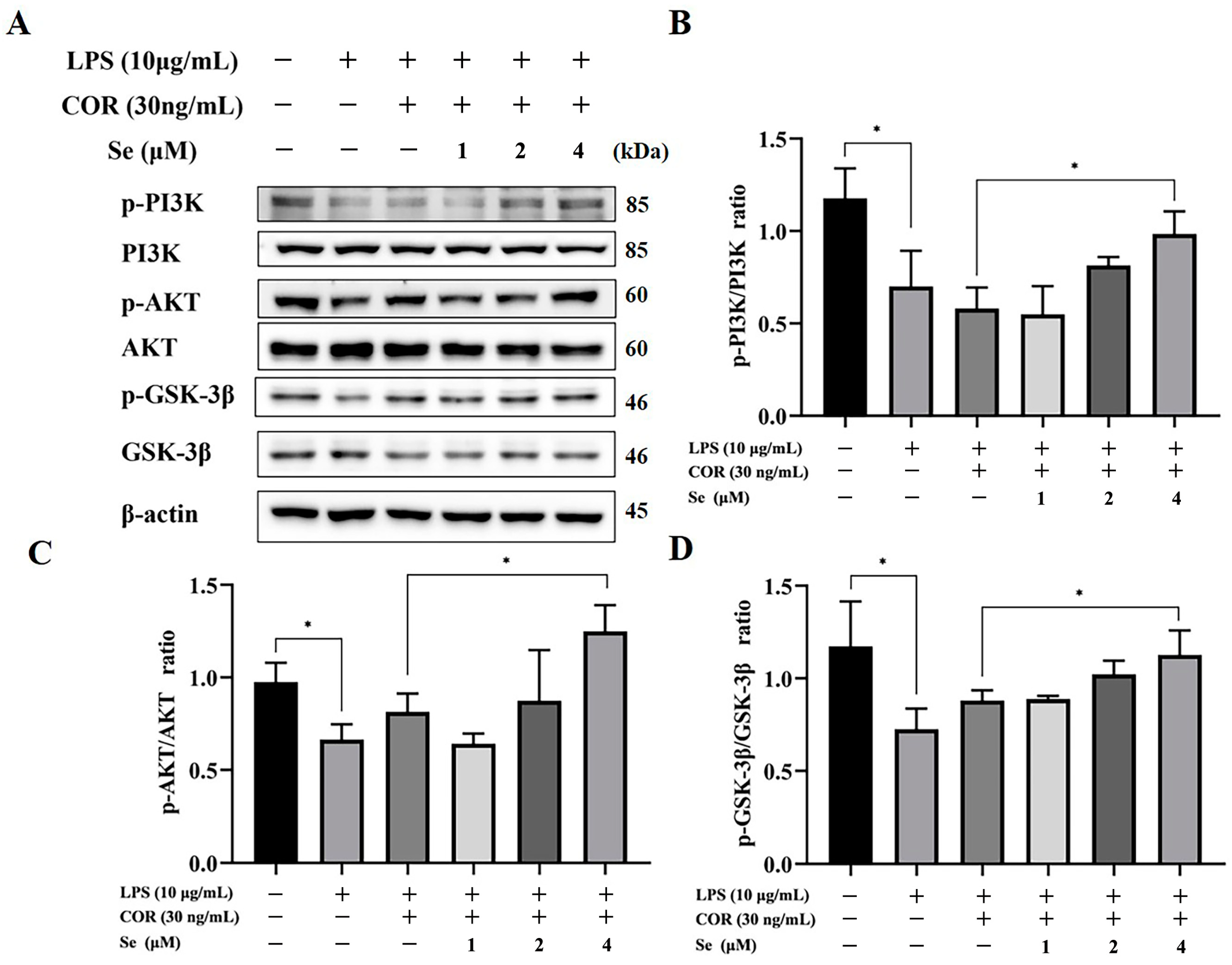
3.5. Se Activated PI3K/AKT/GSK-3β Signaling Pathway of BESCs
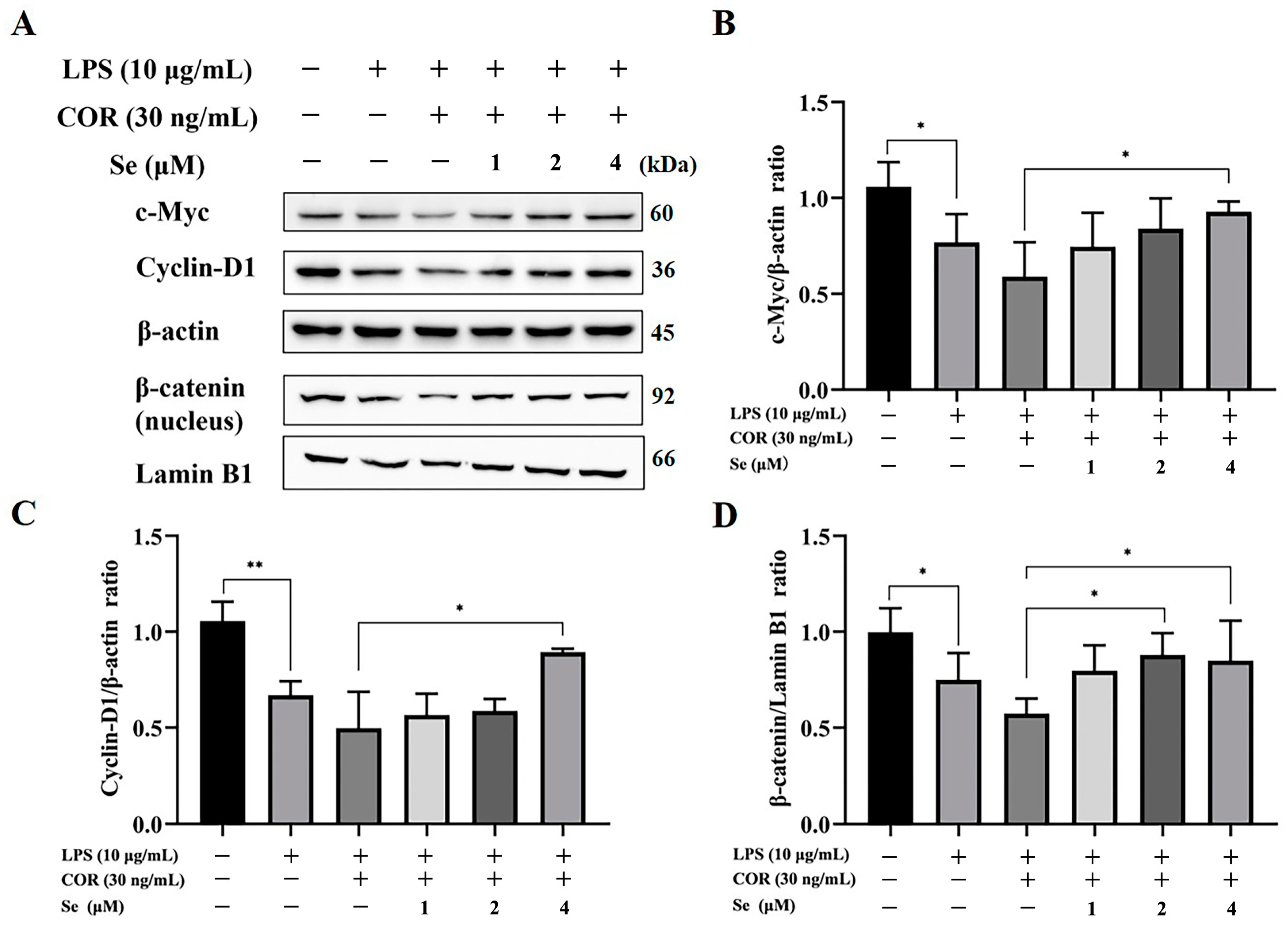
3.6. Se Triggered the Activity of the Wnt/β-Catenin Signaling Pathway in BESCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.; Yang, H.; Ahmad, M.J.; Yang, Y.; Yang, W.; Riaz, H.; Abulaiti, A.; Zhang, S.; Yang, L.; Hua, G. Postpartum Uterine Involution and Embryonic Development Pattern in Chinese Holstein Dairy Cows. Front. Vet. Sci. 2020, 7, 604729. [Google Scholar] [CrossRef]
- Bogado Pascottini, O.; LeBlanc, S.J.; Gnemi, G.; Leroy, J.; Opsomer, G. Genesis of clinical and subclinical endometritis in dairy cows. Reproduction 2023, 166, R15–R24. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Molinari, P.C.C.; Ormsby, T.J.R.; Bromfield, J.J. Preventing postpartum uterine disease in dairy cattle depends on avoiding, tolerating and resisting pathogenic bacteria. Theriogenology 2020, 150, 158–165. [Google Scholar] [CrossRef]
- Dong, J.; Ji, B.; Jiang, Y.; Liu, K.; Guo, L.; Cui, L.; Wang, H.; Li, B.; Li, J. Autophagy activation alleviates the LPS-induced inflammatory response in endometrial epithelial cells in dairy cows. Am. J. Reprod. Immunol. 2024, 91, e13820. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Lv, H.; Deng, L.; Hu, W.; Peng, Z.; Yan, C.; Yang, D.; Tong, C.; Wang, X. Analysis of Transcriptomic Changes in Bovine Endometrial Stromal Cells Treated With Lipopolysaccharide. Front. Vet. Sci. 2020, 7, 575865. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.G.; Turner, M.L.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol. Reprod. 2012, 86, 51. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [PubMed]
- Nagel, C.; Aurich, C.; Aurich, J. Stress effects on the regulation of parturition in different domestic animal species. Anim. Reprod. Sci. 2019, 207, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.T.; Piotrowska-Tomala, K.K.; Acosta, T.J.; Bah, M.M.; Sinderewicz, E.; Majewska, M.; Jankowska, K.; Okuda, K.; Skarzynski, D.J. Effects of cortisol on pregnancy rate and corpus luteum function in heifers: An in vivo study. J. Reprod. Dev. 2012, 58, 223–230. [Google Scholar] [CrossRef]
