Immunomodulatory Role of Rosmarinus officinalis L., Mentha x piperita L., and Lavandula angustifolia L. Essential Oils in Sheep Peripheral Blood Mononuclear Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sample Collection and Extraction of Essential Oils
2.2. Animals and Experimental Treatments
2.3. Isolation of Sheep Peripheral Blood Mononuclear Cells
2.4. XTT Cell Proliferation Assay
2.5. Bromodeoxyuridine Proliferation Assay
2.6. Determination of IL-6, IL-10, and IL-8 in PBMC Supernatant
2.7. Statistical Analysis
3. Results and Discussion
3.1. Essential Oils Composition
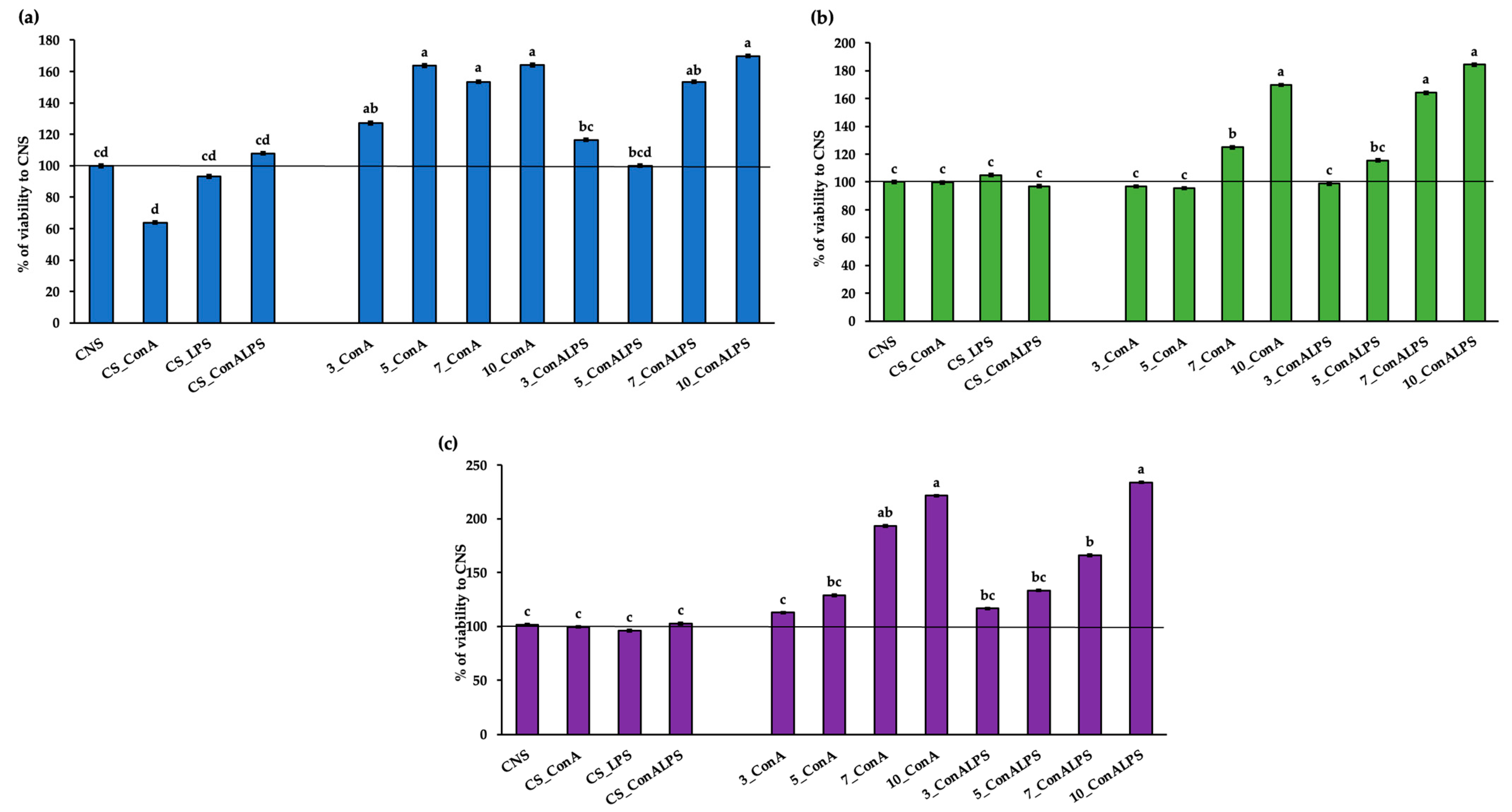
3.2. In Vitro PBMC Viability after EO Treatment
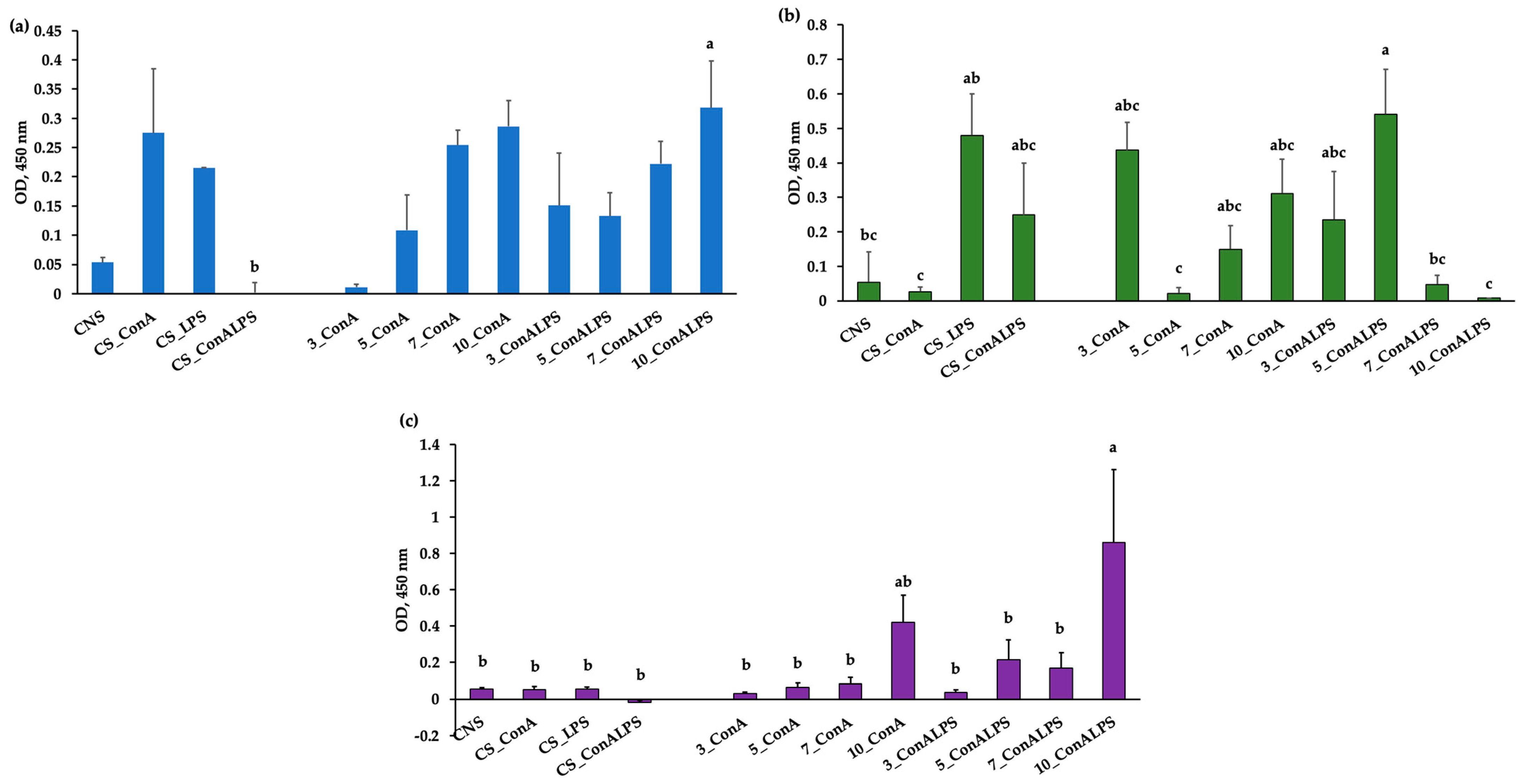
3.3. In Vitro PBMC Proliferative Response to Mitogen Stimulation and EO Treatments
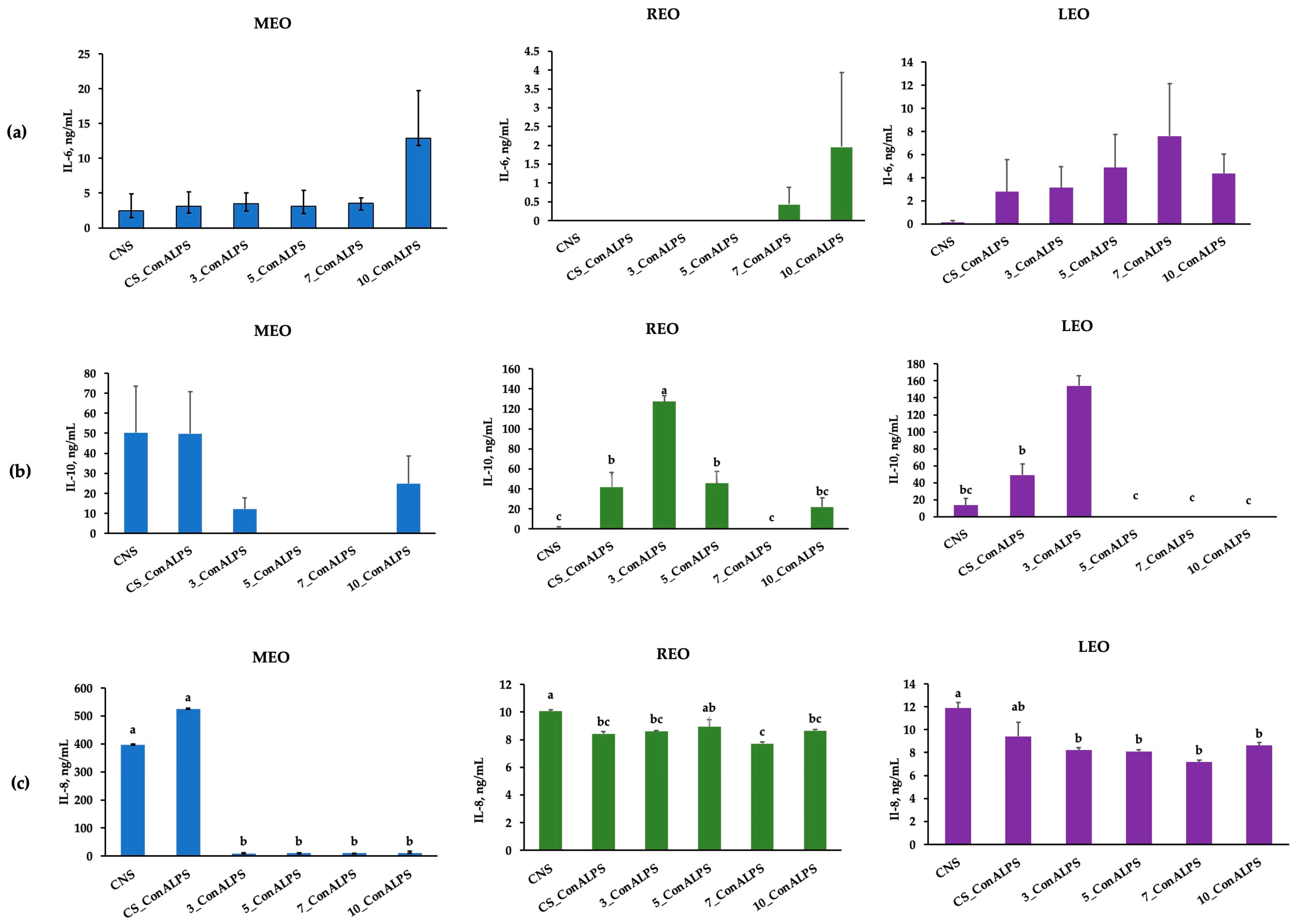
3.4. Cytokine Profiles in PBMC Supernatant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mangalagiri, N.P.; Panditi, S.K.; Jeevigunta, N.L.L. Antimicrobial activity of essential plant oils and their major components. Heliyon 2021, 7, e06835. [Google Scholar] [CrossRef]
- Dorman, H.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.; Yáñez-Ruiz, D.R.; Duval, S.; McEwan, N.; Newbold, C. Plant extracts to manipulate rumen fermentation. Anim. Feed Sci. Technol. 2008, 147, 8–35. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.; Castillejos, L.; Ferret, A. Invited review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [PubMed]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The one health concept: 10 years old and a long road ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Caroprese, M.; Ciliberti, M.G.; Albenzio, M. Application of aromatic plants and their extracts in dairy animals. In Feed Additives; Academic Press: Cambridge, MA, USA, 2020; pp. 261–277. [Google Scholar]
- Caroprese, M.; Ciliberti, M.G.; Marino, R.; Santillo, A.; Sevi, A.; Albenzio, M. Essential oil supplementation in small ruminants: A review on their possible role in rumen fermentation, microbiota, and animal production. Dairy 2023, 4, 497–508. [Google Scholar] [CrossRef]
