Early Neurophysiological Abnormalities in Suspected Acute Canine Polyradiculoneuropathy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
- They had a comprehensive neurological examination conducted by an ECVN resident or an ECVN diplomate.
- They had a history of acute onset of neurological signs with progression to tetraparesis.
- Clinical signs were characterized by tetraparesis or tetraplegia with signs of lower motoneuron involvement in all four limbs (hyporeflexia and hypotonia).
- Complete blood count and biochemical tests showed no abnormalities that could explain the symptoms
- Comprehensive EDX testing, including EMG of appendicular muscles, motor nerve conduction studies (MNCSs), F-wave analysis, repetitive nerve stimulation (RNS), sensory nerve conduction studies (SNCs), and cord dorsum potentials (CDPs), were performed between days 1 and 15 days after the onset of symptoms. All repetitive nerve stimulation testing was within normal limits for study inclusion. Patients were also included if they did not undergo repetitive nerve stimulation, provided that EDX abnormalities were so pronounced that RNS was deemed unnecessary, as they were not consistent with a diagnosis of myasthenia and botulism.
- Significant clinical improvement was observed within a six-month period without the use of specific medical interventions such as steroids, NSAIDs, or pyridostigmine. Supportive care, immunoglobulin treatment, and physiotherapy were frequently administered during this time [9].
- Deceased patients or those euthanized due to respiratory difficulties compatible with paralysis of the intercostal and diaphragm muscles were also included, provided they met all the previous criteria (clinical signs, blood tests, and electrodiagnostic test).
2.2. Electrodiagnostic Study (EDX)
2.2.1. Electromyography (EMG)
- 0: none found
- 1+: Persistent single trains of potential (>2–3 s) in at least two areas
- 2+: Moderate number of potentials at least in 3 areas
- 3+: Several potentials in all areas
- 4+: Full interference pattern of potentials.
2.2.2. Motor Nerve Conduction Studies (MNCSs)
2.2.3. F-Wave
2.2.4. Sensory Nerve Conduction Studies (SNCs) and Cord Dorsum Potentials (CDPs)
2.2.5. Repetitive Nerve Stimulation
2.3. Statistical Analysis
3. Results
3.1. Electrodiagnostic Results
3.1.1. Electromyography (EMG)—Late Diagnostic Group Is Associated with Greater EMG Alterations
3.1.2. Motor Nerve Conduction Studies (MNCSs)
3.1.3. F-Wave
3.1.4. Sensory Nerve Conduction Studies (SNCs) and Cord Dorsum (CDP)