- Amsterdam, A.; Tajima, K.; Sasson, R. Cell-specific regulation of apoptosis by glucocorticoids: Implication to their anti-inflammatory action. Biochem. Pharmacol. 2002, 64, 843–850. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Zust, J.; Hrovatin, B.; Simundić, B. Assessment of selenium and vitamin E deficiencies in dairy herds and clinical disease in calves. Vet. Rec. 1996, 139, 391–394. [Google Scholar] [CrossRef]
- Spears, J.W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef]
- Ceko, M.J.; Hummitzsch, K.; Hatzirodos, N.; Bonner, W.M.; Aitken, J.B.; Russell, D.L.; Lane, M.; Rodgers, R.J.; Harris, H.H. X-Ray fluorescence imaging and other analyses identify selenium and GPX1 as important in female reproductive function. Metallomics 2015, 7, 71–82. [Google Scholar] [CrossRef]
- Wilde, D. Influence of macro and micro minerals in the peri-parturient period on fertility in dairy cattle. Anim. Reprod. Sci. 2006, 96, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cheng, W.; Zhang, L. Maternal selenium deficiency suppresses proliferation, induces autophagy dysfunction and apoptosis in the placenta of mice. Metallomics 2021, 13, mfab058. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Ren, K.K.; Zhang, W.J.; Xiao, L.; Wu, H.Y.; Liu, Q.Y.; Ding, T.; Zhang, X.C.; Nie, W.J.; Ke, Y.; et al. Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem. Cell Res. Ther. 2019, 10, 247. [Google Scholar] [CrossRef]
- Kim, S.M.; Vetrivel, P.; Ha, S.E.; Kim, H.H.; Kim, J.A.; Kim, G.S. Apigetrin induces extrinsic apoptosis, autophagy and G2/M phase cell cycle arrest through PI3K/AKT/mTOR pathway in AGS human gastric cancer cell. J. Nutr. Biochem. 2020, 83, 108427. [Google Scholar] [CrossRef] [PubMed]
- Goad, J.; Ko, Y.A.; Kumar, M.; Syed, S.M.; Tanwar, P.S. Differential Wnt signaling activity limits epithelial gland development to the anti-mesometrial side of the mouse uterus. Dev. Biol. 2017, 423, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, J.; Li, J.; Cui, L.; Meng, X.; Qu, Y.; Wang, H. The proliferative effect of cortisol on bovine endometrial epithelial cells. Reprod. Biol. Endocrinol. 2019, 17, 97. [Google Scholar] [CrossRef]