- Nehme, R.; Andrés, S.; Pereira, R.B.; Ben Jemaa, M.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M.; et al. Essential oils in livestock: From health to food quality. Antioxidants 2021, 10, 330. [Google Scholar] [CrossRef]
- Pereira, R.B.; Rahali, F.Z.; Nehme, R.; Falleh, H.; Jemaa, M.B.; Sellami, I.H.; Ksouri, R.; Bouhallab, S.; Ceciliani, F.; Abdennebi-Najar, L.; et al. Anti-inflammatory activity of essential oils from Tunisian aromatic and medicinal plants and their major constituents in THP-1 macrophages. Food Res. Int. 2023, 167, 112678. [Google Scholar] [CrossRef]
- Catalani, E.; Amadori, M.; Vitali, A.; Lacetera, N. Lymphoproliferative response to lipopolysaccharide and incidence of infections in periparturient dairy cows. J. Dairy Sci. 2013, 96, 7077–7081. [Google Scholar] [CrossRef]
- Muul, L.M.; Heine, G.; Silvin, C.; James, S.P.; Candotti, F.; Radbruch, A.; Worm, M. Measurement of proliferative responses of cultured lymphocytes. Curr. Protoc. Immunol. 2011, 94, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, P.; Saavedra, A.; Petrov, V. In vitro proliferative response of human peripheral blood mononuclear cells to concanavalin A. Clin. Chim. Acta 1997, 264, 91–101. [Google Scholar] [CrossRef]
- McInnes, C.J. Current research on ovine cytokines. Br. Vet. J. 1993, 149, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Budhia, S.; Haring, L.F.; McConnell, I.; Blacklaws, B.A. Quantitation of ovine cytokine mRNA by real-time RT–PCR. J. Immunol. Methods 2006, 309, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Mangan, D.F.; Wahl, S.M. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J. Immunol. 1991, 147, 3408–3412. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, J. Apparatus for the determination of volatile oil. J. Am. Pharm. Assoc. (1912) 1928, 17, 345–349. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Ciliberti, M.; Francavilla, M.; Albenzio, M.; Inghese, C.; Santillo, A.; Sevi, A.; Caroprese, M. Green extraction of bioactive compounds from wine lees and their bio-responses on immune modulation using in vitro sheep model. J. Dairy Sci. 2022, 105, 4335–4353. [Google Scholar] [CrossRef]
- Ciliberti, M.G.; Albenzio, M.; Inghese, C.; Santillo, A.; Marino, R.; Sevi, A.; Caroprese, M. Peripheral blood mononuclear cell proliferation and cytokine production in sheep as affected by cortisol level and duration of stress. J. Dairy Sci. 2017, 100, 750–756. [Google Scholar] [CrossRef]