3.1.5. Repetitive Nerve Stimulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuddon, P.A. Acquired Canine Peripheral Neuropathies. Vet. Clin. N. Am. Small Anim. Pract. 2002, 32, 207–249. [Google Scholar] [CrossRef] [PubMed]
- Añor, S. Acute Lower Motor Neuron Tetraparesis. Vet. Clin. N. Am Small Anim. Pract. 2014, 44, 1201–1222. [Google Scholar] [CrossRef] [PubMed]
- Platt, S.R.; Olby, N.J. BSAVA Manual of Canine and Feline Neurology; British Small Animal Veterinary Association: Gloucester, UK, 2013. [Google Scholar]
- Hirschvogel, K.; Jurina, K.; Steinberg, T.A.; Matiasek, L.A.; Matiasek, K.; Beltrán, E.; Fischer, A. Clinical Course of Acute Canine Polyradiculoneuritis Following Treatment with Human IV Immunoglobulin. J. Am. Anim. Hosp. Assoc. 2012, 48, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.F.; Haas, D.C. Coonhound Paralysis: An Acute Idiopathic Polyradiculoneuritis in Dogs Resembling the Landry-Guillain-Barré Syndrome. J. Neurol. Sci. 1967, 4, 51–81. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.F.; Haas, D.C. Animal Model for Human Disease: Idiopathic Polyneuritis, Guillain-Barré Syndrome. Animal Model: Coonhound Paralysis, Idiopathic Polyradiculoneuritis of Coonhounds. Am. J. Pathol. 1972, 66, 189–192. [Google Scholar] [PubMed]
- Northington, J.W.; Brown, M.J. Acute Canine Idiopathic Polyneuropathy. A Guillain-Barré-like Syndrome in Dogs. J. Neurol. Sci. 1982, 56, 259–273. [Google Scholar] [CrossRef]
- Rupp, A.; Galban-Horcajo, F.; Bianchi, E.; Dondi, M.; Penderis, J.; Cappell, J.; Burgess, K.; Matiasek, K.; McGonigal, R.; Willison, H.J. Anti-GM2 Ganglioside Antibodies Are a Biomarker for Acute Canine Polyradiculoneuritis. J. Peripher. Nerv. Syst. 2013, 18, 75–88. [Google Scholar] [CrossRef]
- Laws, E.J.; Harcourt-Brown, T.R.; Granger, N.; Rose, J.H. An Exploratory Study into Factors Influencing Development of Acute Canine Polyradiculoneuritis in the UK. J. Small Anim. Pract. 2017, 58, 437–443. [Google Scholar] [CrossRef]
- Rutter, C.R.; Rozanski, E.A.; Sharp, C.R.; Powell, L.L.; Kent, M. Outcome and Medical Management in Dogs with Lower Motor Neuron Disease Undergoing Mechanical Ventilation: 14 Cases (2003–2009). J. Vet. Emerg. Crit. Care 2011, 21, 531–541. [Google Scholar] [CrossRef]
- Cuddon, P.A. Electrophysiologic Assessment of Acute Polyradiculoneuropathy in Dogs: Comparison with Guillain-Barré Syndrome in People. J. Vet. Intern. Med. 1998, 12, 294–303. [Google Scholar] [CrossRef]
