A Systematic Review and Meta-Analysis of the Effects of Various Sources and Amounts of Copper on Nursery Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Review and Database Compilation
2.2. Database Coding and Structuring
2.3. Statistical Analysis
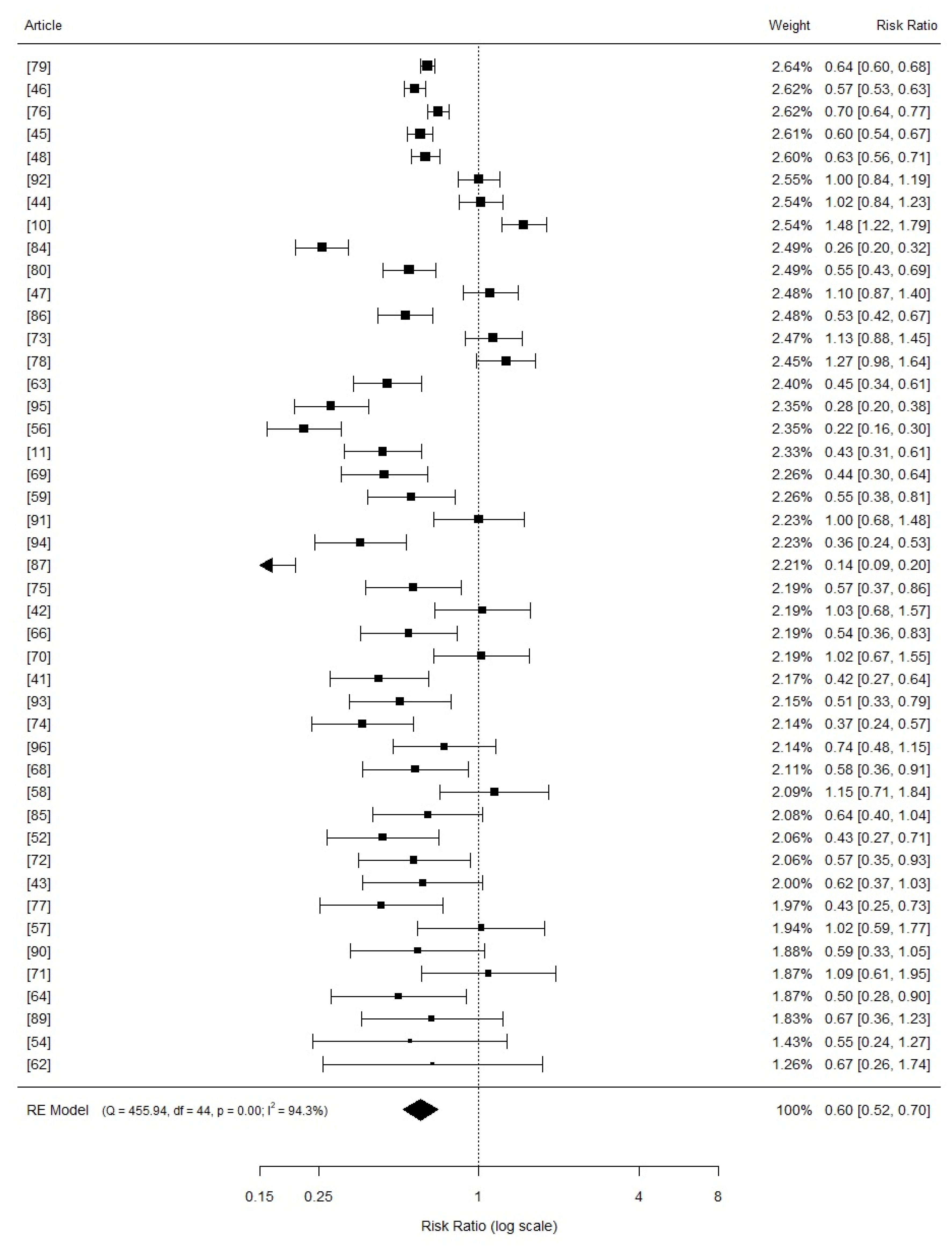
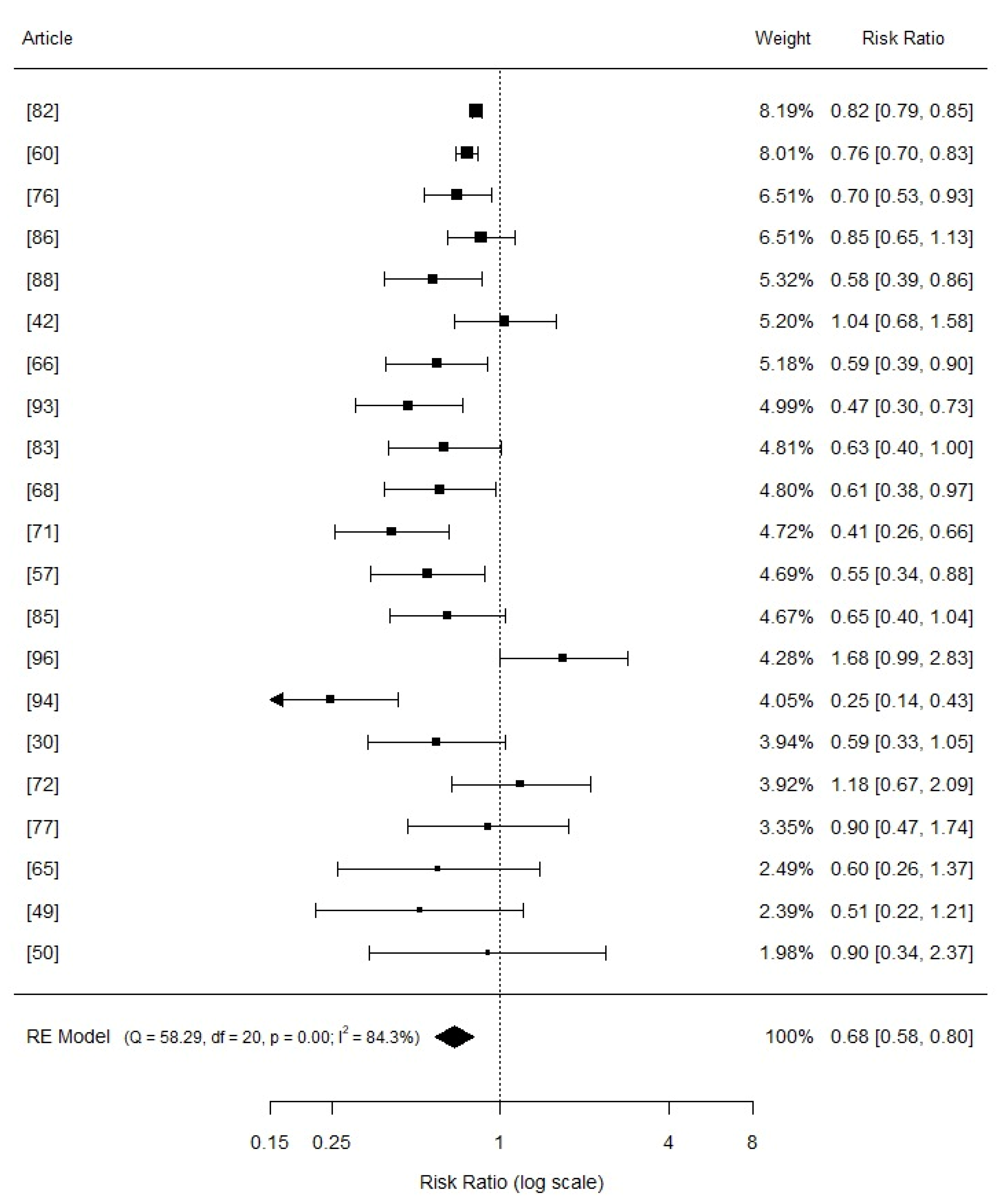
3. Results
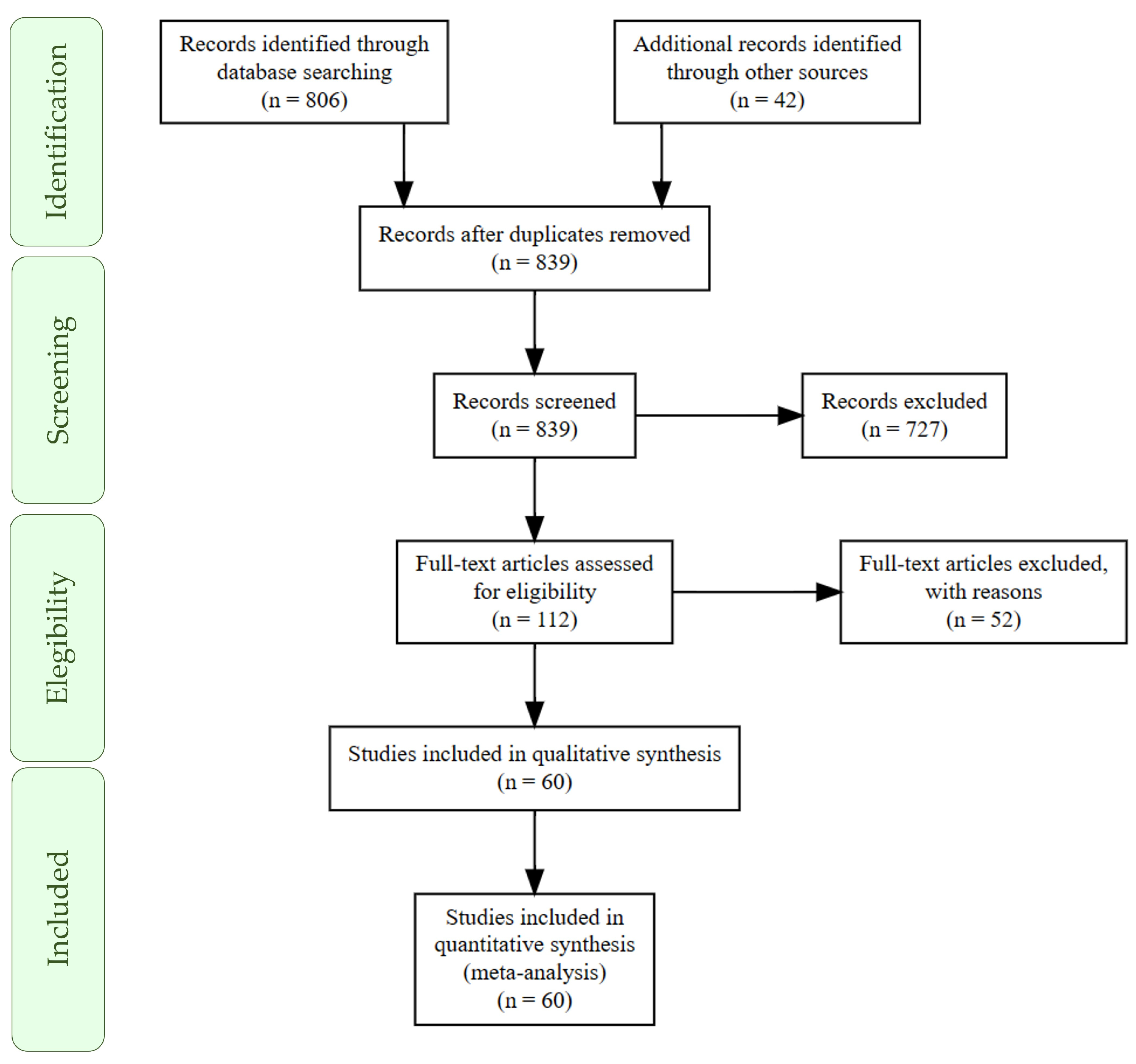
3.1. Literature Search
3.2. Database Description
3.3. Comphreensive Meta-Analysis
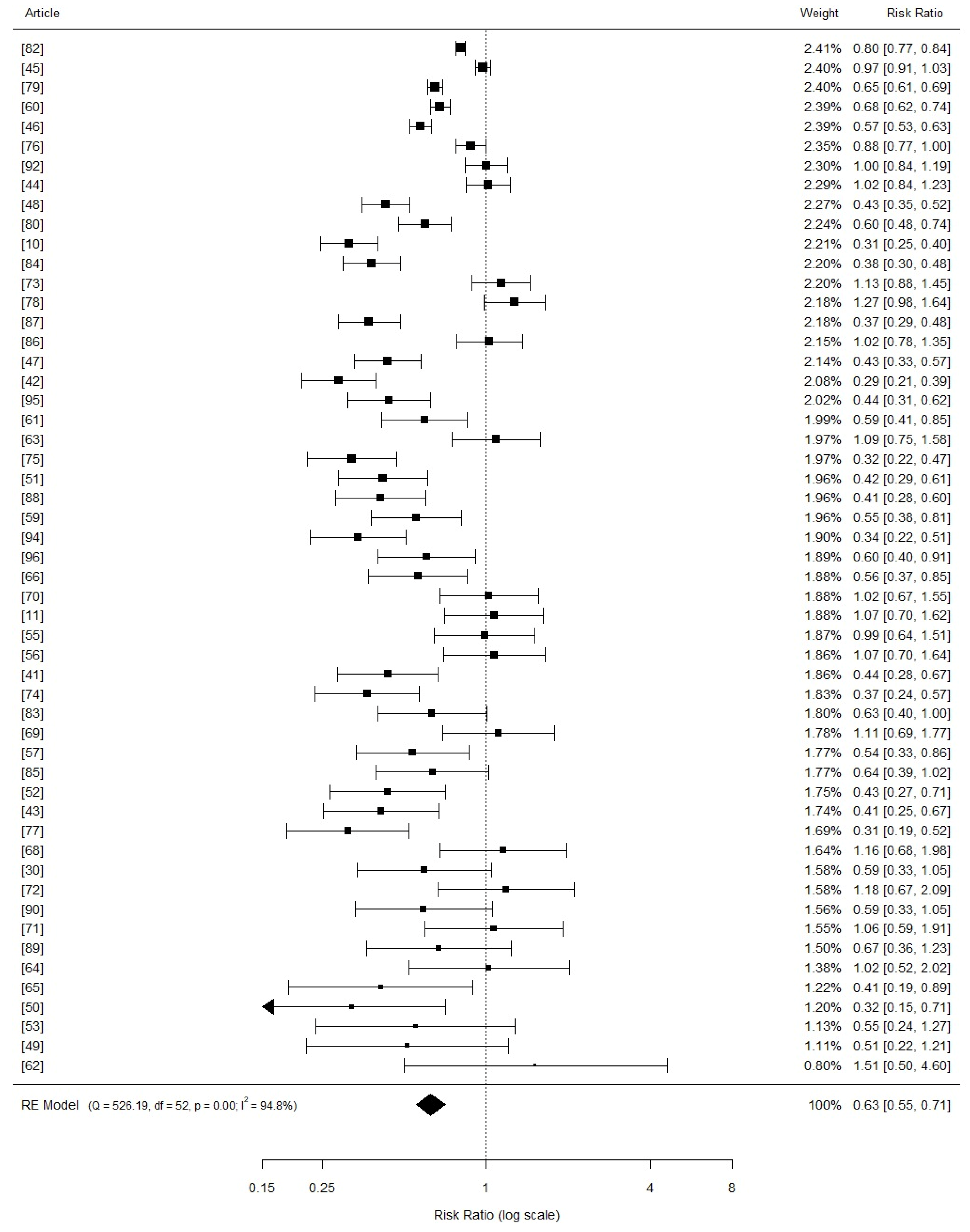
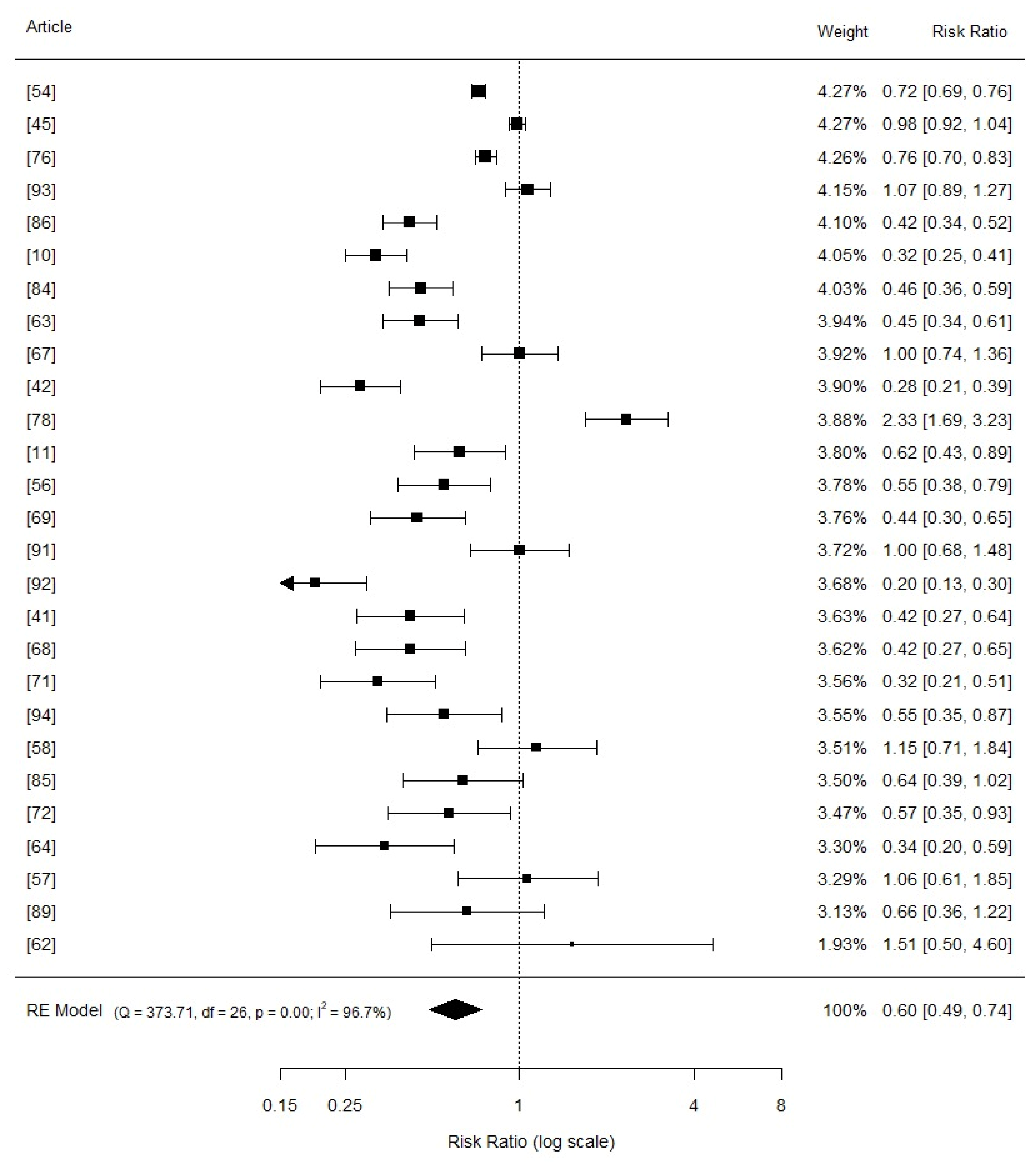
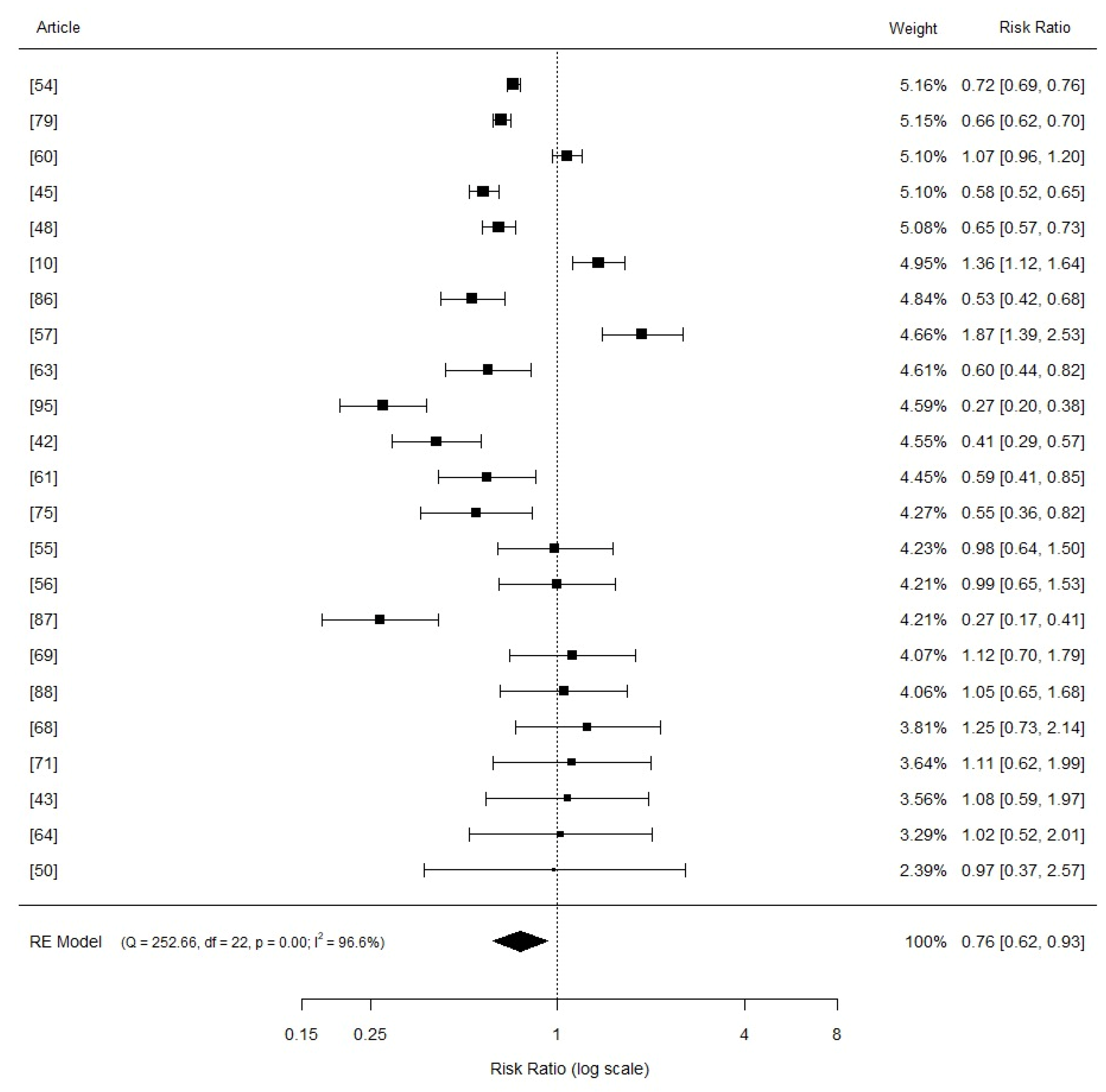
3.4. Effects of Copper Supplementation on Piglets Growth Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, P.; Shu, X.; Tang, M.; Tan, B.; Yin, Y. Effect of Dietary Copper Source (Inorganic vs. Chelated) on Immune Response, Mineral Status, and Fecal Mineral Excretion in Nursery Piglets. Food Agric. Immunol. 2018, 29, 548–563. [Google Scholar] [CrossRef]
- Dalto, D.B.; Da Silva, C.A. A Survey of Current Levels of Trace Minerals and Vitamins Used in Commercial Diets by the Brazilian Pork Industry—A Comparative Study. Transl. Anim. Sci. 2021, 4, txaa195. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Espinosa, C.D.; Fry, R.S.; Kocher, M.E.; Stein, H.H. Effects of Copper Hydroxychloride on Growth Performance and Abundance of Genes Involved in Lipid Metabolism of Growing Pigs. J. Anim. Sci. 2020, 98, skz369. [Google Scholar] [CrossRef]
- Hashimoto, A.; Kambe, T. Mg, Zn and Cu Transport Proteins: A Brief Overview from Physiological and Molecular Perspectives. J. Nutr. Sci. Vitaminol. 2015, 61, S116–S118. [Google Scholar] [CrossRef]
- Wen, Y.; Li, R.; Piao, X.; Lin, G.; He, P. Different copper sources and levels affect growth performance, copper content, carcass characteristics, intestinal microorganism and metabolism of finishing pigs. Anim. Nutr. 2022, 8, 321–330. [Google Scholar] [CrossRef]
- Villagómez-Estrada, S.; Pérez, J.F.; Darwich, L.; Vidal, A.; van Kuijk, S.; Melo-Durán, D.; Solà-Oriol, D. Effects of copper and zinc sources and inclusion levels of copper on weanling pig performance and intestinal microbiota. J. Anim. Sci. 2020, 98, skaa117. [Google Scholar] [CrossRef]
- Revision of the Currently Authorised Maximum Copper Content in Complete Feed. EFSA J. 2016, 14, e04563. [CrossRef]