- Wattegedera, S.; Sills, K.; Howard, C.; Hope, J.; McInnes, C.; Entrican, G. Variability in cytokine production and cell proliferation by mitogen-activated ovine peripheral blood mononuclear cells: Modulation by interleukin (IL)-10 and IL-12. Vet. Immunol. Immunopathol. 2004, 102, 67–76. [Google Scholar] [CrossRef]
- Ciliberti, M.G.; Albenzio, M.; De Palo, P.; Santillo, A.; Caroprese, M. Nexus between immune responses and oxidative stress: The role of dietary hydrolyzed lignin in ex vivo bovine peripheral blood mononuclear cell response. Front. Vet. Sci. 2020, 7, 9. [Google Scholar] [CrossRef]
- Gachkar, L.; Yadegari, D.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem. 2007, 102, 898–904. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Cereti, E.; Barile, D.; Coïsson, J.D.; Arlorio, M.; Dessi, S.; Coroneo, V.; Cabras, P. Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J. Agric. Food Chem. 2004, 52, 3530–3535. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Santoyo, S.; Cavero, S.; Jaime, L.; Ibanez, E.; Senorans, F.; Reglero, G. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. essential oil obtained via supercritical fluid extraction. J. Food Prot. 2005, 68, 790–795. [Google Scholar] [CrossRef]
- Akrout, A.; Hajlaoui, H.; Mighri, H.; Najjaa, H.; Jani, H.E.; Zaidi, S.; Neffati, M. Chemical and biological characteristics of essential oil of Rosmarinus officinalis cultivated in Djerba. J. Essent. Oil Bear. Plants 2010, 13, 398–411. [Google Scholar] [CrossRef]
- Gracindo, L.; Grisi, M.; Silva, D.; Alves, R.; Bizzo, H.; Vieira, R. Chemical characterization of mint (Mentha spp.) germplasm at Federal District, Brazil. Rev. Bras. Plantas Med. 2006, 8, 5–9. [Google Scholar]
- Shahira, M.E.; El-Sayeda, A.E.-K.; Zeinab, A.K.; Mohamed, A.S.; Amany, A.S. Chemical and biological study of Mentha suaveolens Ehrh. cultivated in Egypt. J. Med. Plant Res. 2014, 8, 747–755. [Google Scholar]
- Hayes, J.; Stavanja, M.; Lawrence, B. Mint. The Genus Mentha; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Oumzil, H.; Ghoulami, S.; Rhajaoui, M.; Ilidrissi, A.; Fkih-Tetouani, S.; Faid, M.; Benjouad, A. Antibacterial and antifungal activity of essential oils of Mentha suaveolens. Phytother. Res. 2002, 16, 727–731. [Google Scholar] [CrossRef]