- de Lahunta, A.; Glass, E.; Kent, M. Veterinary Neuroanatomy and Clinical Neurology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2020; Available online: https://shop.elsevier.com/books/de-lahunta-s-veterinary-neuroanatomy-and-clinical-neurology/de-lahunta/978-0-323-69611-1 (accessed on 8 January 2024).
- Holt, N.; Murray, M.; Cuddon, P.A.; Lappin, M.R. Seroprevalence of Various Infectious Agents in Dogs with Suspected Acute Canine Polyradiculoneuritis. J. Vet. Intern. Med. 2011, 25, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Anton, L.; Marenda, M.; Firestone, S.M.; Bushell, R.N.; Child, G.; Hamilton, A.I.; Long, S.N.; Le Chevoir, M.a.R. Investigation of the Role of Campylobacter Infection in Suspected Acute Polyradiculoneuritis in Dogs. J. Vet. Intern. Med. 2018, 32, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.K.; Gourlay, D.S.; Penderis, J.; Bianchi, E.; Dondi, M.; Wessmann, A.; Musteata, M.; Le Chevoir, M.; Martinez-Anton, L.; Bhatti, S.F.M.; et al. Serum Anti-GM2 and Anti-GalNAc-GD1a IgG Antibodies Are Biomarkers for Acute Canine Polyradiculoneuritis. J. Small Anim. Pract. 2022, 63, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Cuddon, P.A. Electrodiagnosis in Veterinary Neurology: Electromyography, Nerve Conduction Studies, and Evoked Responses; Veterinary Specialists of Northhern Colorado: Loveland, CO, USA, 2000. [Google Scholar]
- Preston, D.C. Electromyography and Neuromuscular Disorders: Clinical-Electrophysiological Correlations; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Walker, T.L.; Redding, R.W.; Braund, K.G. Motor Nerve Conduction Velocity and Latency in the Dog. Am. J. Vet. Res. 1979, 40, 1433–1439. [Google Scholar] [PubMed]
- Steiss, J.E. Linear Regression to Determine the Relationship between F-Wave Latency and Limb Length in Control Dogs. Am. J. Vet. Res. 1984, 45, 2649–2650. [Google Scholar] [PubMed]
- Poncelet, L.; Balligand, M. Nature of the Late Potentials and F-Ratio Values in Dogs. Res. Vet. Sci. 1991, 51, 1–5. [Google Scholar] [CrossRef]
- Redding, R.W.; Ingram, J.T.; Colter, S.B. Sensory Nerve Conduction Velocity of Cutaneous Afferents of the Radial, Ulnar, Peroneal, and Tibial Nerves of the Dog: Reference Values. Am. J. Vet. Res. 1982, 43, 517–521. [Google Scholar] [PubMed]
- Cuddon, P.A.; Delauche, A.J.; Hutchison, J.M. Assessment of Dorsal Nerve Root and Spinal Cord Dorsal Horn Function in Clinically Normal Dogs by Determination of Cord Dorsum Potentials. Am. J. Vet. Res. 1999, 60, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Gödde, T.; Jaggy, A.; Vandevelde, M.; Gaillard, C. Evaluation of Repetitive Nerve Stimulation in Young Dogs. J. Small Anim. Pract. 1993, 34, 393–397. [Google Scholar] [CrossRef]