- Yang, P.; Wang, H.; Zhu, M.; Ma, Y. Effects of Choline Chloride, Copper Sulfate and Zinc Oxide on Long-Term Stabilization of Microencapsulated Vitamins in Premixes for Weanling Piglets. Animals 2019, 9, 1154. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Guo, Y.; Liu, B.; Wang, R.; Su, X.; Yu, D.; He, P. Optimal Dietary Copper Requirements and Relative Bioavailability for Weanling Pigs Fed Either Copper Proteinate or Tribasic Copper Chloride. J. Anim. Sci. Biotechnol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Hu, L.; Fu, X.; Lv, M.; Han, X. Dietary Chitosan-Cu Chelate Affects Growth Performance and Small Intestinal Morphology and Apoptosis in Weaned Piglets. Czech, J. Anim. Sci. 2017, 62, 15–21. [Google Scholar] [CrossRef]
- Lovatto, P.A.; Lehnen, C.R.; Andretta, I.; Carvalho, A.D.; Hauschild, L. Meta analysis in scientific research: A methodological approach. Rev. Bras. Zootec. 2007, 36, 285–294. [Google Scholar] [CrossRef]
- St-Pierre, N.R. Meta-Analyses of Experimental Data in the Animal Sciences. R. Bras. Zootec. 2007, 36, 343–358. [Google Scholar] [CrossRef]
- Sauvant, D.; Letourneau-Montminy, M.P.; Schmidely, P.; Boval, M.; Loncke, C.; Daniel, J.B. Review: Use and Misuse of Meta-Analysis in Animal Science. Animal 2020, 14, s207–s222. [Google Scholar] [CrossRef]
- Sales, J. Effects of Pharmacological Concentrations of Dietary Zinc Oxide on Growth of Post-weaning Pigs: A Meta-analysis. Biol. Trace Elem. Res. 2013, 152, 343–349. [Google Scholar] [CrossRef]
- Hauschild, L.; Lovatto, P.A.; Carvalho, A.A.; Andretta, I.; Lehnen, C.R. Relation of plasma zinc and copper with nutritional components and performance of weanling pigs: A meta-analysis. Rev. Bras. Zootec. 2008, 37, 427–432. [Google Scholar] [CrossRef]
- van Kuijk, S.J.A.; Jacobs, M.; Smits, H.M.; Han, Y. The Effect of Hydroxychloride Trace Minerals on the Growth Performance And Carcass Quality of Grower/Finisher Pigs: A Meta-Analysis. J. Anim. Sci. 2019, 97, 4619–4624. [Google Scholar] [CrossRef] [PubMed]
- Ketata, M.A.; Létourneau-Montminy, M.; Guay, F. Estimation of Digestible Zinc and Copper in Pigs: A Meta-Analysis Approach. KeAi Anim. Nutr. 2023. [Google Scholar] [CrossRef]
- Feng, C.; Xie, B.; Wuren, Q.; Gao, M. Meta-Analysis of the Correlation Between Dietary Copper Supply and Broiler Performance. PLoS ONE 2020, 15, e0232876. [Google Scholar] [CrossRef] [PubMed]
- Sauvant, D.; Schmidely, P.; Daudin, J.J.; St-Pierre, N.R. Meta-Analyses of Experimental Data in Animal Nutrition. Animal 2008, 2, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Therrell, A.; Brady, E.; Bowers, K. Association of American Feed Control Officials Committee Reports. In Proceedings of the AAFCO Annual Meeting, St. Louis, Missouri, USA, 4–6 August 2022. [Google Scholar]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses Testing for Heterogeneity. BMJ 2002, 327, 557–560. [Google Scholar] [CrossRef]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The Coefficient of Determination R2 and Intra-Class Correlation Coefficient from Generalized Linear Mixed-Effects Models Revisited and Expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; Clark, J.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 7, 889–896. [Google Scholar]