- Sutour, S.; Bradesi, P.; Casanova, J.; Tomi, F. Composition and chemical variability of Mentha suaveolens ssp. suaveolens and M. suaveolens ssp. insularis from Corsica. Chem. Biodivers. 2010, 7, 1002–1008. [Google Scholar] [CrossRef]
- Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; MELO, D.A.; Donadio, M.V.; Nunes, F.B.; Azambuja, M.S.; Santana, J.C.; Moraes, C.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Acad. Bras. Ciências 2015, 87, 1397–1408. [Google Scholar] [CrossRef]
- Stanojević, L.; Stanković, M.; Cakić, M.; Nikolić, V.; Nikolić, L.; Ilić, D.; Radulović, N. The effect of hydrodistillation techniques on yield, kinetics, composition and antimicrobial activity of essential oils from flowers of Lavandula officinalis L. Hem. Ind. 2011, 65, 455–463. [Google Scholar] [CrossRef]
- Bouzouita, N.; Kachouri, F.; Hamdi, M.; Chaabouni, M.; Aissa, R.B.; Zgoulli, S.; Thonart, P.; Carlier, A.; Marlier, M.; Lognay, G. Volatile constituents and antimicrobial activity of Lavandula stoechas L. oil from Tunisia. J. Essent. Oil Res. 2005, 17, 584–586. [Google Scholar] [CrossRef]
- Imelouane, B.; Elbachiri, A.; Ankit, M.; Benzeid, H.; Khedid, K. Physico-chemical compositions and antimicrobial activity of essential oil of eastern Moroccan Lavandula dentata. Int. J. Agric. Biol. 2009, 11, 113–118. [Google Scholar]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Hristov, A.N. Lavender and hyssop productivity, oil content, and bioactivity as a function of harvest time and drying. Ind. Crops Prod. 2012, 36, 222–228. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of lavender oil and its major components to human skin cells. Cell Prolif. 2004, 37, 221–229. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Jaradat, N.; Hawash, M.; Al-Lahham, S.; Qadi, M.; Shoman, I.; Jaber, S.; Rahem, R.A.; Hussein, F.; Issa, L. Chemical Composition, Antioxidant, Antimicrobial and Anti-Proliferative Activities of Essential Oils of Rosmarinus officinalis from five Different Sites in Palestine. Separations 2022, 9, 339. [Google Scholar] [CrossRef]
