Effects of Vaccination against Recombinant FSH or LH Receptor Subunits on Gonadal Development and Functioning Male Rats
Abstract
Simple Summary
Abstract
1. Implications
2. Introduction
3. Material and Methods
3.1. Experimental Animals
3.2. Vaccine Design and Preparation
3.3. Vaccination and Samples Collection
3.4. Antibody Titer Assays
3.5. Testosterone Detection
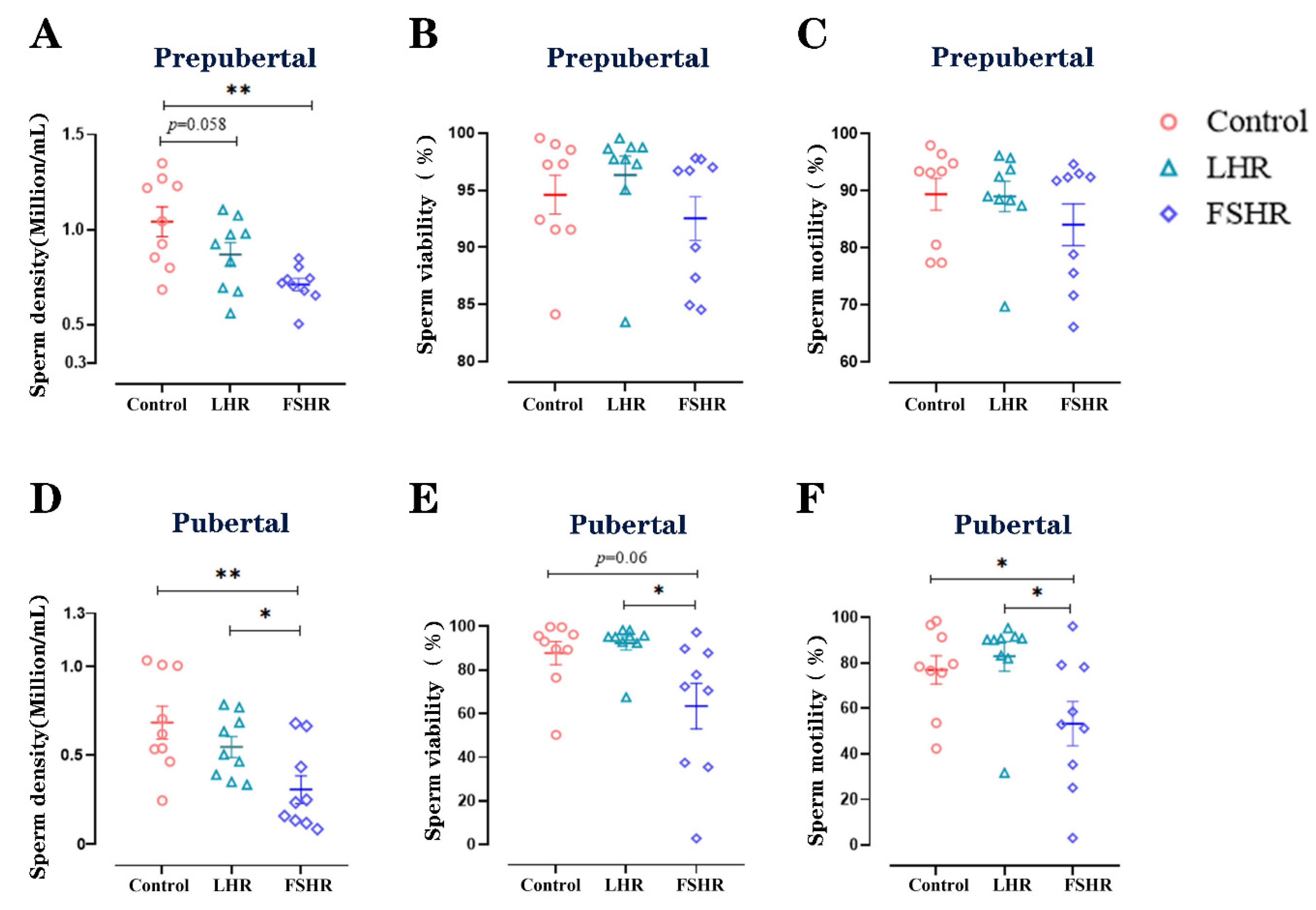
3.6. Sperm Density, Viability, and Motility
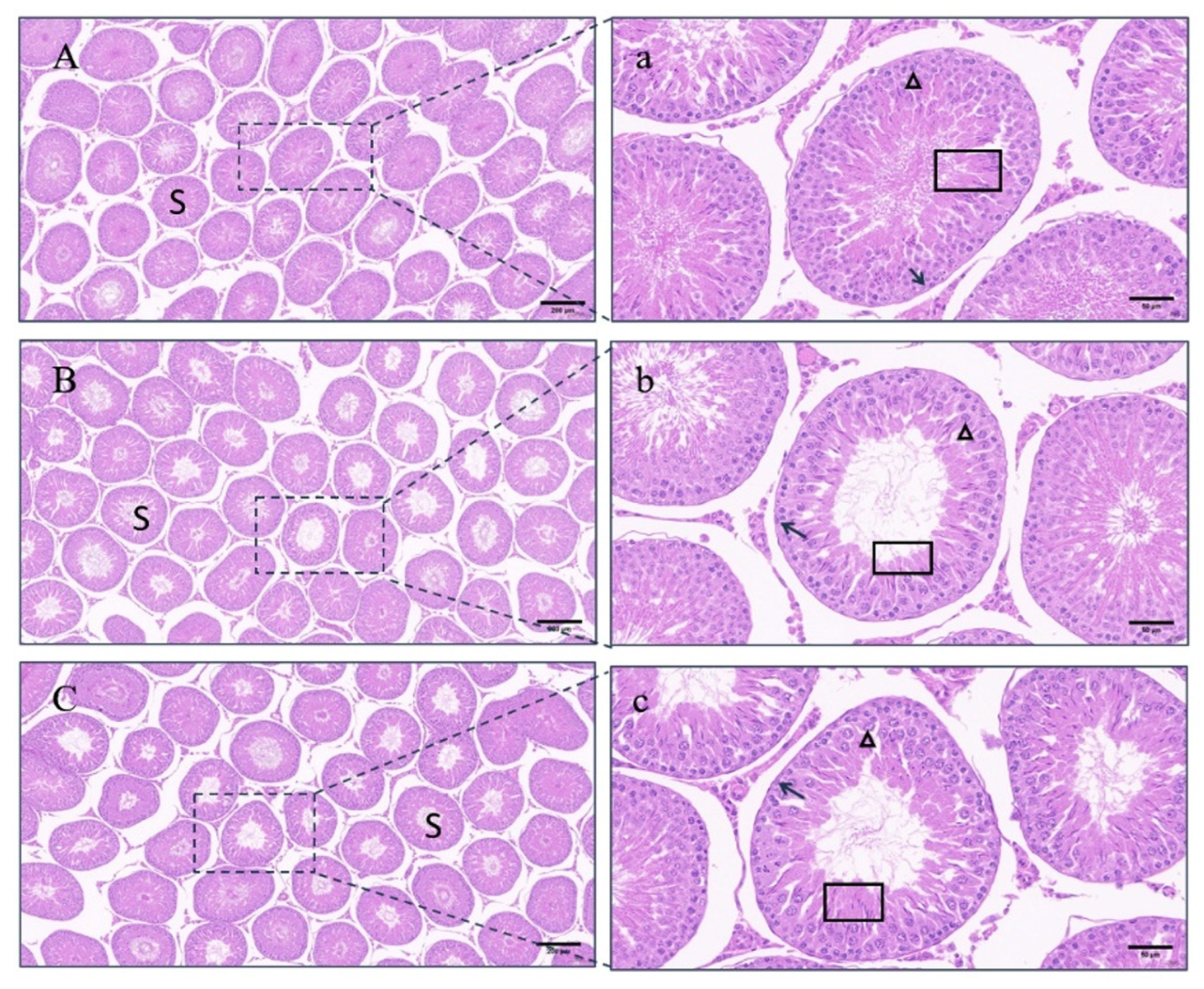
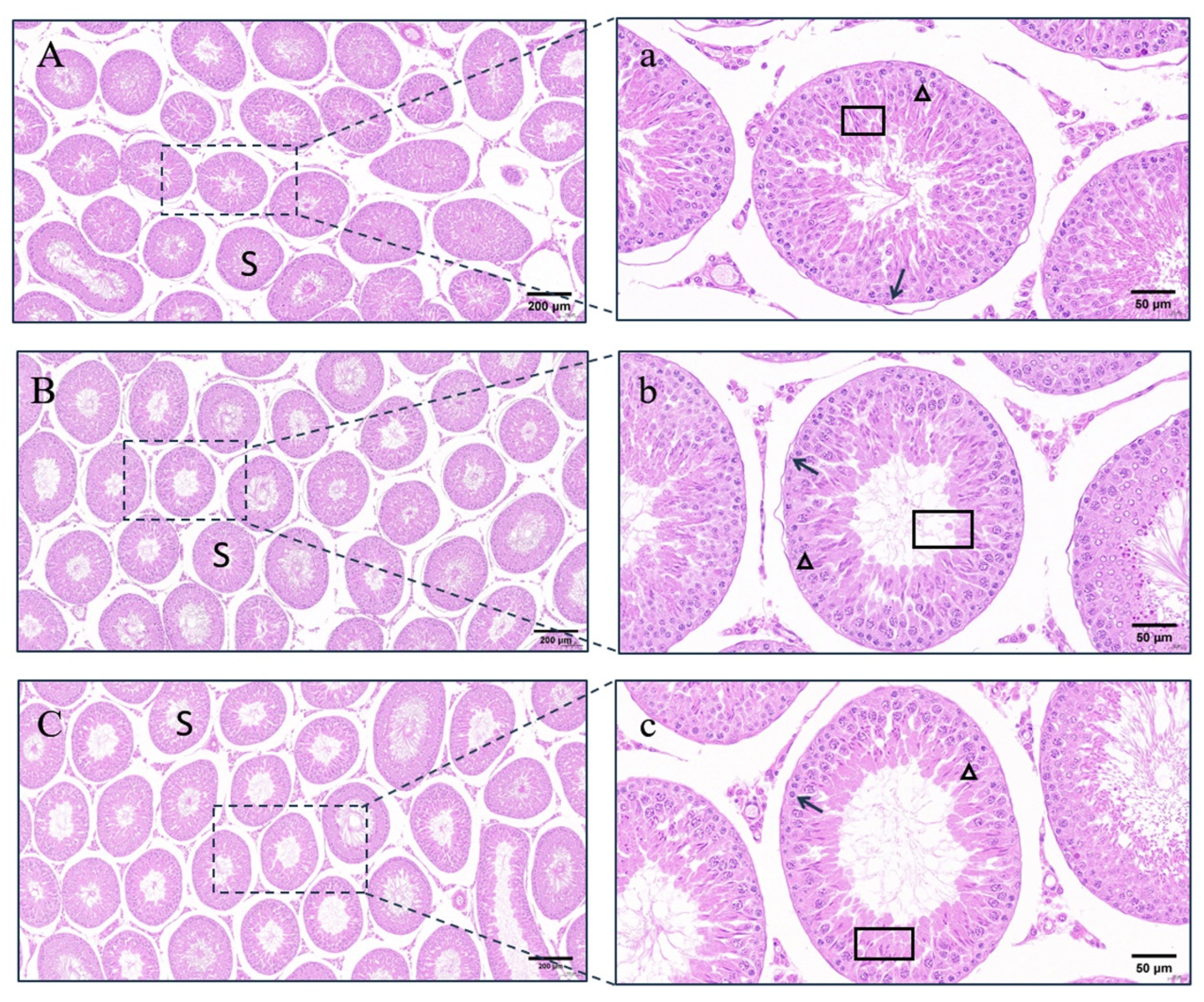
3.7. Histomorphological Observation of Testes
3.8. Statistical Analysis
4. Results
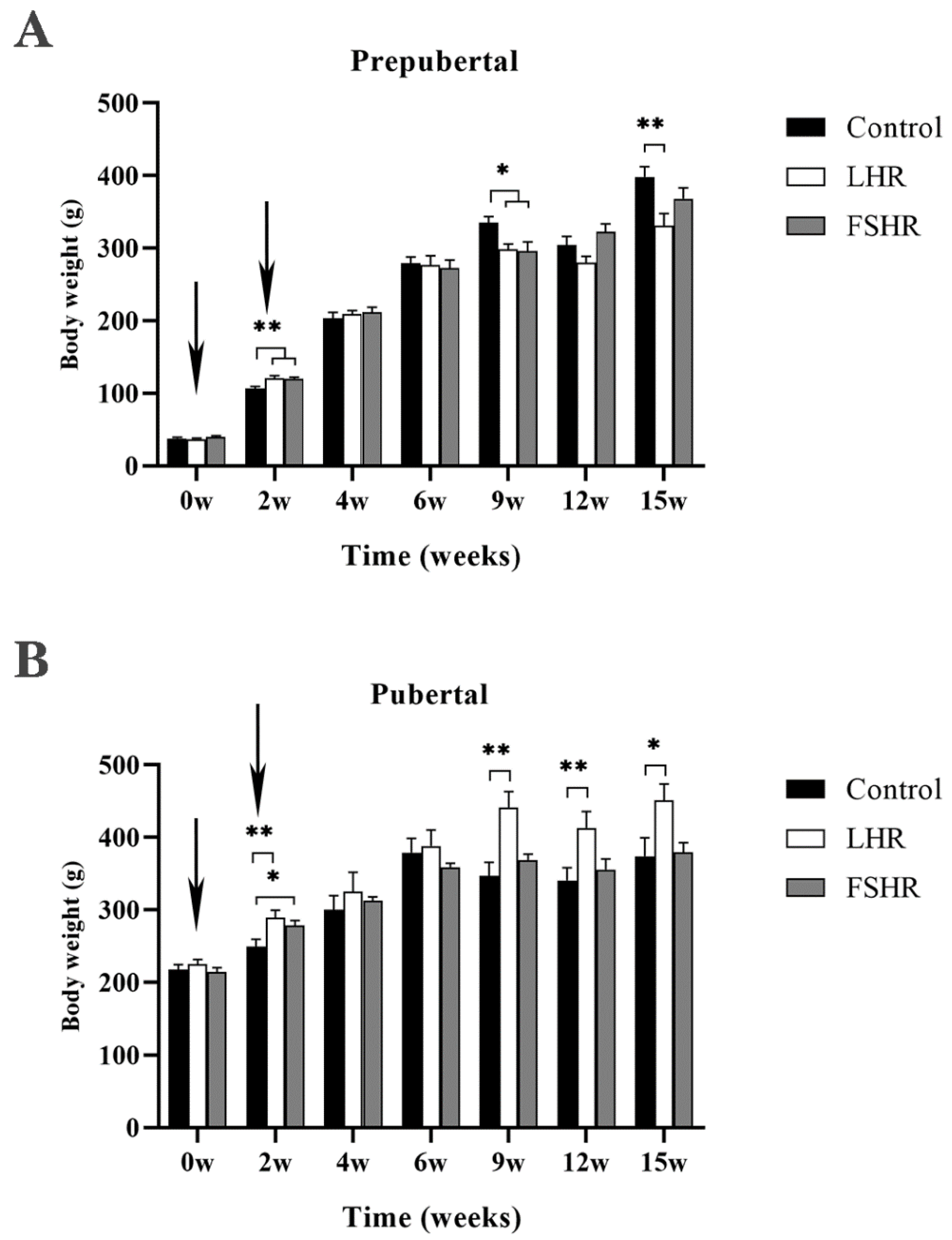
4.1. Effect on Body Weight of Rats
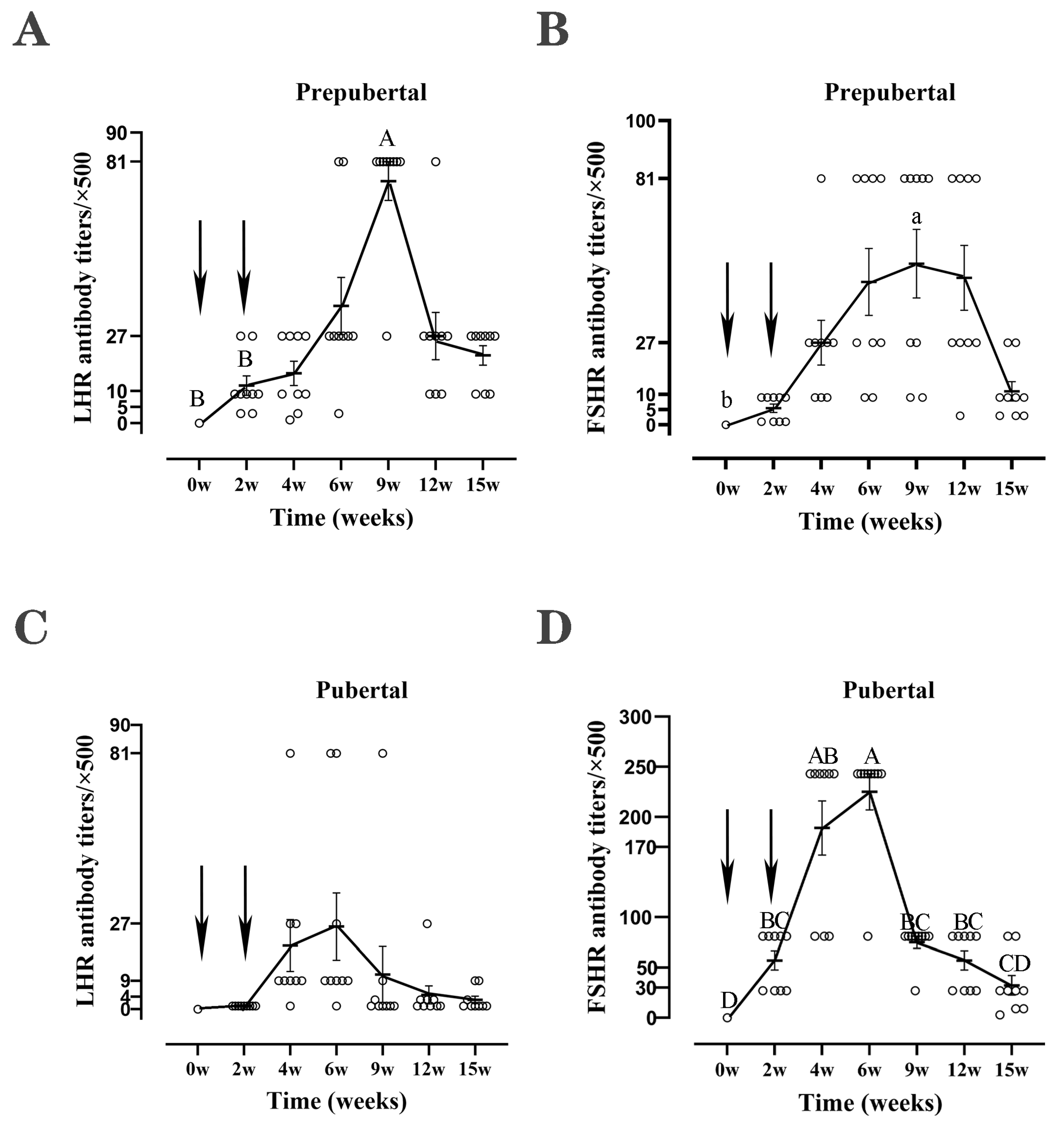
4.2. Antibody Levels
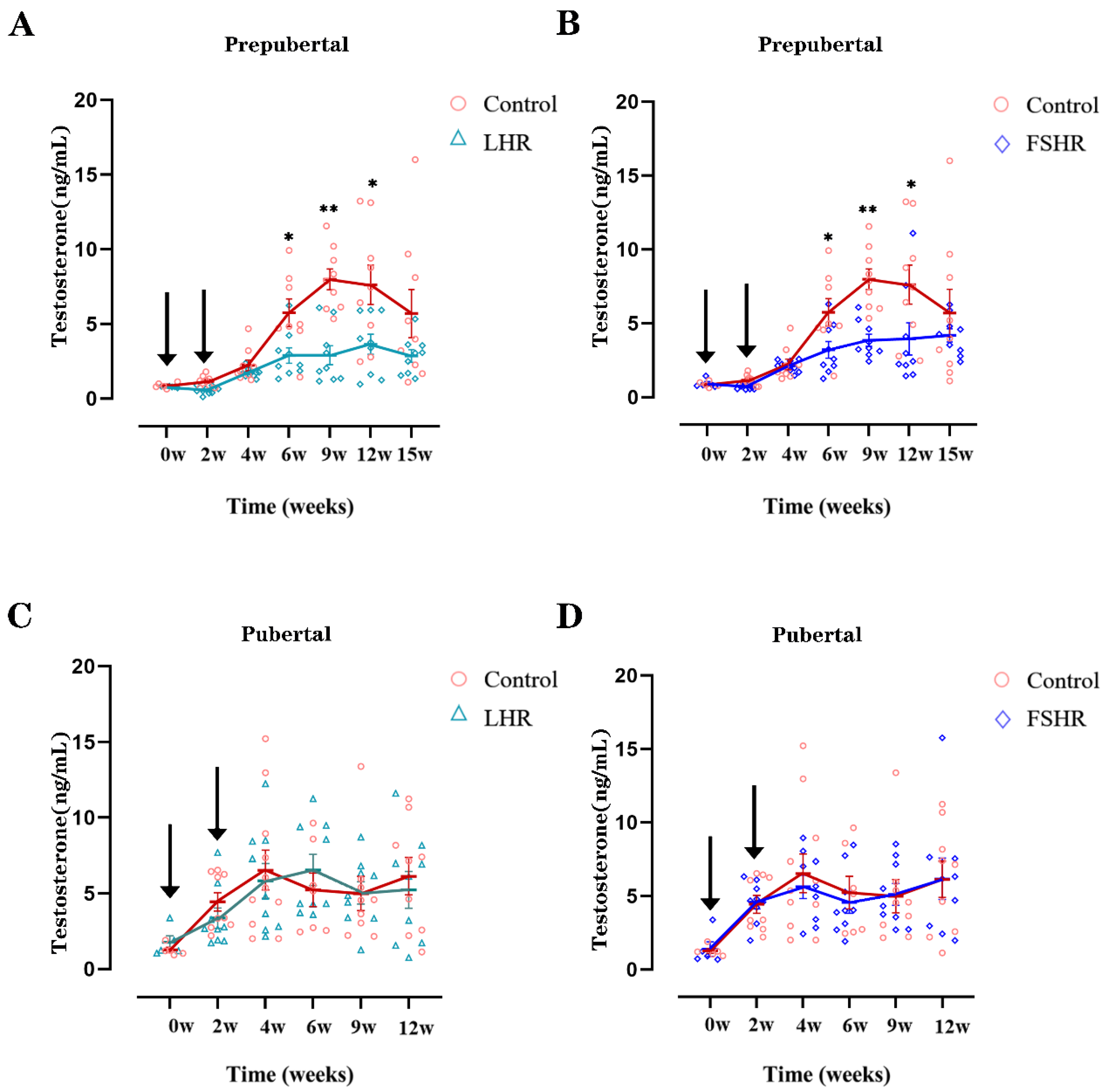
4.3. Testosterone Levels
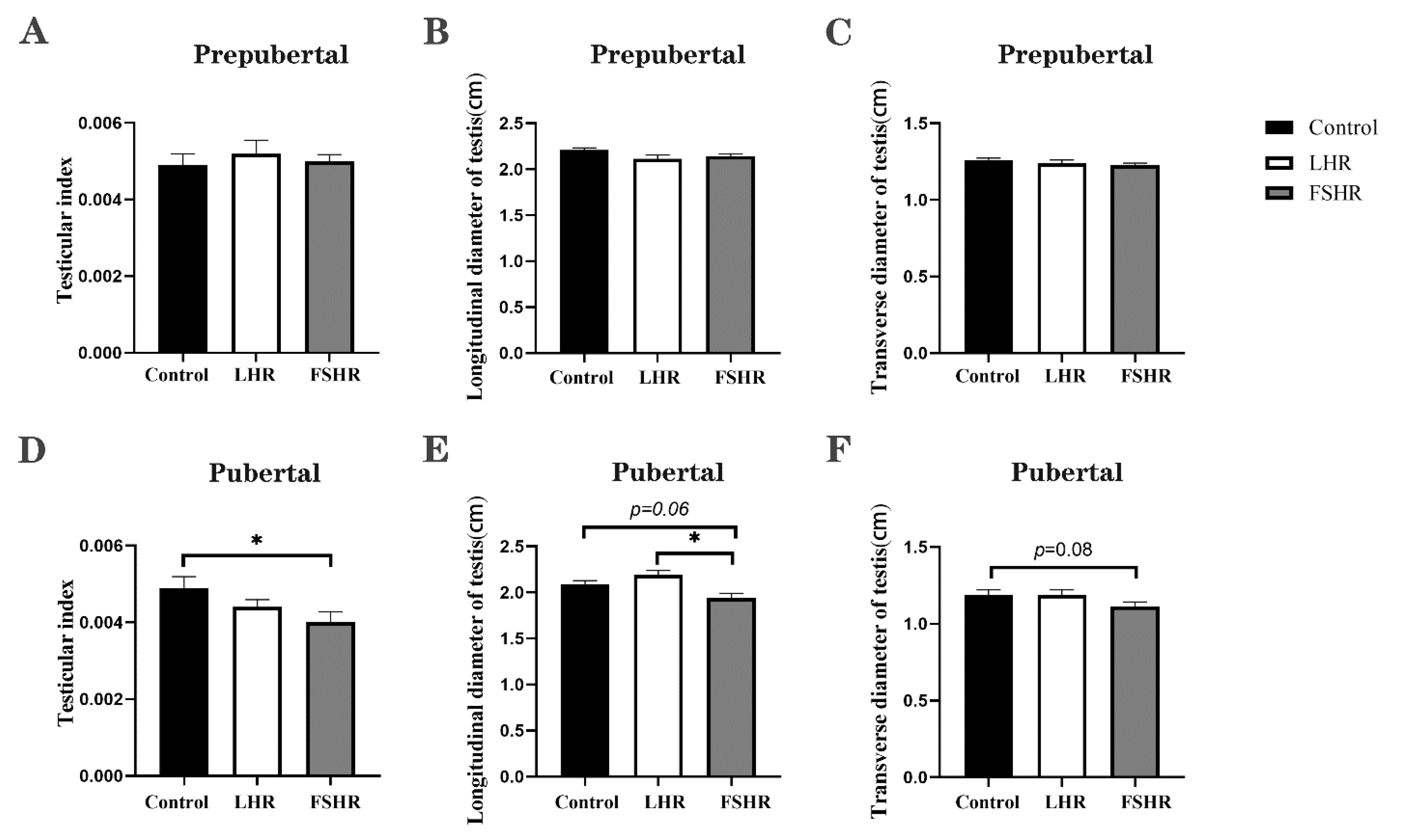
4.4. Testicular Changes
4.5. Sperm Density, Viability, and Motility
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, S.; Jiang, X.; Liu, G.; Sadiq, A.; Farooq, U.; Wassie, T.; Saleem, A.H.; Zubair, M. New trends in immunocastration and its potential to improve animal welfare: A mini review. Trop. Anim. Health Prod. 2022, 54, 369. [Google Scholar] [CrossRef]
- Bonneau, M.; Weiler, U. Pros and Cons of Alternatives to Piglet Castration: Welfare, Boar Taint, and Other Meat Quality Traits. Animals 2019, 9, 884. [Google Scholar] [CrossRef]
- Pesenti Rossi, G.; Dalla Costa, E.; Filipe, J.F.S.; Mazzola, S.M.; Motta, A.; Borciani, M.; Gastaldo, A.; Canali, E.; Pilia, F.; Argenton, M.; et al. Does Immunocastration Affect Behaviour and Body Lesions in Heavy Pigs? Vet. Sci. 2022, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Minhas, V. Wildlife population management: Are contraceptive vaccines a feasible proposition? Front. Biosci. (Sch. Ed.) 2017, 9, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, S.; Brunius, C.; Wallgren, M.; Lundström, K.; Andersson, K.; Zamaratskaia, G.; Rodriguez-Martinez, H. Effects of early vaccination with Improvac(®) on