- de Mello, G.; Antonio Berto, D.; Lo Tierzo, V.; Maria Nascimento Augusto, R.; Maria Ribeiro da Silva, A.; Alves da Trindade Neto, M.; Cordeiro Ensá Junqueira Villela, C.; Vilela Carneiro Girão, L. Sources of Organic Trace Minerals in Diets for Weaned Piglets. Rev. Bras. Zootec. 2012, 41, 1872–1877. [Google Scholar] [CrossRef]
- Chabaev, M.G.; Nekrasov, R.V.; Strekozov, N.I.; Tsis, E.Y.; Klementyev, M.I. Effects of Different Levels and Forms of Chelated Metal Proteinates on Productive Performance and Metabolic Processes in Fattening Young Pigs. Russ. Agric. Sci. 2020, 46, 161–166. [Google Scholar] [CrossRef]
- Dȩbski, B. Supplementation of Pigs Diet with Zinc and Copper as Alternative to Conventional Antimicrobials. Pol. J. Vet. Sci. 2016, 19, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Di Giancamillo, A.; Rossi, R.; Martino, P.A.; Aidos, L.; Maghin, F.; Domeneghini, C.; Corino, C. Copper Sulphate Forms in Piglet Diets: Microbiota, Intestinal Morphology and Enteric Nervous System Glial Cells. Anim. Sci. J. 2018, 89, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Lloyd, K.E.; Flowers, W.L.; Spears, J.W. Effect of Dietary Copper Amount and Source on Copper Metabolism and Oxidative Stress of Weanling Pigs in Short-Term Feeding. J. Anim. Sci. 2015, 93, 2948–2955. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Hao, C.; Liu, L.; Xu, C.; Kuang, H. A Highly Sensitive Enzyme-Linked Immunosorbent Assay for Copper(II) Determination in Drinking Water. Food Agric. Immunol. 2014, 25, 432–442. [Google Scholar] [CrossRef]
- Mayorga, E.J.; Kvidera, S.K.; Horst, E.A.; Al-Qaisi, M.; Dickson, M.J.; Seibert, J.T.; Lei, S.; Keating, A.F.; Ross, J.W.; Rhoads, R.P.; et al. Effects of Zinc Amino Acid Complex on Biomarkers of Gut Integrity and Metabolism during and Following Heat Stress or Feed Restriction in Pigs. J. Anim. Sci. 2018, 96, 4173–4185. [Google Scholar] [CrossRef]
- Chen, F.; Luo, Z.; Chen, G.H.; Shi, X.; Liu, X.; Song, Y.F.; Pan, Y.X. Effects of Waterborne Cu Exposure on Intestinal Copper Transport and Lipid Metabolism of Synechogobius Hasta. Aquat. Toxicol. 2016, 178, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.V.; Nakasone, D.H.; Martinez, C.H.G.; Gemelli, J.L.; Pereira, A.S.C.; Pugine, S.M.P.; De Melo, M.P.; Andrade, A.F.C.; Araújo, L.F.; Augusto, K.Z.; et al. Copper and Zinc Hydroxychloride Cosupplementation Improve Growth Performance and Carcass and Reduce Diarrhea Frequency in Grower-Finisher Pigs. Transl. Anim. Sci. 2021, 5, txab202. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.D.; Stein, H.H. Digestibility and Metabolism of Copper in Diets for Pigs and Influence of Dietary Copper on Growth Performance, Intestinal Health, and Overall Immune Status: A Review. J. Anim. Sci. Biotechnol. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fry, R.S.; Ashwell, M.S.; Lloyd, K.E.; O’Nan, A.T.; Flowers, W.L.; Stewart, K.R.; Spears, J.W. Amount and Source of Dietary Copper Affects Small Intestine Morphology, Duodenal Lipid Peroxidation, Hepatic Oxidative Stress, and MRNA Expression of Hepatic Copper Regulatory Proteins in Weanling Pigs. J. Anim. Sci. 2012, 90, 3112–3119. [Google Scholar] [CrossRef] [PubMed]
- Faccin, J.E.G.; Tokach, M.D.; Goodband, R.D.; Derouchey, J.M.; Woodworth, J.C.; Gebhardt, J.T. Industry Survey of Added Vitamins and Trace Minerals in U.S. Swine Diets. Transl. Anim. Sci. 2023, 7, txad035. [Google Scholar] [CrossRef] [PubMed]
- Byrne, L.; Murphy, R.A. Relative Bioavailability of Trace Minerals in Production Animal Nutrition: A Review. Animals 2022, 12, 1981. [Google Scholar] [CrossRef]
- Dalto, D.B.; Audet, I.; Roy, C.; Kétilim Novais, A.; Deschêne, K.; Goulet, K.; Matte, J.; Lapointe, J. Effects of dietary zinc oxide levels on the metabolism of zinc and copper in weaned pigs. J. Anim. Sci. 2023, 3, skad055. [Google Scholar] [CrossRef]
- Dalto, D.B.; Audet, I.; Jacques Matte, J. Impact of Dietary Zinc: Copper Ratio on the Postprandial Net Portal Appearance of These Minerals in Pigs. J. Anim. Sci. 2019, 97, 3938–3946. [Google Scholar] [CrossRef]
- Apgar, G.A.; Kornegay, E.T.; Lindemann, M.D.; Notter, D.R. Evaluation of Copper Sulfate and A Copper Lysine Complex as Growth Promoters for Weanling Swine. J. Anim. Sci. 1995, 73, 2640–2646. [Google Scholar] [CrossRef]
- Armstrong, T.A.; Spears, J.W.; Van Heugten, E.; Engle, T.E.; Wright, C.L. Effect of Dietary Copper Source (Cupric Citrate and Cupric Sulfate) and Concentration on Growth Performance and Fecal Copper Excretion in Weanling Pigs. J Anim Sci. 2004, 82, 1234–1240. [Google Scholar] [CrossRef]
- Bikker, P.; Jongbloed, A.W.; Van Baal, J. Dose-Dependent Effects of Copper Supplementation of Nursery Diets on Growth Performance and Fecal Consistency in Weaned Pigs. J. Anim. Sci. 2016, 94, 181–186. [Google Scholar] [CrossRef]
