Evaluation of Lab-on-a-Disc Technique Performance for Soil-Transmitted Helminth Diagnosis in Animals in Tanzania
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
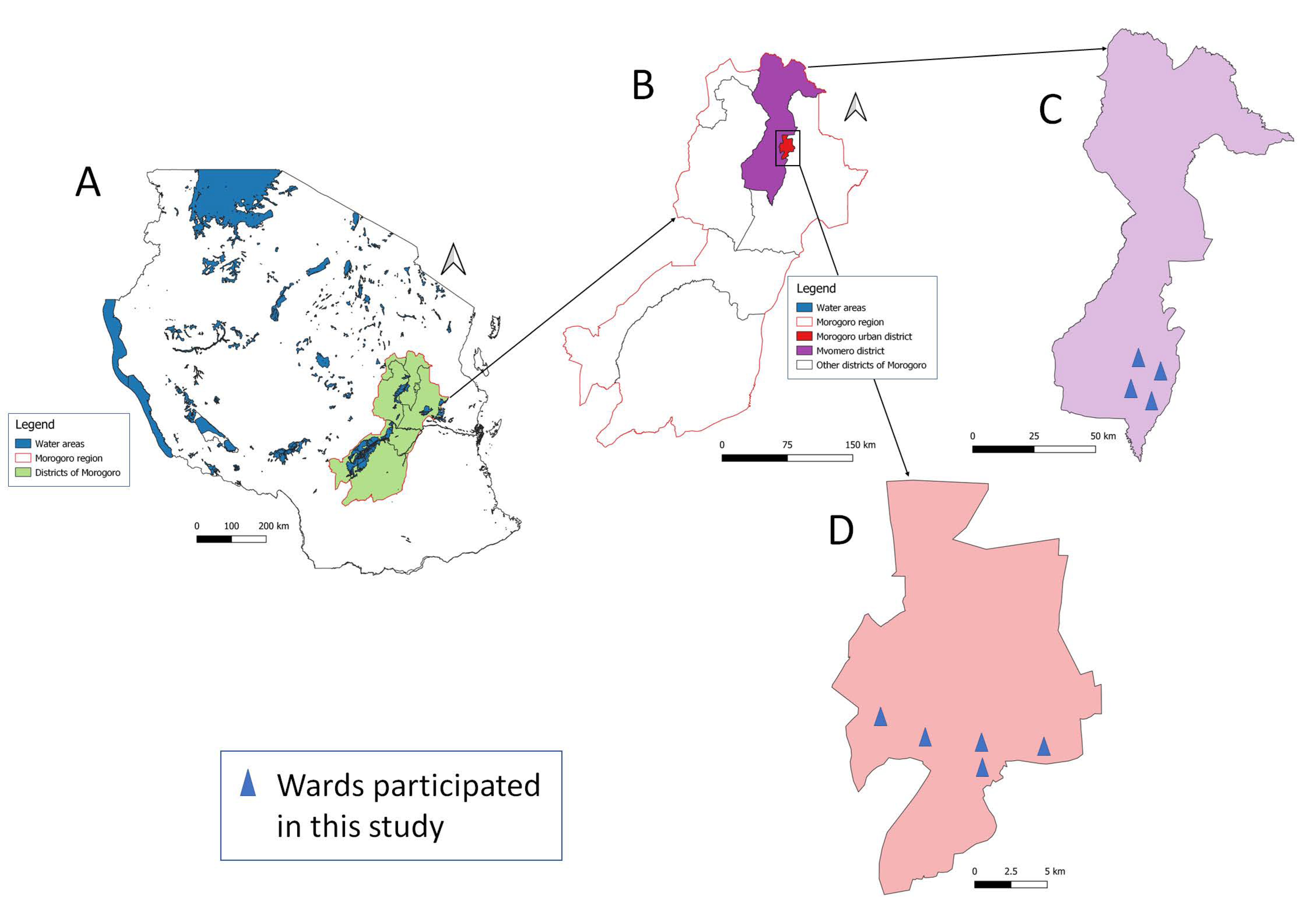
2.1. Study Area
2.2. Study Design and Selection of Study Sites
2.3. Sample Size Estimation
2.4. Data Collection
2.5. Faecal Sample Processing and Analysis
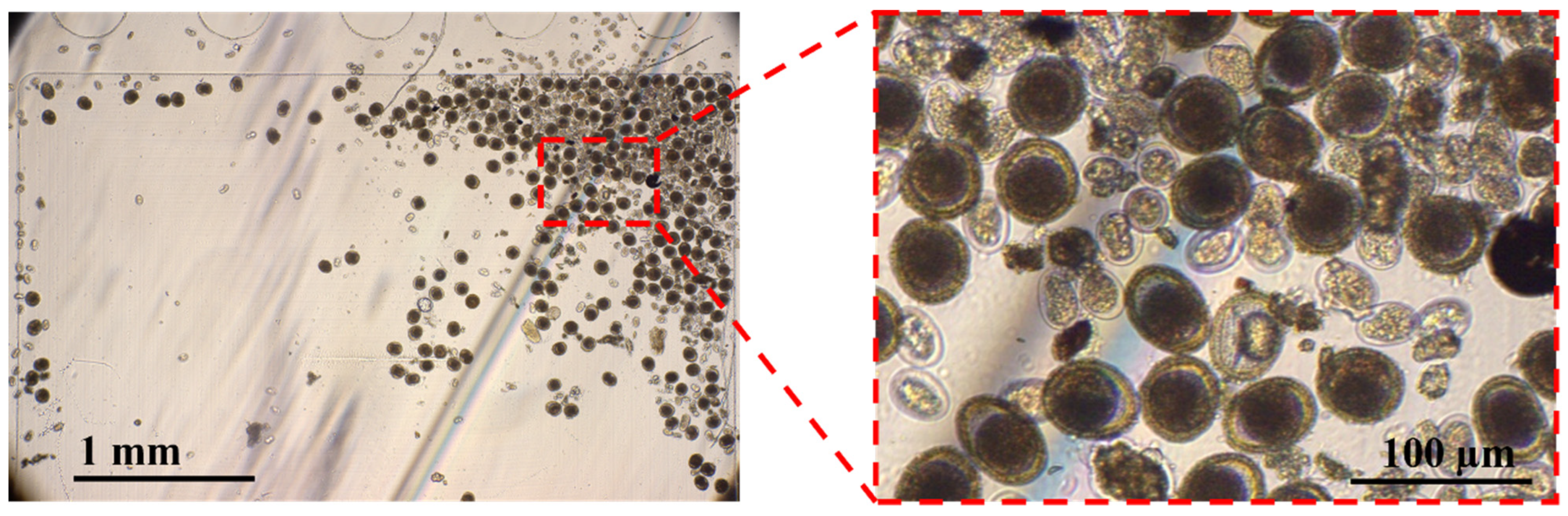
2.5.1. Qualitative Faecal Sample Analysis
2.5.2. Quantitative Faecal Sample Analysis
2.6. Data Analysis
3. Results
3.1. Prevalence of Soil-Transmitted Helminths
3.2. Overall Diagnostic Performance of LoD Technique
3.3. Diagnostic Performance of LoD Technique for STH in Domestic Pigs and Dogs
3.4. LoD Technique Performance for Individual STH Species in Domestic Pigs and Dogs
3.5. Overall Performance of the LoD Technique for Individual STH Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agustina, K.K.; Anthara, M.S.; Anom, N.; Nugraha, A.; Adi, W.; Wiguna, R. Prevalence and distribution of soil-transmitted helminth infection in free-roaming dogs in Bali Province, Indonesia. Vet. World 2021, 14, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, I.C.; Roberts, H.; Fourie, P. Prevalence of gastrointestinal helminths and parasites in smallholder pigs reared in the central Free State Province. Onderstepoort J. Vet. Res. 2019, 86, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jia-Chi, C.; Abdullah, N.A.; Shukor, N.; Jaturas, N.; Richard, R.L.; Majid, M.A.A.; Nissapatorn, V. Soil transmitted helminths in animals—How is it possible for human transmission? Asian Pac. J. Trop. Dis. 2016, 6, 859–863. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Zoonoses and the Human-Animal-Ecosystems Interface; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Cutillas, C.; Callejon, R.; de Rojas, M.; Tewes, B.; Ubeda, J.M.; Ariza, C.; Guevara, D.C. Trichuris suis and Trichuris trichiura are different nematode species. Acta Trop. 2009, 111, 299–307. [Google Scholar] [CrossRef] [PubMed]
- de Silva, N.R.; Brooker, S.; Hotez, P.J.; Montresor, A.; Engels, D.; Savioli, L. Soil-transmitted helminth infections: Updating the global picture. Trends Parasitol. 2003, 19, 547–551. [Google Scholar] [CrossRef]
- Hendrix, C.M. Diagnostic Parasitology for Veterinary Technicians, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 228–241. [Google Scholar]
- Demelash, K.; Abebaw, M.; Negash, A.; Alene, B.; Zemene, M.; Tilahun, M. A Review on Diagnostic Techniques in Veterinary Helminthlogy. Nat. Sci. 2016, 14, 109–118. [Google Scholar] [CrossRef]