the development and function of reproductive organs of male pigs. Anim. Reprod. Sci. 2011, 127, 50–55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baker, D.L.; Powers, J.G.; Ransom, J.I.; McCann, B.E.; Oehler, M.W.; Bruemmer, J.E.; Galloway, N.L.; Eckery, D.C.; Nett, T.M. Reimmunization increases contraceptive effectiveness of gonadotropin-releasing hormone vaccine (GonaCon-Equine) in free-ranging horses (Equus caballus): Limitations and side effects. PLoS ONE 2018, 13, e0201570. [Google Scholar] [CrossRef]
- Theubet, G.; Thun, R.; Hilbe, M.; Janett, F. Effect of vaccination against GnRH (Bopriva®) in the male pubertal calf. Schweiz. Arch. Fur Tierheilkd. 2010, 152, 459–469. [Google Scholar] [CrossRef][Green Version]
- Resta, C.; Moustogiannis, A.; Chatzinikita, E.; Malligiannis Ntalianis, D.; Malligiannis Ntalianis, K.; Philippou, A.; Koutsilieris, M.; Vlahos, N. Gonadotropin-Releasing Hormone (GnRH)/GnRH Receptors and Their Role in the Treatment of Endometriosis. Cureus 2023, 15, e38136. [Google Scholar] [CrossRef]
- Wickings, E.J.; Nieschlag, E. Suppression of spermatogenesis over two years in rhesus monkeys actively immunized with follicle-stimulating hormone. Fertil. Steril. 1980, 34, 269–274. [Google Scholar] [CrossRef]
- Moudgal, N.R.; Ravindranath, N.; Murthy, G.S.; Dighe, R.R.; Aravindan, G.R.; Martin, F. Long-term contraceptive efficacy of vaccine of ovine follicle-stimulating hormone in male bonnet monkeys (Macaca radiata). J. Reprod. Fertil. 1992, 96, 91–102. [Google Scholar] [CrossRef]
- Moudgal, N.R.; Murthy, G.S.; Prasanna Kumar, K.M.; Martin, F.; Suresh, R.; Medhamurthy, R.; Patil, S.; Sehgal, S.; Saxena, B.N. Responsiveness of human male volunteers to immunization with ovine follicle stimulating hormone vaccine: Results of a pilot study. Hum. Reprod. 1997, 12, 457–463. [Google Scholar] [CrossRef]
- Srivastav, A.; Das, R.P. Sperm production and fertility of bonnet monkeys (Macaca radiata) following immunization with ovine follicle stimulating hormone. Indian J. Exp. Biol. 1992, 30, 574–577. [Google Scholar]