- Capps, K.M.; Amachawadi, R.G.; Menegat, M.B.; Woodworth, J.C.; Perryman, K.; Tokach, M.D.; Dritz, S.S.; Derouchey, J.M.; Goodband, R.D.; Bai, J.; et al. Impact of Added Copper, Alone or in Combination with Chlortetracycline, on Growth Performance and Antimicrobial Resistance of Fecal Enterococci of Weaned Piglets. J. Anim. Sci. 2020, 98. [Google Scholar] [CrossRef] [PubMed]
- Coffey, R.D.; Cromwelf, G.L.; Monegue, H.J. Efficacy of a Copper-Lysine Complex as a Growth Promotant for Weanling Pigs. J. Anim. Sci. 1994, 72, 2880–2886. [Google Scholar] [CrossRef]
- Cromwell, G.L.; Lindemann, M.D.; Monegue, H.J.; Hall, D.D.; Orr, D.E. Tribasic Copper Chloride and Copper Sulfate as Copper Sources for Weanling Pigs. J. Anim. Sci. 1998, 76, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Maxwell, C.V.; Brown, D.C.; De Rodas, B.Z.; Johnson, Z.B.; Kegley, E.B.; Hellwig, D.H.; Dvorak, R.A. Effect of Dietary Mannan Oligosaccharides And(or) Pharmacological Additions of Copper Sulfate on Growth Performance and Immunocompetence of Weanling and Growing/Finishing Pigs. J. Anim. Sci. 2002, 80, 2887–2894. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Q.; Xu, H.; Yu, X.; Chen, L.; Wang, Z.; Feng, J. Selection of Copper and Zinc Dosages in Pig Diets Based on the Mutual Benefit of Animal Growth and Environmental Protection. Ecotoxicol. Environ. Saf. 2021, 216, 112177. [Google Scholar] [CrossRef]
- Dove, C.R.; Ewan, R.C. Effect of Excess Dietary Copper, Iron or Zinc on the Tocopherol and Selenium Status of Growing Pigs. J. Anim. Sci. 1990, 68, 2407–2413. [Google Scholar] [CrossRef]
- Dove, C.R.; Ewan, R.C. Effect of Vitamin E and Copper on the Vitamin E Status and Performance of Growing Pigs. J. Anim. Sci. 1991, 69, 2516–2523. [Google Scholar] [CrossRef]
- Dove, C.R. The Effect of Copper Level on Nutrient Utilization of Weanling Pigs. J. Anim. Sci. 1995, 73, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.D.; Fry, R.S.; Usry, J.L.; Stein, H.H. Copper Hydroxychloride Improves Growth Performance and Reduces Diarrhea Frequency of Weanling Pigs Fed a Corn-Soybean Meal Diet but Does Not Change Apparent Total Tract Digestibility of Energy and Acid Hydrolyzed Ether Extract. J. Anim. Sci. 2017, 95, 5447–5454. [Google Scholar] [CrossRef]
- Espinosa, C.D.; Fry, R.S.; Kocher, M.E.; Stein, H.H. Effects of Copper Hydroxychloride and Dietary Fiber on Intestinal Permeability, Growth Performance, and Blood Characteristics of Nursery Pigs. Anim. Feed. Sci. Technol. 2020, 263, 114447. [Google Scholar] [CrossRef]
- Federizzi, K.C. Efeito da Suplementação De Complexo Metal-Aminoácido De Zinco, Manganês E Cobre Sobre O Desempenho Zootécnico E Integridade Do Aparelho Locomotor De Suínos. Palotina 2014. Available online: https://acervodigital.ufpr.br/handle/1884/36503 (accessed on 6 October 2023).