- Nicholls, J.; Obendorf, D. Application of a composite fecal egg procedure in diagnostic parasitology. Vet. Parasitol. 1994, 52, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, G.; Rinaldi, L.; Maurelli, M.P.; Utzinger, J. FLOTAC: New multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010, 5, 503–515. [Google Scholar] [CrossRef]
- Kong, L.X.; Perebikovsky, A.; Moebius, J.; Kulinsky, L.; Madou, M. Lab-on-a-CD: A Fully Integrated Molecular Diagnostic System. J. Lab. Autom. 2016, 21, 323–355. [Google Scholar] [CrossRef]
- Madou, M.; Zoval, J.; Jia, G.; Kido, H.; Kim, J.; Kim, N. Lab on a CD. Annu. Rev. Biomed. Eng. 2006, 8, 601–628. [Google Scholar] [CrossRef]
- Misko, V.R.; Kryj, A.; Ngansop, A.M.T.; Yazdani, S.; Briet, M.; Basinda, N.; Mazigo, H.D.; Malsche, W.D. Migration Behavior of Low-Density Particles in Lab-on-a-Disc Devices: Effect of Walls. Micromachines 2021, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Sukas, S.; Van Dorst, B.; Kryj, A.; Lagatie, O.; Malsche, W.D. Development of a Lab-on-a-Disk Platform with Digital Imaging for Identification and Counting of Parasite Eggs in Human and Animal Stool. Micromachines 2019, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, N.K.; Singh, H.; Rath, S.S. Copro-prevalence and risk factor assessment of gastrointestinal parasitism in Indian domestic pigs. Helminthologia 2020, 57, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Chidumayo, N.N. Epidemiology of canine gastrointestinal helminths in sub-Saharan Africa. Parasit. Vectors 2018, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Kabululu, M.L.; Ngowi, H.A.; Kimera, S.I.; Lekule, F.P.; Kimbi, E.C.; Johansen, M.V. Risk factors for prevalence of pig parasitoses in Mbeya Region, Tanzania. Vet. Parasitol. 2015, 212, 460–464. [Google Scholar] [CrossRef]
- Roepstorff, A.; Nansen, P. Epidemiology, Diagnosis and Control of Helminth Parasites of Swine; FAO Animal Health Manual; M-27; FAO: Rome, Italy, 1998; ISBN 92-5-104220-9. [Google Scholar]
- Soulsby, E.J. Helminth, Arthropods and Protozoa of Domesticated Animals, 7th ed.; Bailliere Tindall: London, UK, 1982; pp. 212–252. [Google Scholar]
- MAFF. Manual of Veterinary Parasitology Laboratory Techniques, 1st ed.; Debere Zeit Ethiopia; Ministry of Agriculture Fisheries and Food: Canberra, ACT, Australia, 2006; pp. 10–16.
- Demeke, G.; Fenta, A.; Dilnessa, T. Evaluation of Wet Mount and Concentration Techniques of Stool Examination for Intestinal Parasites Identification at Debre Markos Comprehensive Specialized Hospital, Ethiopia. Infect. Drug Resist. 2021, 14, 1357–1362. [Google Scholar] [CrossRef]