- Ferreira, H. Assessment Report on Rosmarinus officinalis L., Aetheroleum and Rosmarinus officinalis L., Folium; Committee on Herbal Medicinal Products (HMPC): Amsterdam, The Netherlands, 2010. [Google Scholar]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Safety assessment of Rosmarinus officinalis (rosemary)-derived ingredients as used in cosmetics. Int. J. Toxicol. 2018, 37, 12S–50S. [Google Scholar] [CrossRef]
- Silva, W.; Bona, N.; Pedra, N.; Cunha, K.D.; Fiorentini, A.; Stefanello, F.; Zavareze, E.; Dias, A. Risk assessment of in vitro cytotoxicity, antioxidant and antimicrobial activities of Mentha piperita L. essential oil. J. Toxicol. Environ. Health Part A 2022, 85, 230–242. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Chandrakanthan, M.; Handunnetti, S.M.; Premakumara, G.S.A.; Kathirgamanathar, S. Topical anti-Inflammatory activity of essential oils of Alpinia calcarata Rosc., its main constituents, and possible mechanism of action. Evid.-Based Complement. Altern. Med. 2020, 2020, 2035671. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.-S. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory activities of selected essential oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef]
- Gong, X.; Wang, B.; Yan, L.; Lu, X.; Zhao, X. Linalool inhibits the growth of human T cell acute lymphoblastic leukemia cells with involvement of the MAPK signaling pathway. Oncol. Lett. 2020, 20, 181. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Wang, Y.; Qing, C.; Wang, W.; Yang, Y. In vitro and in vivo efficacy studies of Lavender angustifolia essential oil and its active constituents on the proliferation of human prostate cancer. Integr. Cancer Ther. 2017, 16, 215–226. [Google Scholar] [CrossRef]
- Becer, E.; Altundağ, E.M.; Güran, M.; Vatansever, H.S.; Ustürk, S.; Hanoğlu, D.Y.; Başer, K.H.C. Composition and antibacterial, anti-inflammatory, antioxidant, and anticancer activities of Rosmarinus officinalis L. essential oil. S. Afr. J. Bot. 2023, 160, 437–445. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef]
- Cosentino, M.; Bombelli, R.; Conti, A.; Colombo, M.L.; Azzetti, A.; Bergamaschi, A.; Marino, F.; Lecchini, S. Antioxidant properties and in vitro immunomodulatory effects of peppermint (Mentha piperita L.) essential oils in human leukocytes. J. Pharm. Sci. Res 2009, 1, 33–43. [Google Scholar]