- Gonzales-Eguia, A.; Fu, C.M.; Lu, F.Y.; Lien, T.F. Effects of Nanocopper on Copper Availability and Nutrients Digestibility, Growth Performance and Serum Traits of Piglets. Livest. Sci. 2009, 126, 122–129. [Google Scholar] [CrossRef]
- Gonzalez-Esquerra, R.; Araujo, R.B.; Haese, D.; Kill, J.L.; Cunha, A.F.; Monzani, P.S.; Lima, C.G. Effect of Dietary Copper Sources on Performance, Gastric Ghrelin-RNA Expression, and Growth Hormone Concentrations in Serum in Piglets. J. Anim. Sci. 2019, 97, 4242–4247. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, M.P.L. Avaliação De Fontes De Cobre Sobre O Desempenho De Leitões De 24 a 70 Dias De Idade. Master Dissertation/Thesis, University of Vila Velha, Vila Velha, ES, Brazil, 2014. Available online: https://repositorio.uvv.br//handle/123456789/302 (accessed on 6 October 2023).
- Hauschild, L.; Lovatto, P.A.; Lehnen, C.R.; Andretta, I.; Garcia, G.G.; Daniel, E. Piglets Feeding with Diets Containing Milk Fermented and Zinc And Copper Organic. Arch. Zoot. 2012, 61, 71–77. [Google Scholar] [CrossRef]
- Hedemann, M.S.; Jensen, B.B.; Poulsen, H.D. Influence of Dietary Zinc and Copper on Digestive Enzyme Activity and Intestinal Morphology in Weaned Pigs. J. Anim. Sci. 2006, 84, 3310–3320. [Google Scholar] [CrossRef]
- Hill, G.M.; Cromwell, G.L.; Crenshaw, T.D.; Dove, C.R.; Ewan, R.C.; Knabe, D.A.; Lewis, A.J.; Libal, G.W.; Mahan, D.C.; Shurson, G.C.; et al. Growth Promotion Effects and Plasma Changes from Feeding High Dietary Concentrations of Zinc and Copper to Weanling Pigs (Regional Study). J. Anim. Sci. 2000, 78, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.F.; Zhang, Q.H.; Wu, H.; Wang, C.C.; Cao, S.T.; Feng, J.; Hu, C.H. Influences of Copper/Zinc-Loaded Montmorillonite on Growth Performance, Mineral Retention, Intestinal Morphology, Mucosa Antioxidant Capacity, and Cytokine Contents in Weaned Piglets. Biol. Trace Elem. Res. 2018, 185, 356–363. [Google Scholar] [CrossRef]
- Liao, P.; Li, M.; Li, Y.; Tan, X.; Zhao, F.; Shu, X.; Yin, Y. Effects of Dietary Supplementation with Cupreous N-Carbamylglutamate (NCG) Chelate and Copper Sulfate on Growth Performance, Serum Biochemical Profile and Immune Response, Tissue Mineral Levels and Fecal Excretion of Mineral in Weaning Piglets. Food Agric. Immunol. 2017, 28, 1315–1329. [Google Scholar] [CrossRef]
- Liu, H.; Tang, X.P.; Fang, R.J.; Yi, F.; Zhang, C.; Yang, R.Q.; Sun, F.; Zhou, S.Y. Effects of Copper Amino Acids Complex on Growth Performance and Serum Cu-Zn Sod Activity in Piglets. Pak. J. Zool. 2020, 52, 1977–1984. [Google Scholar] [CrossRef]
- Lima, I.A.V.; Miyada, V.S. Organic and Inorganic Copper as Growth Promoters of Weanling Pigs. Rev. Bras. Zoot. 2003, 32. [Google Scholar] [CrossRef]
- Luo, X.G.; Dove, C.R. Effect of Dietary Copper and Fat on Nutrient Utilization, Digestive Enzyme Activities, and Tissue Mineral Levels in Weanling Pigs. J. Anim. Sci. 1996, 74, 1888–1896. [Google Scholar] [CrossRef]
- Mei, S.F.; Yu, B.; Ju, C.F.; Zhu, D.; Chen, D.W. Effect of Different Levels of Copper on Growth Performance and Cecal Ecosystem of Newly Weaned Piglets. Ital. J. Anim. Sci. 2009, 9, 378–381. [Google Scholar] [CrossRef]
- Ma, Y.L.; Lindemann, M.D.; Webb, S.F.; Rentfrow, G. Evaluation of Trace Mineral Source and Preharvest Deletion of Trace Minerals from Finishing Diets on Tissue Mineral Status in Pigs. Asian-Australas J. Anim. Sci. 2012, 31, 252–262. [Google Scholar] [CrossRef]
- Ma, Y.L.; Zanton, G.I.; Zhao, J.; Wedekind, K.; Escobar, J.; Vazquez-Añón, M. Multitrial Analysis of the Effects of Copper Level and Source on Performance in Nursery Pigs. J. Anim. Sci. 2015, 93, 606–614. [Google Scholar] [CrossRef]
- Martin, R.E.; Mahan, D.C.; Hill, G.M.; Link, J.E.; Jolliff, J.S. Effect of Dietary Organic Microminerals on Starter Pig Performance, Tissue Mineral Concentrations, and Liver and Plasma Enzyme Activities. J. Anim. Sci. 2011, 89, 1042–1055. [Google Scholar] [CrossRef]
- Mendonça, M.V. Effects of the Association of Different Copper and Zinc Sources in the Diet of Weaned Piglets. Master Dissertation/Thesis, University of São Paulo, Pirassununga, SP, Brazil, 2018. [Google Scholar] [CrossRef]
- Mello, G.; Berto, D.A.; Lo Tierzo, V.; Augusto, R.M.N.; Da Silva, A.M.R.; Trindade Neto, M.A.; Villela, C.C.V.; Girão, L.V.C. Sources of Organic Trace Minerals in Diets for Weaned Piglets. Rev. Bras. Zoot. 2012, 41. [Google Scholar] [CrossRef]
- Muniz, M.H.B.; Berto, D.A.; Hauptli, L.; Fracarolli, C.; Trindade Neto, M.A.; Tamassia, L.F.M.; Wechsler, F.S. Organic and Inorganic Source of Zinc and Cooper as Growth Promoters for Weanling Piglets. Rev. Bras. Zoot. 2010, 41. [Google Scholar] [CrossRef]
- Namkung, H.; Gong, J.; Yu, H.; de Lange, C.F.M. Effect of Pharmacological Intakes of Zinc and Copper on Growth Performance, Circulating Cytokines and Gut Microbiota of Newly Weaned Piglets Challenged with Coliform Lipopolysaccharides. Can. J. Anim. Sci. 2006, 86, 511–522. [Google Scholar] [CrossRef]