- Mignan, T.; Targett, M.; Lowrie, M. Classification of Myasthenia Gravis and Congenital Myasthenic Syndromes in Dogs and Cats. J. Vet. Intern. Med. 2020, 34, 1707–1717. [Google Scholar] [CrossRef]
- Gross, S.; Fischer, A.; Rosati, M.; Matiasek, L.; Corlazzoli, D.; Cappello, R.; Porcarelli, L.; Harcourt-Brown, T.; Jurina, K.; Garosi, L.; et al. Nodo-Paranodopathy, Internodopathy and Cleftopathy: Target-Based Reclassification of Guillain–Barré-like Immune-Mediated Polyradiculoneuropathies in Dogs and Cats. Neuromuscul. Disord. 2016, 26, 825–836. [Google Scholar] [CrossRef]
- Holliday, T.A. Electrodiagnostic Examination: Somatosensory Evoked Potentials and Electromyography. Vet. Clin. N. Am. Small Anim. Pract. 1992, 22, 833–857. [Google Scholar] [CrossRef]
- Rasera, A.; Romito, S.; Segatti, A.; Concon, E.; Alessandrini, L.; Basaldella, F.; Badari, A.; Bonetti, B.; Squintani, G. Very Early and Early Neurophysiological Abnormalities in Guillain-Barré Syndrome: A 4-Year Retrospective Study. Eur. J. Neurol. 2021, 28, 3768–3773. [Google Scholar] [CrossRef]
- Jin, J.; Hu, F.; Qin, X.; Liu, X.; Li, M.; Dang, Y.; Dang, J. Very Early Neurophysiological Study in Guillain-Barre Syndrome. Eur. Neurol. 2018, 80, 100–105. [Google Scholar] [CrossRef]
- Cummings, J.F.; de Lahunta, A.; Holmes, D.F.; Schultz, R.D. Coonhound Paralysis. Further Clinical Studies and Electron Microscopic Observations. Acta Neuropathol. 1982, 56, 167–178. [Google Scholar] [CrossRef]
- Winer, J.B. Guillain Barré Syndrome. Mol. Pathol. 2001, 54, 381–385. [Google Scholar] [CrossRef]
- Lorenz, M.D.; Coates, J.R.; Kent, M. Front Matter. In Handbook of Veterinary Neurology, 5th ed.; W.B. Saunders: Saint Louis, MO, USA, 2011; p. iii. ISBN 978-1-4377-0651-2. [Google Scholar]
- Kimura, J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice; Oxford University Press: Oxford, UK, 2013; ISBN 978-0-19-935316-3. [Google Scholar]
- Inada, S.; Sugano, S.; Ibaraki, T. Electromyographic Study on Denervated Muscles in the Dog. Nihon Juigaku Zasshi 1963, 25, 327–336. [Google Scholar] [CrossRef]
- Hughes, R.A.C.; Cornblath, D.R. Guillain-Barré Syndrome. Lancet 2005, 366, 1653–1666. [Google Scholar] [CrossRef]

| Early Group (n = 24) | Late Group (n = 47) | p-Value * | |
|---|---|---|---|
| Breed (n, %) | 0.121 a | ||
| Mongrel | 5 (20.8%) | 19 (40.4%) | |
| German Shepherd | 2 (8.3%) | 2 (4.3%) | |
| Labrador Retriever | 1 (4.2%) | 4 (8.5%) | |
| Jack Russel Terrier | 1 (4.2%) | 3 (6.4%) | |
| Poodle | 1 (4.2%) | 2 (4.3%) | |