- Ciesielska-Figlon, K.; Daca, A.; Kokotkiewicz, A.; Łuczkiewicz, M.; Zabiegała, B.; Witkowski, J.M.; Lisowska, K.A. The influence of Nigella sativa essential oil on proliferation, activation, and apoptosis of human T lymphocytes in vitro. Biomed. Pharmacother. 2022, 153, 113349. [Google Scholar] [CrossRef]
- Caldefie-Chézet, F.; Fusillier, C.; Jarde, T.; Laroye, H.; Damez, M.; Vasson, M.P.; Guillot, J. Potential anti-inflammatory effects of Melaleuca alternifolia essential oil on human peripheral blood leukocytes. Phytother. Res. 2006, 20, 364–370. [Google Scholar] [CrossRef]
- Caroprese, M.; Albenzio, M.; Marzano, A.; Schena, L.; Annicchiarico, G.; Sevi, A. Relationship between cortisol response to stress and behavior, immune profile, and production performance of dairy ewes. J. Dairy Sci. 2010, 93, 2395–2403. [Google Scholar] [CrossRef]
- Caroprese, M.; Ciliberti, M.G.; Albenzio, M.; Sevi, A. Heat stress associated changes in the immune system related responses in sheep. In Climate Change and Livestock Production: Recent Advances and Future Perspectives; Springer: Berlin/Heidelberg, Germany, 2022; pp. 49–58. [Google Scholar]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef]
- Hopkins, S.J. The pathophysiological role of cytokines. Leg. Med. 2003, 5, S45–S57. [Google Scholar] [CrossRef]
- Chouhan, S.; Guleria, S. Anti-inflammatory activity of medicinal plants: Present status and future perspectives. In Botanical Leads for Drug Discovery; Springer: Singapore, 2020; pp. 67–92. [Google Scholar]
- Inouye, S.; Abe, S. Nouvelle approache de l’aromathérapie anti-infectieuse. Phytothérapie 2007, 1, 2–4. [Google Scholar] [CrossRef]
- Huang, M.-Y.; Liao, M.-H.; Wang, Y.-K.; Huang, Y.-S.; Wen, H.-C. Effect of lavender essential oil on LPS-stimulated inflammation. Am. J. Chin. Med. 2012, 40, 845–859. [Google Scholar] [CrossRef]
- Ku, C.-M.; Lin, J.-Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Chrousos, G.P. Stress, cytokine patterns and susceptibility to disease. Best Pract. Res. Clin. Endocrinol. Metab. 1999, 13, 583–595. [Google Scholar] [CrossRef]