- Okiyama, W.H.E. Influence of Sources and Levels of Copper on the Performance of Weaned Pigs. Master Dissertation/Thesis, University of São Paulo, Pirassununga, SP, Brazil, 2017. [Google Scholar] [CrossRef]
- Pastorelli, G.; Rossi, R.; Zanardi, E.; Ghidini, S.; Corino, C. Two Different Forms and Levels of Cuso4 in Piglet Feeding: Liver, Plasma and Faeces Copper Status. J. Anim. Feed Sci. 2014, 23, 52–57. [Google Scholar] [CrossRef]
- Pérez, V.G.; Waguespack, A.M.; Bidner, T.D.; Southern, L.L.; Fakler, T.M.; Ward, T.L.; Steidinger, M.; Pettigrew, J.E. Additivity of Effects from Dietary Copper and Zinc on Growth Performance and Fecal Microbiota of Pigs After Weaning. J. Anim. Sci. 2011, 89, 414–425. [Google Scholar] [CrossRef]
- Possobon, R.M. O Cobre Como Estimulante Do Crescimento De Suínos Em Recria. Master Dissertation/Thesis, University of São Paulo, Piracicaba, SP, Brazil, 1991. [Google Scholar] [CrossRef]
- Ren, P.; Chen, J.; Hancock, D.; Vazquez-Añón, M. Interactive Effects of Copper Sources and a High Level of Phytase in Phosphorus-Deficient Diets on Growth Performance, Nutrient Digestibility, Tissue Mineral Concentrations, and Plasma Parameters in Nursery Pigs. Biol. Trace Elem. Res. 2021, 199, 4582–4592. [Google Scholar] [CrossRef]
- Schaaf, S. Effect of Dietary Source and Concentrations of Copper, Manganese, and Zinc on Growth Performance and Immune Response of Nursery Pigs. Master Dissertation/Thesis, Oklahoma State University, Stillwater, OK, USA, 2017. [Google Scholar]
- Shelton, N.W.; Tokach, M.D.; Nelssen, J.L.; Goodband, R.D.; Dritz, S.S.; Derouchey, J.M.; Hill, G.M. Effects of Copper Sulfate, Tri-Basic Copper Chloride, and Zinc Oxide on Weanling Pig Performance. J. Anim. Sci. 2011, 89, 2440–2451. [Google Scholar] [CrossRef]
- Shurson, G.C.; Ku, P.K.; Waxler, G.L.; Yokoyama, M.T.; Miller, E.R. Physiological Relationships Between Microbiological Status and Dietary Copper Levels in the Pig. J. Anim. Sci. 1990, 68, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L.; Richert, B.T. Effects of the Interrelationship Between Zinc Oxide and Copper Sulfate on Growth Performance of Early-Weaned Pigs. J. Anim. Sci. 1997, 75, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Y.L.; Hu, C.H. Effects of Copper-Exchanged Montmorillonite, as Alternative to Antibiotic, on Diarrhea, Intestinal Permeability and Proinflammatory Cytokine of Weanling Pigs. Appl. Clay. Sci. 2013, 77–78, 52–55. [Google Scholar] [CrossRef]
- Stansbury, W.F.; Tribble, L.F.; Orr, D.E. Effect of Chelated Copper Sources on Performance of Nursery and Growing Pigs. J. Anim. Sci. 1990, 68, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, M.C.; Watanabe, P.H.; Pascoal, L.A.F.; Assis, M.M.; Ruiz, U.S.; Amorim, A.B.; Silva, S.Z.; Almeida, V.V.; Melo, G.M.P.; Robles-Huaynate, R.A. Inorganic and Organic Trace Mineral Supplementation in Weanling Pig Diets. An Acad. Bras. Cienc. 2015, 87, 1071–1081. [Google Scholar] [CrossRef]
- Veum, T.L.; Carlson, M.S.; Wu, C.W.; Bollinger, D.W.; Ellersieck, M.R. Copper Proteinate in Weanling Pig Diets for Enhancing Growth Performance and Reducing Fecal Copper Excretion Compared with Copper Sulfate. J. Anim. Sci. 2004, 82, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Windisch, W.M.; Gotterbarm, G.G.; Roth, F.X. Effect of Potassium Diformate in Combination With Different Amounts and Sources of Excessive Dietary Copper on Production Performance in Weaning Piglets. Arch. Anim. Nutr. 2001, 54, 87–100. [Google Scholar] [CrossRef]
- Xia, M.S.; Hu, C.H.; Xu, Z.R. Effects of Copper Bearing Montmorillonite on the Growth Performance, Intestinal Microflora and Morphology of Wean-Ling Pigs. Anim. Feed Sci. Technol. 2005, 118, 307–317. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Liu, L.; Zhu, X.; Wang, X.; Liu, Z.; Wang, Z.; Yang, L.; Liu, G. Effect of High Dietary Copper on Somatostatin and Growth Hormone-Releasing Hormone Levels in the Hypothalami of Growing Pigs. Biol. Trace Elem. Res. 2011, 143, 893–900. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Dong, Z.; Li, G.; Wang, J.; Li, Y.; Wan, D.; Yang, H.; Yin, Y. Effect of Dietary Copper on Intestinal Microbiota and Antimicrobial Resistance Profiles of Escherichia coli in Weaned Piglets. Front Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Cho, J.H.; Kim, I.H. Effects of Chelated Copper and Zinc Supplementation on Growth Performance, Nutrient Digestibility, Blood Profiles, and Fecal Noxious Gas Emission in Weanling Pigs. J. Anim. Sci. Technol. 2013, 55, 295–301. [Google Scholar] [CrossRef]