- Salam, M.M.; Maqbool, A.; Naureen, A.; Lateef, M. Comparison of different diagnostic techniques against Fasciolosis in Buffaloes. Vet. World 2009, 2, 129–132. [Google Scholar]
- Cringoli, G.; Maurelli, M.P.; Levecke, B.; Bosco, A.; Vercruysse, J.; Utzinger, J.; Rinaldi, L. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat. Protoc. 2017, 12, 1723–1732. [Google Scholar] [CrossRef]
- Ballweber, L.R. Diagnostic Methods for Parasitic Infections in Livestock. Vet. Clin. Food Anim. 2006, 22, 695–705. [Google Scholar] [CrossRef]
- Foreyt, W. Diagnostic Parasitology. Vet. Clin. N. Am. Small Anim. Pract. 1989, 19, 979–1000. [Google Scholar] [CrossRef]
- Nausch, N.; Dawson, E.M.; Midzi, N.; Mduluza, T.; Mutapi, F.; Doenhoff, M.J. Field evaluation of a new antibody-based diagnostic for Schistosoma haematobium and S. mansoni at the point-of-care in northeast Zimbabwe. BMC Infect. Dis. 2014, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Lamberton, P.H.L.; Kabatereine, N.B.; Oguttu, D.W.; Fenwick, A.; Webster, J.P. Sensitivity and specifcity of multiple kato-katz thick smears and acirculating cathodic antigen test for Schistosoma mansoni diagnosis pre- and post-repeated-praziquantel treatment. PLoS Neglected Trop. Dis. 2014, 8, e3139. [Google Scholar] [CrossRef] [PubMed]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar] [PubMed]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]

| Technique | Sensitivity | Specificity | PPV | NPV | TA |
|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | Kappa Values | |
| McM | 93.51 (91.39–95.63) | 60.89 (56.69–65.10) | 81.91 (78.60–85.23) | 83.21 (79.99–86.43) | 0.5808 |
| Flotation | 93.18 (91.00–95.35) | 59.67 (55.44–63.89) | 81.14 (77.77–84.51) | 82.44 (79.17–85.72) | 0.5645 |
| Technique | Sensitivity | Specificity | PPV | NPV | TA |
|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | Kappa Value | |
| McMaster | |||||
| LoD | 90.40 (86.7–94.2) | 33.33 (27.3–39.3) | 87.32 (83.1–91.6) | 40.63 (34.4–46.9) | 0.2558 |
| Flotation | 98.99 (97.7–100) | 84.62 (80.0–89.2) | 97.03 (94.9–99.2) | 94.29 (91.3–97.2) | 0.8720 |
| Flotation | |||||
| LoD | 90.10 (86.3–93.9) | 34.29 (28.2–40.3) | 88.78 (84.8–92.8) | 37.50 (31.3–43.7) | 0.2528 |
| McM | 97.0 (94.9–99.19) | 94.3 (91.3–97.2) | 98.99 (97.7–100.3) | 84.62 (80.0–89.2) | 0.8720 |
| Technique | Sensitivity | Specificity | PPV | NPV | TA/Kappa Value |