- Schanbacher, B.D. Effects of active immunization of the ram and bull against luteinizing hormone. Theriogenology 1985, 24, 59–71. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shrestha, A.; Minhas, V. Milestones in contraceptive vaccines development and hurdles in their application. Hum. Vaccines Immunother. 2014, 10, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.M.; Dias, J.A.; Van Roey, P. Three-dimensional structure of human follicle-stimulating hormone. Mol. Endocrinol. 2001, 15, 378–389. [Google Scholar] [CrossRef]
- Lapthorn, A.J.; Harris, D.C.; Littlejohn, A.; Lustbader, J.W.; Canfield, R.E.; Machin, K.J.; Morgan, F.J.; Isaacs, N.W. Crystal structure of human chorionic gonadotropin. Nature 1994, 369, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Thau, R.B.; Sundaram, K.; Thornton, Y.S.; Seidman, L.S. Effects of immunization with the beta-subunit of ovine luteinizing hormone on corpus luteum function in the rhesus monkey. Fertil. Steril. 1979, 31, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Talwar, G.P.; Singh, O.; Singh, V.; Rao, D.N.; Sharma, N.C.; Das, C.; Rao, L.V. Enhancement of antigonadotropin response to the beta-subunit of ovine luteinizing hormone by carrier conjugation and combination with the beta-subunit of human chorionic gonadotropin. Fertil. Steril. 1986, 46, 120–126. [Google Scholar] [CrossRef]
- Santa Coloma, T.A.; Dattatreyamurty, B.; Reichert, L.E., Jr. A synthetic peptide corresponding to human FSH beta-subunit 33-53 binds to FSH receptor, stimulates basal estradiol biosynthesis, and is a partial antagonist of FSH. Biochemistry 1990, 29, 1194–1200. [Google Scholar] [CrossRef]
- Lal, D.; Mahale, S.D.; Nandedkar, T.D.; Iyer, K.S. Identification of bioneutralization epitopes of human follicle stimulating hormone in the regions 31-52 and 66-75 of its beta-subunit. J. Reprod. Immunol. 1997, 33, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moudgal, N.R.; Sairam, M.R.; Dighe, R.R. Relative ability of ovine follicle stimulating hormone and itsβ-subunit to generate antibodies having bioneutralization potential in nonhuman primates. J. Biosci. 1989, 14, 91–100. [Google Scholar] [CrossRef]
