Ex Vivo Immune Function and Modulatory Effects of Calcitriol in Dogs with Naturally Occurring Diabetes Mellitus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Criteria for Selection of Dogs and Study Design
2.2. Data and Sample Collection
2.3. Calcitriol
2.4. Blood Sample Collection and Calcitriol Treatment
2.5. Leukocyte Cytokine Production
2.6. Phagocytosis of E. coli
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
3.1. Animal Population
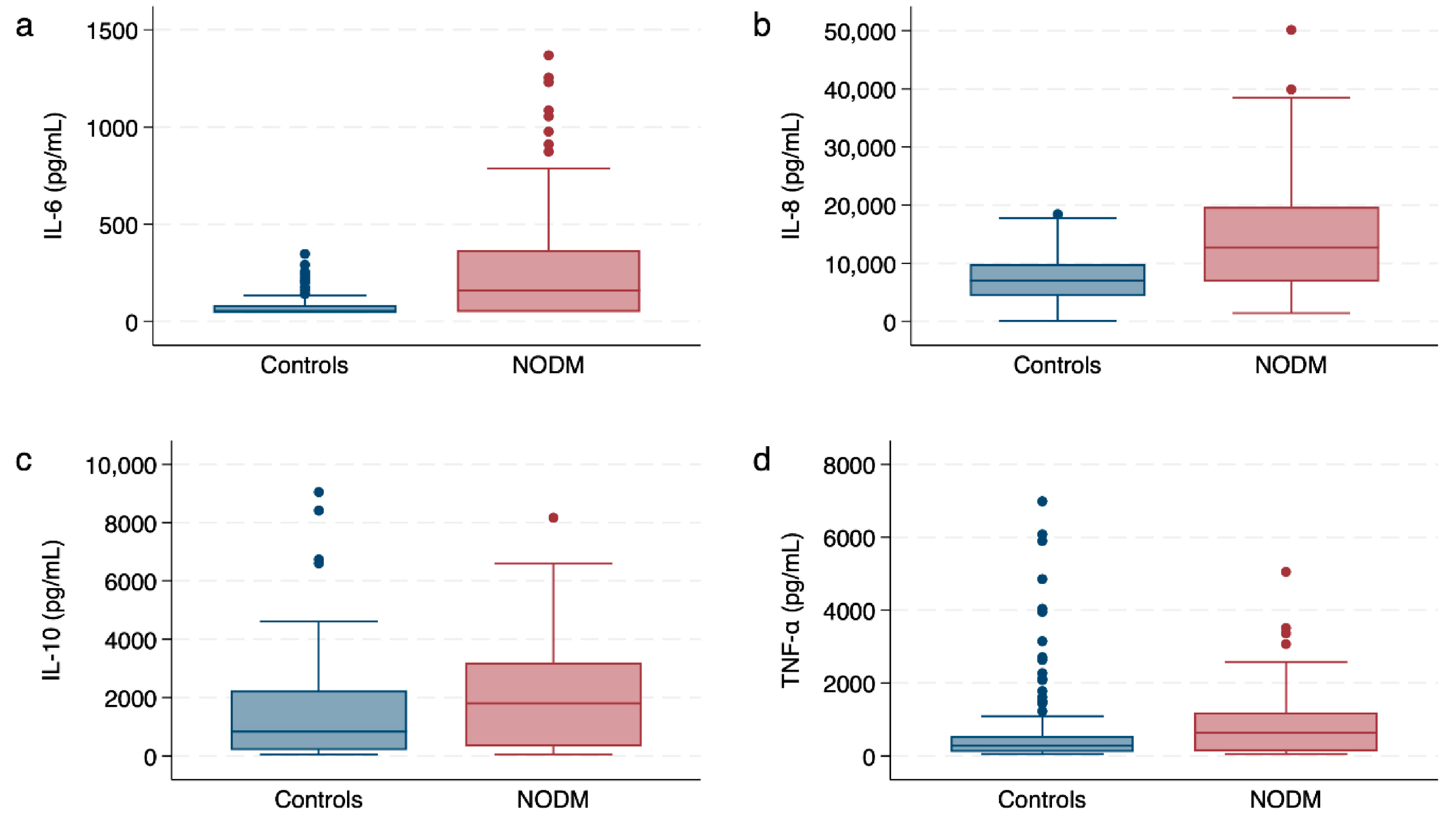
3.2. Leukocyte Cytokine Responses
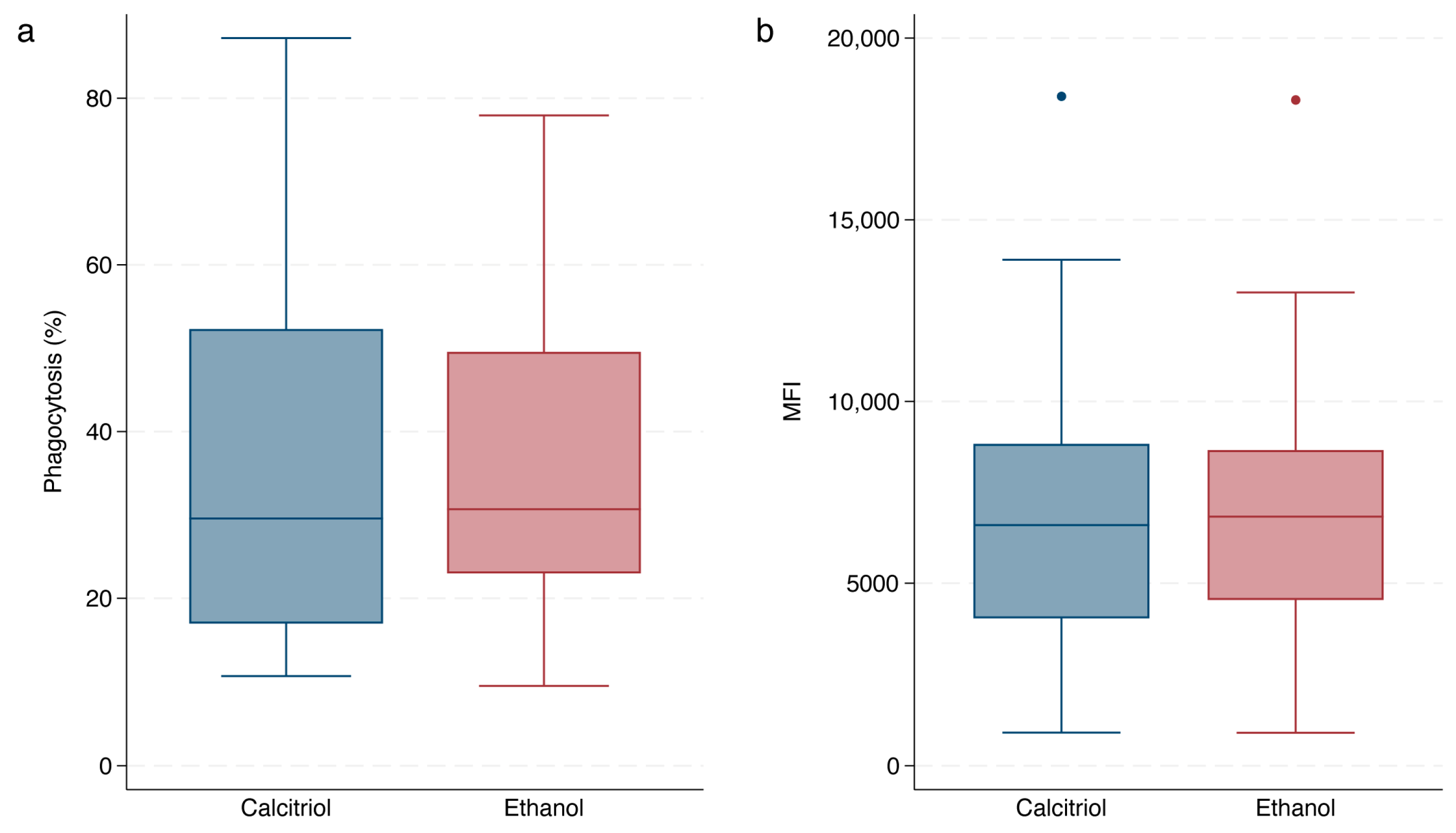
3.3. Effect of Calcitriol
3.4. Phagocytic Capacity of Opsonized-E. coli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hess, R.S.; Kass, P.H.; Ward, C.R. Breed distribution of dogs with diabetes mellitus admitted to a tertiary care facility. J. Am. Vet. Med. Assoc. 2000, 216, 1414–1417. [Google Scholar] [CrossRef] [PubMed]
- Guptill, L.; Glickman, L.; Glickman, N. Time trends and risk factors for diabetes mellitus in dogs: Analysis of veterinary medical data base records (1970–1999). Vet. J. 2003, 165, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Fracassi, F.; Pietra, M.; Boari, A.; Aste, G.; Giunti, M.; Famigli-Bergamini, P. Breed distribution of canine diabetes mellitus in Italy. Vet. Res. Commun. 2004, 28 (Suppl. 1), 339–342. [Google Scholar] [CrossRef] [PubMed]