- Yoshimura, T.; Matsushima, K.; Tanaka, S.; Robinson, E.A.; Appella, E.; Oppenheim, J.J.; Leonard, E.J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc. Natl. Acad. Sci. USA 1987, 84, 9233–9237. [Google Scholar] [CrossRef]
- Wang, P.; Wu, P.; Siegel, M.I.; Egan, R.W.; Billah, M.M. Interleukin (IL)-10 inhibits nuclear factor кB (NFкB) activation in human monocytes: IL-10 and IL-4 suppress cytokine synthesis by different mechanisms (∗). J. Biol. Chem. 1995, 270, 9558–9563. [Google Scholar] [CrossRef]
- Li, P.; Piao, X.; Ru, Y.; Han, X.; Xue, L.; Zhang, H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas. J. Anim. Sci. 2012, 25, 1617. [Google Scholar] [CrossRef]
- Su, G.; Zhou, X.; Wang, Y.; Chen, D.; Chen, G.; Li, Y.; He, J. Effects of plant essential oil supplementation on growth performance, immune function and antioxidant activities in weaned pigs. Lipids Health Dis. 2018, 17, 139. [Google Scholar] [CrossRef]
- Xu, Y.; Lahaye, L.; He, Z.; Zhang, J.; Yang, C.; Piao, X. Micro-encapsulated essential oils and organic acids combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88+). Anim. Nutr. 2020, 6, 269–277. [Google Scholar] [CrossRef]
| No. | Mentha x piperita L. | Percentage (%) | No. | Rosmarinus officinalis L. | Percentage (%) | No. | Lavandula angustifolia L. | Percentage (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 1.6 | 1 | Tricyclene | 0.4 | 1 | α-Tujene | 0.2 |
| 2 | Sabinene | 1.5 | 2 | α-Thujene | 0.3 | 2 | α-Pinene | 0.7 |
| 3 | β-Pinene | 2.7 | 3 | α-Pinene | 20.0 | 3 | Camfene | 0.4 |
| 4 | Myrcene | 4.2 | 4 | Camphene | 10.8 | 4 | Sabinene | 0.3 |
| 5 | 3-Octanol | 0.3 | 5 | β-Pinene | 4.9 | 5 | β-Pinene | 0.6 |
| 6 | Limonene | 11.8 | 6 | 3-Octanone | 1.2 | 6 | 1-octen-3-ol | 0.6 |
| 7 | 1,8-Cineole | 17.4 | 7 | β-Myrcene | 5.1 | 7 | 3-octanone | 0.2 |
| 8 | Linalol | 0.2 | 8 | α-Phellandrene | 2.4 | 8 | β-Myrcene | 1.0 |
| 9 | α-Terpineol | 0.3 | 9 | Δ3-Carene | 0.1 | 9 | α-Phellandrene | 0.1 |
| 10 | Piperitone oxide | 0.4 | 10 | α-Terpinene | 1.0 | 10 | Δ3-Carene | 0.2 |
| 11 | Carvone | 0.2 | 11 | p-Cymene | 1.1 | 11 | α-Terpinene | 0.1 |
| 12 | Pipertitenone oxide | 51.25 | 12 | 1,8-Cineole | 25.2 | 12 | p-Cymene | 4.7 |
| 13 | β-Caryophyllene | 3.3 | 13 | β-cis-Ocimene | 0.1 | 13 | 1,8-Cineol | 11 |