| Epagneul Breton | 1 (4.2%) | 1 (2.1%) | |
| Spitz | 1 (4.2%) | 1 (2.1%) | |
| West Highland White Terrier | 1 (4.2%) | 1 (2.1%) | |
| Bolognese | 2 (8.3%) | - | |
| Border Collie | 2 (8.3%) | - | |
| Rough Collie | 2 (8.3%) | - | |
| Beagle | 1 (4.2%) | - | |
| Cavalier King Charles Spaniel | 1 (4.2%) | - | |
| Italian Spinone | 1 (4.2%) | - | |
| Berger de Beauce | 1 (4.2%) | - | |
| Springer Spaniel | 1 (4.2%) | - | |
| Cocker Spaniel | - | 4 (8.5%) | |
| Fox Terrier | - | 2 (4.3%) | |
| Golden Retriever | - | 2 (4.3%) | |
| Australian Shepherd | - | 1 (2.1%) | |
| Belgian Shepherd | - | 1 (2.1%) | |
| Chihuahua | - | 1 (2.1%) | |
| Dachshund | - | 1 (2.1%) | |
| Shi-Tzu | - | 1 (2.1%) | |
| Siberian Husky | - | 1 (2.1%) | |
| Age (months) (Mean ± SD) | 91.3 ± 36.3 | 96.9 ± 43.9 | 0.591 b |
| Weight (kg) (Median (IQR)) | 13.25 (7–21.2) | 12 (7.3–23.2) | 0.971 c |
| Gender, n (%) | 0.588 a | ||
| Female | 10 (41.7%) | 16 (34.0%) | |
| Male | 10 (41.7%) | 20 (42.6%) | |
| Spayed Female | 2 (8.3%) | 9 (19.2%) | |
| Neutered Male | 2 (8.3%) | 2 (4.3%) |
| Early Group n = 24, (%) | Late Group n = 47, (%) | p-Value * | |
|---|---|---|---|
| Severity of Symptoms | 0.094 | ||
| Amb. Tetraparesis, hyporeflexia | 3 (12.5%) | 15 (31.9%) | |
| Amb. Tetraparesis, hyporeflexia with palpebral deficit | 0 (0%) | 1 (2.1%) | |
| Non-amb. Tetraparesis, hyporeflexia | 20 (83.3%) | 25 (53.2%) | |
| Non-amb. Tetraparesis, hyporeflexia with palpebral deficit | 1 (4.2%) | 1 (2.1%) | |
| Tetraplegia, hyporeflexia | 0 (0%) | 5 (10.6%) | |
| Ancillary tests | |||
| Ultrasound | 12 (50.0) | 17 (36.2) | 0.262 |
| Thorax X-ray | 14 (58.3) | 17 (36.2) | 0.075 |
| MRI | 2 (8.3) | 5 (10.6) | 0.758 |
| Total Body CT | 0 (0) | 2 (4.3) | 0.305 |
| Serology | 0 (0) | 7 (14.9) | 0.046 |
| Thyroid panel | 1 (4.2) | 4 (8.5) | 0.499 |
| Botulin test | 1 (4.2) | 1 (2.1) | 0.623 |
| CSF analysis | 0 (0) | 1 (2.1) | 0.472 |
| Echocardiogram | 1 (4.2) | 0 (0) | 0.159 |
| Electrophoresis | 0 (0) | 1 (2.1) | 0.472 |
| Muscle biopsy | 0 (0) | 1 (2.1) | 0.472 |
| Treatment | 0.413 | ||
| Physiotherapy | 11 (45.8%) | 29 (61.7%) | |
| Physiotherapy + Vit B + L-carnitine | 10 (41.7%) | 12 (25.5%) | |
| Physiotherapy + Immunoglobulins | 0 (0%) | 1 (2.1%) | |
| Immunoglobulins | 1 (4.2%) | 0 (0%) | |
| Intensive Care | 2 (8.3%) | 4 (8.5%) | |
| None | 0 (0%) | 1 (2.2%) | |
| Follow-up status | 0.980 | ||
| Return to Normal | 22 (91.7%) | 43 (91.50%) | |
| Euthanasia | 2 (8.3%) | 4 (8.5%) |