|---|---|---|---|---|---|
| McMaster | |||||
| LoD | 97.87 (96.2–99.6) | 68.57 (63.1–74.0) | 75.82 (70.8–80.8) | 96.97 (95.0–99.0) | 0.6651 |
| Flotation | 94.33 (91.6–97.0) | 98.57 (97.2–100) | 98.5 (97.1–99.9) | 94.5 (91.9–97.2) | 0.9288 |
| Flotation | |||||
| LoD | 97.78 (96.1–99.50) | 65.8 (60.2–71.3) | 72.5 (67.3–77.8) | 96.97 (95.0–99.0) | 0.6271 |
| McM | 98.52 (97.1–99.9) | 94.52 (91.9–97.2) | 94.33 (91.6–97.0) | 98.57 (97.1–100) | 0.9288 |
| STH Species | Strongyle | Ascarid | Whipworm | |||
|---|---|---|---|---|---|---|
| Animal Species | Dog | Pig | Dog | Pig | Dog | Pig |
| Sensitivity | 97% | 88.36% | 97.14% | 71.43% | 42.9% | 72.73% |
| Specificity | 69.59% | 33.33% | 93.90% | 95.07% | 96.4% | 93.63% |
| PPV | 74.14% | 83.92% | 69.39% | 47.62% | 23.08% | 64.86% |
| NPV | 96.26% | 42.11% | 99.57% | 98.15% | 98.51% | 95.50% |
| STH Species | Strongyle | Ascarid | Whipworm |
|---|---|---|---|
| Sensitivity | 91.93% | 89.80% | 67.50% |
| Specificity | 60.71% | 94.46% | 95.19% |
| PPV | 79.36% | 62.86% | 54.00% |
| NPV | 82.07% | 98.88% | 97.22% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubagumya, S.L.; Nzalawahe, J.; Misinzo, G.; Mazigo, H.D.; Briet, M.; Misko, V.R.; De Malsche, W.; Legein, F.; Justine, N.C.; Basinda, N.; et al. Evaluation of Lab-on-a-Disc Technique Performance for Soil-Transmitted Helminth Diagnosis in Animals in Tanzania. Vet. Sci. 2024, 11, 174. https://doi.org/10.3390/vetsci11040174
Rubagumya SL, Nzalawahe J, Misinzo G, Mazigo HD, Briet M, Misko VR, De Malsche W, Legein F, Justine NC, Basinda N, et al. Evaluation of Lab-on-a-Disc Technique Performance for Soil-Transmitted Helminth Diagnosis in Animals in Tanzania. Veterinary Sciences. 2024; 11(4):174. https://doi.org/10.3390/vetsci11040174
Chicago/Turabian StyleRubagumya, Sarah L., Jahashi Nzalawahe, Gerald Misinzo, Humphrey D. Mazigo, Matthieu Briet, Vyacheslav R. Misko, Wim De Malsche, Filip Legein, Nyanda C. Justine, Namanya Basinda, and et al. 2024. "Evaluation of Lab-on-a-Disc Technique Performance for Soil-Transmitted Helminth Diagnosis in Animals in Tanzania" Veterinary Sciences 11, no. 4: 174. https://doi.org/10.3390/vetsci11040174
APA StyleRubagumya, S. L., Nzalawahe, J., Misinzo, G., Mazigo, H. D., Briet, M., Misko, V. R., De Malsche, W., Legein, F., Justine, N. C., Basinda, N., & Mafie, E. (2024). Evaluation of Lab-on-a-Disc Technique Performance for Soil-Transmitted Helminth Diagnosis in Animals in Tanzania. Veterinary Sciences, 11(4), 174. https://doi.org/10.3390/vetsci11040174