- Piras, C.; Guo, Y.; Soggiu, A.; Chanrot, M.; Greco, V.; Urbani, A.; Charpigny, G.; Bonizzi, L.; Roncada, P.; Humblot, P. Changes in protein expression profiles in bovine endometrial epithelial cells exposed to E. coli LPS challenge. Mol. Biosyst. 2017, 13, 392–405. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Cui, L.; Liu, K.; Guo, L.; Li, J.; Dong, J. The effect of selenium on the proliferation of bovine endometrial epithelial cells in a lipopolysaccharide-induced damage model. BMC Vet. Res. 2024, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Cui, L.; Liu, K.; Shao, X.; Sun, W.; Li, J.; Wang, H.; Qian, C.; Li, J.; Dong, J. Cortisol inhibits lipopolysaccharide-induced inflammatory response in bovine endometrial stromal cells via NF-κB and MAPK signaling pathways. Dev. Comp. Immunol. 2022, 133, 104426. [Google Scholar] [CrossRef]
- Siqueira, L.C.; Favaretto, B.; Moraes, B.T.; de Freitas, V.O.; Bicalho, R.C.; Horn, R.C.; Bassuino, D.M.; Wolkmer, P. Bovine Endometritis and the Inflammatory Peripheral Cholinergic System. Appl. Biochem. Biotechnol. 2020, 190, 1242–1256. [Google Scholar] [CrossRef]
- Nakamura, A.; Kimura, F.; Tsuji, S.; Hanada, T.; Takebayashi, A.; Takahashi, A.; Kitazawa, J.; Morimune, A.; Amano, T.; Kushima, R.; et al. Bovine lactoferrin suppresses inflammatory cytokine expression in endometrial stromal cells in chronic endometritis. J. Reprod. Immunol. 2022, 154, 103761. [Google Scholar] [CrossRef] [PubMed]
- Monika, P.; Waiker, P.V.; Chandraprabha, M.N.; Rangarajan, A.; Murthy, K.N.C. Myofibroblast progeny in wound biology and wound healing studies. Wound. Repair. Regen. 2021, 29, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.J.; Mardaryev, A.N.; Poterlowicz, K.; Sharova, T.Y.; Aziz, A.; Sharpe, D.T.; Botchkareva, N.V.; Sharov, A.A. Bone morphogenetic protein signaling suppresses wound-induced skin repair by inhibiting keratinocyte proliferation and migration. J. Invest. Dermatol. 