| 14 | Germacrene D | 0.6 | 14 | γ-Terpinene | 1.8 | 14 | Trans Ocimene | 3.3 |
| 15 | Bicyclogermacrene | 0.1 | 15 | Terpinalene | 1.0 | 15 | Cis Ocimene | 1.3 |
| 16 | Caryophyllene oxide | 0.8 | 16 | Linalol | 0.7 | 16 | γ-Terpinene | 0.4 |
| 17 | Camphor | 16.2 | 17 | Terpinolene | 0.4 | |||
| 18 | Borneol | 1.7 | 18 | Linalol | 44.5 | |||
| 19 | Terpinen-4-olo | 0.4 | 19 | Canfor | 3.6 | |||
| 20 | α-Terpineol | 0.7 | 20 | Borneol | 10.9 | |||
| 21 | Verbenone | 1.4 | 21 | Terpinen-4-ol | 9.8 | |||
| 22 | Bornyl acetate | 0.7 | 22 | α-Terpineol | 0.9 | |||
| 23 | β-Caryophyllene | 1.6 | 23 | Lavandulyl Acetate | 1.3 | |||
| 24 | α-Humulene | 0.4 | 24 | Geranyl Acetate | 0.7 | |||
| 25 | β-Farnesene | 0.7 | ||||||
| % Total identified | 96.65 | % Total identified | 99.2 | % Total identified | 97.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciliberti, M.G.; Albenzio, M.; Sevi, A.; Frabboni, L.; Marino, R.; Caroprese, M. Immunomodulatory Role of Rosmarinus officinalis L., Mentha x piperita L., and Lavandula angustifolia L. Essential Oils in Sheep Peripheral Blood Mononuclear Cells. Vet. Sci. 2024, 11, 157. https://doi.org/10.3390/vetsci11040157
Ciliberti MG, Albenzio M, Sevi A, Frabboni L, Marino R, Caroprese M. Immunomodulatory Role of Rosmarinus officinalis L., Mentha x piperita L., and Lavandula angustifolia L. Essential Oils in Sheep Peripheral Blood Mononuclear Cells. Veterinary Sciences. 2024; 11(4):157. https://doi.org/10.3390/vetsci11040157
Chicago/Turabian StyleCiliberti, Maria Giovanna, Marzia Albenzio, Agostino Sevi, Laura Frabboni, Rosaria Marino, and Mariangela Caroprese. 2024. "Immunomodulatory Role of Rosmarinus officinalis L., Mentha x piperita L., and Lavandula angustifolia L. Essential Oils in Sheep Peripheral Blood Mononuclear Cells" Veterinary Sciences 11, no. 4: 157. https://doi.org/10.3390/vetsci11040157
APA StyleCiliberti, M. G., Albenzio, M., Sevi, A., Frabboni, L., Marino, R., & Caroprese, M. (2024). Immunomodulatory Role of Rosmarinus officinalis L., Mentha x piperita L., and Lavandula angustifolia L. Essential Oils in Sheep Peripheral Blood Mononuclear Cells. Veterinary Sciences, 11(4), 157. https://doi.org/10.3390/vetsci11040157