- Moudgal, N.R.; Sairam, M.R.; Krishnamurthy, H.N.; Sridhar, S.; Krishnamurthy, H.; Khan, H. Immunization of male bonnet monkeys (M. radiata) with a recombinant FSH receptor preparation affects testicular function and fertility. Endocrinology 1997, 138, 3065–3068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fuchs, T.; Thun, R.; Parvizi, N.; Nathues, H.; Koehrmann, A.; Andrews, S.; Brock, F.; Klein, G.; Sudhaus, N.; Grosse Beilage, E. Effect of a gonadotropin-releasing factor vaccine on follicle-stimulating hormone and luteinizing hormone concentrations and on the development of testicles and the expression of boar taint in male pigs. Theriogenology 2009, 72, 672–680. [Google Scholar] [CrossRef]
- Sambroni, E.; Abdennebi-Najar, L.; Remy, J.J.; Le Gac, F. Delayed sexual maturation through gonadotropin receptor vaccination in the rainbow trout Oncorhynchus mykiss. Gen. Comp. Endocrinol. 2009, 164, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Maenhoudt, C.; Santos, N.R.; Fontbonne, A. Suppression of fertility in adult dogs. Reprod. Domest. Anim. 2014, 49 (Suppl. S2), 58–63. [Google Scholar]
- Saxena, B.B.; Clavio, A.; Singh, M.; Rathnam, P.; Bukharovich, Y.; Reimers, T., Jr.; Saxena, A.; Perkins, S. Modulation of ovarian function in female dogs immunized with bovine luteinizing hormone receptor. Reprod. Domest. Anim. 2002, 37, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Diethelm-Okita, B.M.; Okita, D.K.; Banaszak, L.; Conti-Fine, B.M. Universal epitopes for human CD4+ cells on tetanus and diphtheria toxins. J. Infect. Dis. 2000, 181, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Chenal, A.; Nizard, P.; Forge, V.; Pugnière, M.; Roy, M.O.; Mani, J.C.; Guillain, F.; Gillet, D. Does fusion of domains from unrelated proteins affect their folding pathways and the structural changes