2014, 134, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, J.; Cui, L.; Liu, K.; Guo, L.; Li, J.; Wang, H. The effect and mechanism of selenium supplementation on the proliferation capacity of bovine endometrial epithelial cells exposed to lipopolysaccharide in vitro under high cortisol background. J. Anim. Sci. 2024, 102, skae021. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, Z.; Liu, Q.; Zhang, R.; Ni, J. Se-methylselenocysteine inhibits apoptosis induced by clusterin knockdown in neuroblastoma N2a and SH-SY5Y cell lines. Int. J. Mol. Sci. 2014, 15, 21331–21347. [Google Scholar] [CrossRef]
- Hu, J.; Pan, D.; Li, G.; Chen, K.; Hu, X. Regulation of programmed cell death by Brd4. Cell Death Dis. 2022, 13, 1059. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Mohan, S.; Abdelwahab, S.I.; Kamalidehghan, B.; Syam, S.; May, K.S.; Harmal, N.S.; Shafifiyaz, N.; Hadi, A.H.; Hashim, N.M.; Rahmani, M.; et al. Involvement of NF-κB and Bcl2/Bax signaling pathways in the apoptosis of MCF7 cells induced by a xanthone compound Pyranocycloartobiloxanthone A. Phytomedicine 2012, 19, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum. Reprod. 2014, 29, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Shukla, N.; Naik, A.; Moryani, K.; Soni, M.; Shah, J.; Dave, H. TGF-β at the crossroads of multiple prognosis in breast cancer, and beyond. Life Sci. 2022, 310, 121011. [Google Scholar] [CrossRef]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-β1 Signaling and Tissue Fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10, a022293. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, R.; Wang, G.; Zhang, Y.; Liu, F. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res. Ther. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Nafiu, A.B.; Rahman, M.T. Selenium added unripe carica papaya pulp extracts enhance wound repair through TGF-β1 and VEGF-a signalling pathway. BMC Complement. Altern. Med. 2015, 15, 369. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Sun, Y.; Qiu, X.; Huang, J.; Wang, A.; Zhang, Q.; Pang, S.; Huang, Q.; Zhou, R.; Li, L. The Intracellular Interaction of Porcine β-Defensin 2 with VASH1 Alleviates Inflammation via Akt Signaling Pathway. J. Immunol. 2022, 208, 2795–2805. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shan, C.; Wu, Z.; Yu, H.; Yang, A.; Tan, B. Emodin alleviated pulmonary inflammation in rats with LPS-induced acute lung injury through inhibiting the mTOR/HIF-1α/VEGF signaling pathway. Inflamm. Res. 2020, 69, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lin, J.; He, W.; Huang, K. Selenium and Taurine Combination Is Better Than Alone in Protecting Lipopolysaccharide-Induced Mammary Inflammatory Lesions via Activating PI3K/Akt/mTOR Signaling Pathway by Scavenging Intracellular ROS. Oxid. Med. Cell. Longev. 2021, 2021, 5048375. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Zhao, J.; Su, J.; Chen, J.; Cui, X.; Sun, M.; Zhang, X. Curcumin induces mitochondrial apoptosis in human hepatoma cells through BCLAF1-mediated modulation of PI3K/AKT/GSK-3β signaling. Life Sci. 2022, 306, 120804. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Sun, Y.; Hu, X.; Liu, L. Gigantol inhibits proliferation and enhanced oxidative stress-mediated apoptosis through modulating of Wnt/β-catenin signaling pathway in HeLa cells. J. Biochem. Mol. Toxicol. 2022, 36, e22944. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X. Selenium Antagonizes the Lead-Induced Apoptosis of Chicken Splenic Lymphocytes In Vitro by Activating the PI3K/Akt Pathway. Biol. Trace. Elem. Res. 2018, 182, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Zhang, J.; Qi, Y.H.; Kong, M.; Liu, S.A.; Hu, J.J. Linc-ROR promotes endometrial cell proliferation by activating the PI3K-Akt pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2218–2225. [Google Scholar] [PubMed]
- Wu, X.; Li, S.; Xue, P.; Li, Y. Liraglutide Inhibits the Apoptosis of MC3T3-E1 Cells Induced by Serum Deprivation through cAMP/PKA/β-Catenin and PI3K/AKT/GSK3β Signaling Pathways. Mol. Cells 2018, 41, 234–243. [Google Scholar]
- Yu, J.; Liu, D.; Sun, X.; Yang, K.; Yao, J.; Cheng, C.; Wang, C.; Zheng, J. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/β-catenin signaling via transactivation of GSK-3β and Axin2 expression. Cell Death Dis. 2019, 10, 26. [Google Scholar] [CrossRef]