- Zhao, J.; Allee, G.; Gerlemann, G.; Ma, L.; Gracia, M.I.; Parker, D.; Vazquez-Anon, M.; Harrell, R.J. Effects of a Chelated Copper as Growth Promoter on Performance and Carcass Traits in Pigs. Asian-Australas J. Anim. Sci. 2014, 27, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Harper, A.F.; Estienne, M.J.; Webb, K.E.; Mcelroy, A.P.; Denbow, D.M. Growth Performance and Intestinal Morphology Responses in Early Weaned Pigs to Supplementation of Antibiotic-Free Diets with an Organic Copper Complex and Spray-Dried Plasma Protein in Sanitary and Nonsanitary Environments. J. Anim. Sci. 2007, 85, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Kornegay, E.T.; Van Laarp, H.; Swinkelss, J.W.G.M.; Wong, E.A.; Lindemann, M.D. The Role of Feed Consumption and Feed Efficiency in Copper-Stimulated Growth. J. Anim. Sci. 1994, 72, 2385–2394. [Google Scholar] [CrossRef]
- Zhou, W.; Kornegay, E.T.; Lindemann, M.D.; Swinkels, J.W.; Welten, M.K.; Wong, E.A. Stimulation of Growth by Intravenous Injection of Copper in Weanling Pigs. J. Anim. Sci. 1994, 72, 2395–2403. [Google Scholar] [CrossRef]
- Zhu, D.; Yu, B.; Ju, C.; Mei, S.; Chen, D. Effect of High Dietary Copper on the Expression of Hypothalamic Appetite Regulators in Weanling Pigs. J. Anim. Feed Sci. 2011, 20, 60–70. [Google Scholar] [CrossRef]
| Nutrient 1 | Mean | Minimum | Maximum |
|---|---|---|---|
| DE, kcal/kg | 3388.0 | 3071.6 | 3843.1 |
| ME, kcal/kg | 3240.5 | 2947.6 | 3666.3 |
| CP, % | 20.0 | 11.67 | 25.29 |
| Ca, % | 0.85 | 0.134 | 1.64 |
| P total, % | 0.70 | 0.25 | 0.94 |
| Ca:P | 2.26 | 0.40 | 4.50 |
| Lys, % | 1.22 | 0.79 | 1.88 |
| Met, % | 0.34 | 0.25 | 0.70 |
| Met + Cys, % | 0.64 | 0.31 | 0.97 |
| Thr, % | 0.80 | 0.48 | 0.97 |
| Trp, % | 0.22 | 0.13 | 0.30 |
| Copper, mg/kg | 120.9 | 5.00 | 1500 |
| Zinc, mg/kg | 665.4 | 45.0 | 3125 |
| Zn:Cu | 13.92 | 0.05 | 250 |
| N 1 | ADFI 2, kg/d | ADG 3, kg/d | F: G 4 | |
|---|---|---|---|---|
| Copper Sources | ||||
| Basal | 194 | 0.561 ± 0.08 | 0.344 ± 0.05 a | 1.65 ± 0.03 a |
| Inorganic | 429 | 0.596 ± 0.09 | 0.374 ± 0.06 b | 1.61 ± 0.03 b |
| Organic | 323 | 0.589 ± 0.08 | 0.372 ± 0.06 b | 1.58 ± 0.02 b |
| Copper levels | ||||
| 1–15 mg/kg | 194 | 0.597 ± 0.09 | 0.343 ± 0.06 a | 1.70 ± 0.02 a |
| 16–80 mg/kg | 130 | 0.595 ± 0.09 | 0.363 ± 0.05 a | 1.64 ± 0.03 a |
| 81–200 mg/kg | 429 | 0.584 ± 0.08 | 0.375 ± 0.05 b | 1.57 ± 0.03 b |
| >201 mg/kg | 125 | 0.586 ± 0.12 | 0.384 ± 0.07 b | 1.54 ± 0.04 b |
| Effects | p-value 5 | |||
| Sources | 0.663 | 0.013 | 0.020 | |
| Level | 0.166 | 0.005 | <0.001 | |
| Sources * Level | 0.478 | 0.210 | 0.515 | |
| Weaning Age, d | <0.001 | <0.001 | 0.005 | |
| Initial body weight, kg | <0.001 | <0.001 | <0.001 | |
| ADFI (kg/d) | ADG (kg/d) | F: G | |
|---|---|---|---|
| Model 1 (SE) | Model 2 (SE) | Model 3 (SE) | |
| Intercept | −0.0059 (0.0284) | 0.0433 (0.0187) * | 1.381 (0.0369) *** |
| Ingested Cu, mg/d | 0.0022 (0.0004) *** | 0.0013 (0.003) *** | 0.00002 (0.0004) * |
| Ingested Zn, mg/d | 0.0003 (0.00005) *** | 0.00001 (0.00011) *** | 0.00007 (0.00005) |
| Zn/Cu ratio | 0.00005 (0.00017) | −0.00001(0.00001) | 0.00005 (0.0003) |
| Initial BW, kg | 0.02681 (0.0027) *** | 0.0128(0.002) *** | 0.0154 (0.0032) *** |
| Initial age | 0.0107 (0.0010) *** | 0.006 (0.0006) *** | 0.00297 (0.0012) * |
| Inorganic Cu | 0.0222 (0.0157) | −0.0024(0.009) | 0.07437 (0.0182) *** |
| Organic Cu | 0.0084 (0.0132) | 0.00352(0.008) | 0.0179 (0.0153) |
| AIC 1 | −1600.6 | −798.3 | −539.1 |
| BIC | −1553.3 | −751.1 | −491.9 |
| Observations | 834 | 834 | 834 |
| Variance | 0.01 | 0.02 | 0.04 |
| Residual variance | 0.01 | 0.02 | 0.02 |
| Conditional R2 | 0.737 | 0.735 | 0.654 |
| Marginal R2 | 0.365 | 0.455 | 0.080 |
| RMSE | 0.081 | 0.133 | 0.153 |
| ICC | 0.586 | 0.514 | 0.623 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galiotto Miranda, P.A.; Remus, A.; Dalto, D.B.; Hilgemberg, R.; Beber Jasluk, G.; Rosário Silva, B.C.; Lehnen, C.R. A Systematic Review and Meta-Analysis of the Effects of Various Sources and Amounts of Copper on Nursery Piglets. Vet. Sci. 2024, 11, 68. https://doi.org/10.3390/vetsci11020068
Galiotto Miranda PA, Remus A, Dalto DB, Hilgemberg R, Beber Jasluk G, Rosário Silva BC, Lehnen CR. A Systematic Review and Meta-Analysis of the Effects of Various Sources and Amounts of Copper on Nursery Piglets. Veterinary Sciences. 2024; 11(2):68. https://doi.org/10.3390/vetsci11020068
Chicago/Turabian StyleGaliotto Miranda, Pedro Augusto, Aline Remus, Danyel Bueno Dalto, Rafaela Hilgemberg, Guilherme Beber Jasluk, Brena Cristine Rosário Silva, and Cheila Roberta Lehnen. 2024. "A Systematic Review and Meta-Analysis of the Effects of Various Sources and Amounts of Copper on Nursery Piglets" Veterinary Sciences 11, no. 2: 68. https://doi.org/10.3390/vetsci11020068
APA StyleGaliotto Miranda, P. A., Remus, A., Dalto, D. B., Hilgemberg, R., Beber Jasluk, G., Rosário Silva, B. C., & Lehnen, C. R. (2024). A Systematic Review and Meta-Analysis of the Effects of Various Sources and Amounts of Copper on Nursery Piglets. Veterinary Sciences, 11(2), 68. https://doi.org/10.3390/vetsci11020068