involved in their function? A case study with the diphtheria toxin T domain. Protein Eng. 2002, 15, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Phalipon, A.; Kaczorek, M. Genetically engineered diphtheria toxin fusion proteins carrying the hepatitis B surface antigen. Gene 1987, 55, 255–263. [Google Scholar] [CrossRef]
- Phalipon, A.; Crainic, R.; Kaczorek, M. Expression of a poliovirus type 1 neutralization epitope on a diphtheria toxin fusion protein. Vaccine 1989, 7, 132–136. [Google Scholar] [CrossRef]
- Johnson, N.; Pickett, M.A.; Watt, P.J.; Clarke, I.N.; Heckels, J.E. Construction of an epitope vector utilising the diphtheria toxin B-subunit. FEMS Microbiol. Lett. 1997, 146, 91–96. [Google Scholar] [CrossRef]
- Alvine, T.D.; Osei-Owusu, P.; Condry, D.L.; Nilles, M.L. Expression and Purification of N-Terminally His-Tagged Recombinant Type III Secretion Proteins. Methods Mol. Biol. 2017, 1531, 183–191. [Google Scholar] [PubMed]
- Schäfer, F.; Seip, N.; Maertens, B.; Block, H.; Kubicek, J. Purification of GST-Tagged Proteins. Methods Enzymol. 2015, 559, 127–139. [Google Scholar] [PubMed]
- Morote, J.; Comas, I.; Ferrer, R.; Planas, J.; Celma, A.; Regis, L. Accuracy of serum luteinizing hormone and serum testosterone measurements to assess the efficacy of medical castration in prostate cancer patients. J. Biomed. Sci. 2017, 24, 81. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Du, H.; Tian, W.; Xie, H.; Zhang, B.; Fu, W.; Li, Y.; Ling, Y.; Zhang, Y.; Fang, F.; et al. Effect of GnRH immunocastration on immune function in male rats. Front. Immunol. 2022, 13, 1023104. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.R. The hematoxylin and eosin stain in anatomic pathology-An often-neglected focus of quality assurance in the laboratory. Semin. Diagn. Pathol. 2019, 36, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Ladics, G.S. Primary immune response to sheep red blood cells (SRBC) as the conventional T-cell dependent antibody response (TDAR) test. J. Immunotoxicol. 