- Feng, M.; Wang, K.; Fu, S.; Wei, H.; Mu, X.; Li, L.; Zhang, S. Tubulin TUBB4B Is Involved in Spermatogonia Proliferation and Cell Cycle Processes. Genes 2022, 13, 1082. [Google Scholar] [CrossRef]
- Kapoor, S.; Padwad, Y.S. Phloretin induces G2/M arrest and apoptosis by suppressing the β-catenin signaling pathway in colorectal carcinoma cells. Apoptosis 2023, 28, 810–829. [Google Scholar] [CrossRef] [PubMed]


| Names | Primers (5′→3′) | Product Sizes (bp) | Accession Number |
|---|---|---|---|
| β-actin-F | CATCACCATCGGCAATGAGC | 156 | NM_173979.3 |
| β-actin-R | AGCACCGTGTTGGCGTAGAG | ||
| VEGF-F | CCTGATGCGGTGCGGGGGCT | 372 | NM_001316992.1 |
| VEGF-R | TGGTGGTGGCGGCGGCTATG | ||
| CTGF-F | AGCTGACCTGGAGGAGAACA | 139 | NM_174030.2 |
| CTGF-R | GTCTGTGCACACTCCGCAGA | ||
| TGF-β1-F | CGAGCCCTGGACACCAACTA | 137 | NM_001166068.1 |
| TGF-β1-R | AGGCAGAAATTGGCGTGGTA | ||
| TGF-β3-F | CTGTGCGTGAATGGCTCTTG | 153 | XM_005212206.5 |
| TGF-β3-R | CATCATCGCTGTCCACACCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Wang, Z.; Fei, F.; Jiang, Y.; Jiang, Y.; Guo, L.; Liu, K.; Cui, L.; Meng, X.; Li, J.; et al. Selenium Enhances the Growth of Bovine Endometrial Stromal Cells by PI3K/AKT/GSK-3β and Wnt/β-Catenin Pathways. Vet. Sci. 2024, 11, 674. https://doi.org/10.3390/vetsci11120674
Dong J, Wang Z, Fei F, Jiang Y, Jiang Y, Guo L, Liu K, Cui L, Meng X, Li J, et al. Selenium Enhances the Growth of Bovine Endometrial Stromal Cells by PI3K/AKT/GSK-3β and Wnt/β-Catenin Pathways. Veterinary Sciences. 2024; 11(12):674. https://doi.org/10.3390/vetsci11120674
Chicago/Turabian StyleDong, Junsheng, Zi Wang, Fan Fei, Yeqi Jiang, Yongshuai Jiang, Long Guo, Kangjun Liu, Luying Cui, Xia Meng, Jianji Li, and et al. 2024. "Selenium Enhances the Growth of Bovine Endometrial Stromal Cells by PI3K/AKT/GSK-3β and Wnt/β-Catenin Pathways" Veterinary Sciences 11, no. 12: 674. https://doi.org/10.3390/vetsci11120674
APA StyleDong, J., Wang, Z., Fei, F., Jiang, Y., Jiang, Y., Guo, L., Liu, K., Cui, L., Meng, X., Li, J., & Wang, H. (2024). Selenium Enhances the Growth of Bovine Endometrial Stromal Cells by PI3K/AKT/GSK-3β and Wnt/β-Catenin Pathways. Veterinary Sciences, 11(12), 674. https://doi.org/10.3390/vetsci11120674