| Muscle | Early Group n (%) | Late Group n (%) | p-Value * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | N/A | 0 | 1 | 2 | 3 | 4 | N/A | ||
| Supraspinatus | 16 (66.7) | 1 (4.2) | 0 (0) | 3 (12.5) | 2 (8.3) | 2 (8.3) | 8 (17.0) | 8 (17.0) | 9 (19.2) | 11 (23.4) | 3 (6.4) | 8 (17.0) | 0.004 |
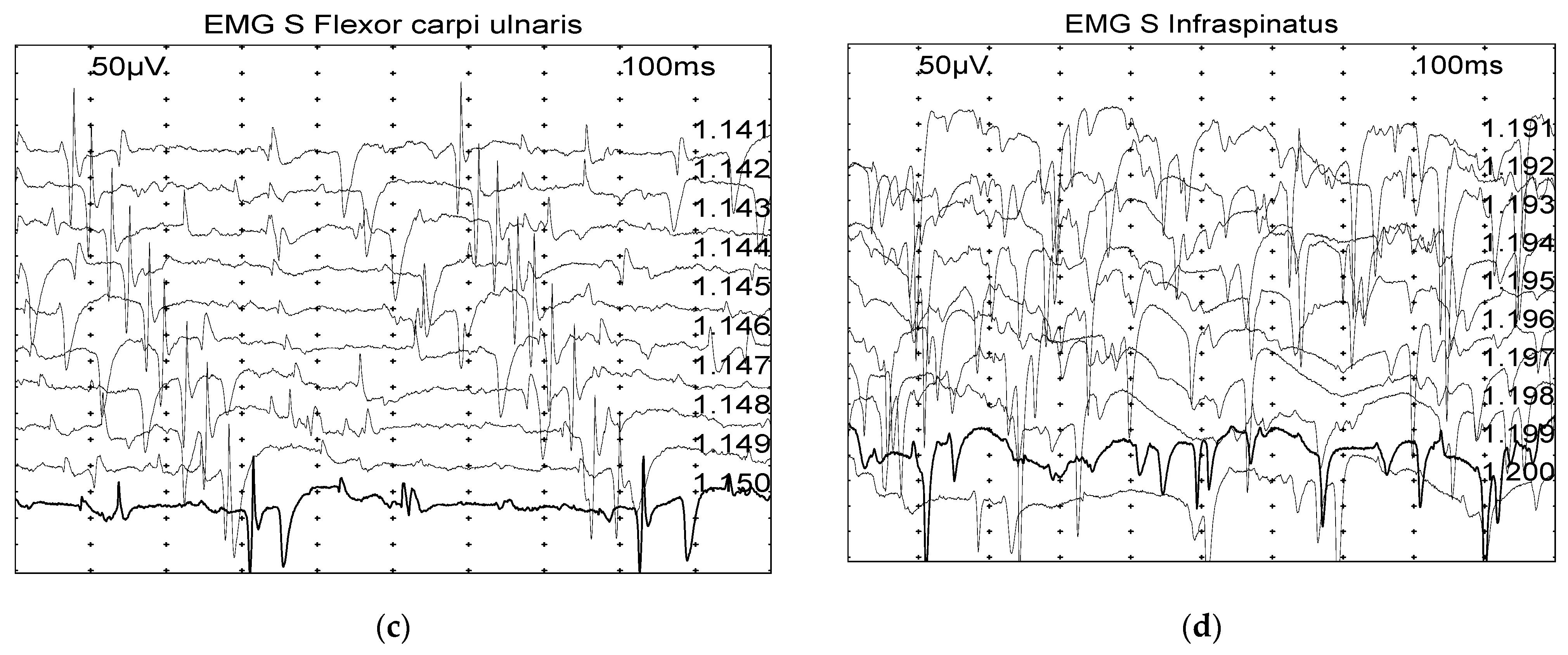
| Infraspinatus | 5 (20.8) | 3 (12.5) | 1 (4.2) | 1 (4.2) | 2 (8.33) | 12 (50.0) | 5 (10.6) | 2 (4.3) | 4 (8.5) | 3 (6.4) | 6 (12.8) | 27 (57.5) | 0.173 |
| Triceps Brachii | 13 (54.2) | 3 (12.5) | 1 (4.2) | 6 (25.0) | 1 (4.2) | 0 (0) | 5 (10.6) | 8 (17.0) | 12 (25.5) | 11 (23.4) | 9 (19.2) | 2 (4.3) | 0.002 |
| Biceps Brachii | 12 (50.0) | 4 (16.7) | 2 (8.3) | 3 (12.5) | 3 (12.5) | 0 (0) | 4 (8.5) | 3 (6.4) | 13 (27.7) | 12 (25.5) | 13 (27.7) | 2 (4.3) | <0.001 |
| Extensor Carpi Radialis | 11 (45.8) | 5 (20.8) | 3 (12.5) | 2 (8.3) | 3 (12.5) | 0 (0) | 5 (10.6) | 1 (2.1) | 6 (12.8) | 16 (34.0) | 18 (38.3) | 1 (2.1) | <0.001 |
| Thoracic Limb Flexors | 7 (29.2) | 2 (8.3) | 2 (8.3) | 2 (8.3) | 4 (16.7) | 7 (29.2) | 4 (8.5) | 3 (6.4) | 5 (10.6) | 9 (19.2) | 12 (25.5) | 14 (29.8) | 0.044 |
| Interosseous | 2 (8.3) | 6 (25.0) | 8 (33.3) | 4 (16.7) | 4 (16.7) | 0 (0) | 2 (4.26) | 3 (6.4) | 4 (8.5) | 15 (31.9) | 22 (46.8) | 1 (2.1) | 0.001 |
| Gluteal | 17 (70.8) | 1 (4.2) | 3 (12.5) | 0 (0) | 3 (12.5) | 0 (0) | 14 (29.8) | 9 (19.2) | 6 (12.8) | 7 (14.9) | 6 (12.8) | 5 (10.6) | 0.015 |
| Vastus Lateralis | 14 (58.3) | 1 (4.2) | 4 (16.7) | 1 (4.2) | 2 (8.3) | 2 (8.3) | 5 (10.6) | 7 (14.9) | 8 (17.0) | 13 (27.6) | 6 (12.8) | 8 (17.0) | 0.001 |
| Semitendinosus | 16 (66.7) | 1 (4.2) | 2 (8.3) | 3 (12.5) | 0 (0) | 2 (8.3) | 7 (14.9) | 7 (14.9) | 11 (23.4) | 6 (12.8) | 10 (21.3) | 6 (12.8) | <0.001 |
| Gastrocnemius | 6 (25.0) | 6 (25.0) | 2 (8.3) | 6 (25.0) | 4 (16.7) | 0 (0) | 1 (2.1) | 1 (2.1) | 12 (25.5) | 11 (23.4) | 21 (44.7) | 1 (2.1) | 0.001 |
| Cranial Tibial | 8 (33.3) | 8 (33.3) | 1 (4.2) | 6 (25.0) | 1 (4.2) | 0 (0) | 2 (4.3) | 2 (4.3) | 11 (23.4) | 15 (31.9) | 16 (34.0) | 1 (2.1) | <0.001 |
| Plantaris interosseous | 1 (4.2) | 7 (29.2) | 7 (29.2) | 7 (29.2) | 2 (8.3) | 0 (0) | 1 (2.1) | 4 (8.5) | 5 (10.6) | 16 (34.0) | 20 (42.6) | 1 (2.1) | <0.001 |
| Ulnar Nerve | Sciatic–Tibial Nerve | |||||
|---|---|---|---|---|---|---|
| Early Group n (%) | Late Group n (%) | p-Value | Early Group n (%) | Late Group n (%) | p-Value | |
| CMAP amplitude abnormalities | 0.316 | 0.089 | ||||
| Yes | 14 (58.3) | 25 (53.2) | 20 (83.3) | 30 (63.8) | ||
| No | 6 (25.0) | 19 (40.4) | 4 (16.7) | 17 (36.2) | ||
| N/A | 4 (16.7) | 3 (6.4) | - | |||