2007, 4, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Kodama, R.; Okazaki, T.; Sato, T.; Iwashige, S.; Tanigawa, Y.; Fujishima, J.; Moriyama, A.; Yamashita, N.; Sasaki, Y.; Yoshikawa, T.; et al. Age Difference in Morphology and Immunohistology inthe Thymus and Spleen in Crl:CD (SD) Rats. J. Toxicol. Pathol. 2012, 25, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.A.; Rao, C.V. Recent progress in luteinizing hormone/human chorionic gonadotrophin hormone research. Mol. Hum. Reprod. 2009, 15, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef]
- Yang, L.H.; Li, J.T.; Yan, P.; Liu, H.L.; Zeng, S.Y.; Wu, Y.Z.; Liang, Z.Q.; He, W. Follicle-stimulating hormone receptor (FSHR)-derived peptide vaccine induced infertility in mice without pathological effect on reproductive organs. Reprod. Fertil. Dev. 2011, 23, 544–550. [Google Scholar] [CrossRef]
- Yan, P.; He, W.; Wu, Y.; Chen, Z.; He, H.; Ni, B.; Zhang, J.; Yang, X.; Shen, Z.; Fu, X.; et al. Enhanced Suppression of Fertility Can be Achieved by Priming with FSHR and Eppin and Further Boosting with Their B-cell Epitope Peptides. Am. J. Reprod. Immunol. 2015, 74, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.; Bachelot, A.; Cocca, M.P.; Vasseur, C.; Rodien, P.; Kuttenn, F.; Touraine, P.; Misrahi, M. Molecular pathology of the FSH receptor: New insights into FSH physiology. Mol. Cell. Endocrinol. 2008, 282, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, H.; Babu, P.S.; Morales, C.R.; Sairam, M.R. Delay in sexual maturity of the follicle-stimulating hormone receptor knockout male mouse. Biol. Reprod. 2001, 65, 522–531. [Google Scholar] [CrossRef]
- Strehler, E.; Sterzik, K.; De Santo, M.; Abt, M.; Wiedemann, R.; Bellati, U.; Collodel, G.; Piomboni, P.; Baccetti, B. The effect of follicle-stimulating hormone therapy on sperm quality: An ultrastructural mathematical evaluation. J. Androl. 