| Decrease in MNCV | 0.009 | 0.453 | ||||
| Yes | 7 (29.2) | 30 (63.8) | 10 (41.7) | 24 (51.1) | ||
| No | 13 (54.2) | 13 (27.7) | 14 (58.3) | 23 (48.9) | ||
| N/A | 4 (16.7) | 4 (8.5) | - | - | ||
| Conduction Block | 0.339 | 0.085 | ||||
| Yes | 5 (20.8) | 16 (34.0) | 2 (8.3) | 12 (25.5) | ||
| No | 15 (62.5) | 27 (57.5) | 22 (91.7) | 35 (74.5) | ||
| N/A | 4 (16.7) | 4 (8.5) | - | - | ||
| Temporal Dispersion | 0.39 | 0.055 | ||||
| Yes | 7 (29.2) | 20 (42.6) | 16 (66.7) | 20 (42.6) | ||
| No | 13 (54.2) | 23 (48.9) | 8 (33.3) | 27 (57.5) | ||
| N/A | 4 (16.7) | 4 (8.5) | - | - | ||
| Ulnar Nerve | Tibial Nerve | |||||
|---|---|---|---|---|---|---|
| Early Group n (%) | Late Group n (%) | p-Value | Early Group n (%) | Late Group n (%) | p-Value | |
| F-wave (at least one abnormality) | 0.945 | 0.275 | ||||
| Yes | 12 (50.0) | 26 (55.3) | 21 (87.5) | 36 (76.6) | ||
| No | 8 (33.3) | 18 (38.3) | 3 (12.5) | 11 (23.4) | ||
| Not performed | 4 (16.7) | 3 (6.4) | - | - | ||
| F-wave latency abnormalities | 0.697 | 0.603 | ||||
| Yes | 6 (25.0) | 16 (34.0) | 9 (37.5) | 17 (36.2) | ||
| No | 7 (29.2) | 11 (23.4) | 5 (20.8) | 13 (27.7) | ||
| No F-wave detected | 6 (25.0) | 10 (21.3) | 9 (37.5) | 12 (25.5) | ||
| Not performed | 5 (20.8) | 10 (21.3) | 1 (4.2) | 5 (10.6) | ||
| Increased F Ratio | 0.490 | |||||
| Yes | 8 (33.3) | 16 (34.0) | ||||
| No | 7 (29.2) | 16 (34.0) | ||||
| No F-wave detected | 9 (37.5) | 12 (25.5) | ||||
| Not performed | - | 3 (6.4) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcarelli, L.; Cauduro, A.; Bianchi, E.; Pauciulo, C.; Maurelli, C.; Corlazzoli, D. Early Neurophysiological Abnormalities in Suspected Acute Canine Polyradiculoneuropathy. Vet. Sci. 2024, 11, 178. https://doi.org/10.3390/vetsci11040178
Porcarelli L, Cauduro A, Bianchi E, Pauciulo C, Maurelli C, Corlazzoli D. Early Neurophysiological Abnormalities in Suspected Acute Canine Polyradiculoneuropathy. Veterinary Sciences. 2024; 11(4):178. https://doi.org/10.3390/vetsci11040178
Chicago/Turabian StylePorcarelli, Laura, Alberto Cauduro, Ezio Bianchi, Claudia Pauciulo, Chiara Maurelli, and Daniele Corlazzoli. 2024. "Early Neurophysiological Abnormalities in Suspected Acute Canine Polyradiculoneuropathy" Veterinary Sciences 11, no. 4: 178. https://doi.org/10.3390/vetsci11040178
APA StylePorcarelli, L., Cauduro, A., Bianchi, E., Pauciulo, C., Maurelli, C., & Corlazzoli, D. (2024). Early Neurophysiological Abnormalities in Suspected Acute Canine Polyradiculoneuropathy. Veterinary Sciences, 11(4), 178. https://doi.org/10.3390/vetsci11040178