1997, 18, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Abel, M.H.; Baker, P.J.; Charlton, H.M.; Monteiro, A.; Verhoeven, G.; De Gendt, K.; Guillou, F.; O’Shaughnessy, P.J. Spermatogenesis and sertoli cell activity in mice lacking sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology 2008, 149, 3279–3285. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, P.J.; Monteiro, A.; Abel, M. Testicular development in mice lacking receptors for follicle stimulating hormone and androgen. PLoS ONE 2012, 7, e35136. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.J.; Ramachandra, S.G.; Ramesh, V.; Couture, L.; Abdennebi, L.; Salesse, R.; Remy, J.J. Induction of infertility in adult male bonnet monkeys by immunization with phage-expressed peptides of the extracellular domain of FSH receptor. Reprod. Biomed. Online 2004, 8, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Smith, C.E.; Gregory, M.; Cyr, D.G.; Sairam, M.R.; Hermo, L. Effects of FSH receptor deletion on epididymal tubules and sperm morphology, numbers, and motility. Mol. Reprod. Dev. 2005, 72, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Themmen, A.P.N.; Huhtaniemi, I.T. Mutations of gonadotropins and gonadotropin receptors: Elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr. Rev. 2000, 21, 551–583. [Google Scholar] [CrossRef]
- Narula, A.; Gu, Y.Q.; O’Donnell, L.; Stanton, P.G.; Robertson, D.M.; McLachlan, R.I.; Bremner, W.J. Variability in sperm suppression during testosterone administration to adult monkeys is related to follicle stimulating hormone suppression and not to intratesticular androgens. J. Clin. Endocrinol. Metab. 2002, 87, 3399–3406. [Google Scholar] [CrossRef] [PubMed]
- Oduwole, O.O.; Peltoketo, H.; Huhtaniemi, I.T. Role of Follicle-Stimulating Hormone in Spermatogenesis. Front. Endocrinol. 2018, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Amory, J.K.; Bremner, W. Endocrine regulation of testicular function in men: Implications for contraceptive development. Mol. Cell. Endocrinol. 2001, 182, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Schlatt, S.; Ehmcke, J. Regulation of spermatogenesis: An evolutionary biologist’s perspective. Semin. Cell Dev. Biol. 2014, 29, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.; Moffat, L.; Belling, K.; de França, L.R.; Segatelli, T.M.; Saunders, P.T.; Sharpe, R.M.; Smith, L.B. Androgen receptor signalling in peritubular myoid cells is essential for normal differentiation and function of adult Leydig cells. Int. J. Androl. 2012, 35, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; He, W.; Liang, Z.; Chen, Z.; Shang, X.; He, H.; Tang, Y.; Ni, B.; Zhang, J.; Shen, Z.; et al. A novel dominant B-cell epitope of FSHR identified by molecular docking induced specific immune response and suppressed fertility. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2009, 25, 828–838. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, F.; Fu, W.; Zhang, B.; Han, M.; Xie, H.; Yi, Q.; Qian, W.; Cui, J.; Cao, M.; Li, Y.; et al. Effects of Vaccination against Recombinant FSH or LH Receptor Subunits on Gonadal Development and Functioning Male Rats. Vet. Sci. 2024, 11, 176. https://doi.org/10.3390/vetsci11040176
Pan F, Fu W, Zhang B, Han M, Xie H, Yi Q, Qian W, Cui J, Cao M, Li Y, et al. Effects of Vaccination against Recombinant FSH or LH Receptor Subunits on Gonadal Development and Functioning Male Rats. Veterinary Sciences. 2024; 11(4):176. https://doi.org/10.3390/vetsci11040176
Chicago/Turabian StylePan, Fuqiang, Wanzhen Fu, Bochao Zhang, Mengdi Han, Huihui Xie, Qing Yi, Wei Qian, Jiankun Cui, Meng Cao, Yanqiuhong Li, and et al. 2024. "Effects of Vaccination against Recombinant FSH or LH Receptor Subunits on Gonadal Development and Functioning Male Rats" Veterinary Sciences 11, no. 4: 176. https://doi.org/10.3390/vetsci11040176
APA StylePan, F., Fu, W., Zhang, B., Han, M., Xie, H., Yi, Q., Qian, W., Cui, J., Cao, M., Li, Y., Jia, Y., Fang, F., Ling, Y., Li, Y., & Liu, Y. (2024). Effects of Vaccination against Recombinant FSH or LH Receptor Subunits on Gonadal Development and Functioning Male Rats. Veterinary Sciences, 11(4), 176. https://doi.org/10.3390/vetsci11040176