- Davison, L.J.; Herrtage, M.E.; Catchpole, B. Study of 253 dogs in the United Kingdom with diabetes mellitus. Vet. Rec. 2005, 156, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Fall, T.; Hamlin, H.H.; Hedhammar, A.; Kampe, O.; Egenvall, A. Diabetes mellitus in a population of 180,000 insured dogs: Incidence, survival, and breed distribution. J. Vet. Intern. Med. 2007, 21, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, B.; Ristic, J.M.; Fleeman, L.M.; Davison, L.J. Canine diabetes mellitus: Can old dogs teach us new tricks? Diabetologia 2005, 48, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Fleeman, L.M.; Wilson, B.J.; Mansfield, C.S.; McGreevy, P. Epidemiological study of dogs with diabetes mellitus attending primary care veterinary clinics in Australia. Vet. Rec. 2020, 187, e22. [Google Scholar] [CrossRef] [PubMed]
- Brito-Casillas, Y.; Melian, C.; Holder, A.; Wiebe, J.C.; Navarro, A.; Quesada-Canales, O.; Exposito-Montesdeoca, A.B.; Catchpole, B.; Wagner, A.M. Studying the heterogeneous pathogenesis of canine diabetes: Observational characterization of an island population. Vet. Med. Sci. 2021, 7, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Mattin, M.; O'Neill, D.; Church, D.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D. An epidemiological study of diabetes mellitus in dogs attending first opinion practice in the UK. Vet. Rec. 2014, 174, 349. [Google Scholar] [CrossRef]
- Moshref, M.; Tangey, B.; Gilor, C.; Papas, K.K.; Williamson, P.; Loomba-Albrecht, L.; Sheehy, P.; Kol, A. Concise Review: Canine Diabetes Mellitus as a Translational Model for Innovative Regenerative Medicine Approaches. Stem Cells Transl. Med. 2019, 8, 450–455. [Google Scholar] [CrossRef]
- Gilor, C.; Niessen, S.J.; Furrow, E.; DiBartola, S.P. What’s in a Name? Classification of Diabetes Mellitus in Veterinary Medicine and Why It Matters. J. Vet. Intern. Med. 2016, 30, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Behrend, E.; Holford, A.; Lathan, P.; Rucinsky, R.; Schulman, R. 2018 AAHA diabetes management guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 2018, 54, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, E.; Monteagudo, P.T.; Marrocos, M.S.; Campa, A. Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin. Exp. Immunol. 2006, 146, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Kulseng, B.; Skjak-Braek, G.; Folling, I.; Espevik, T. TNF production from peripheral blood mononuclear cells in diabetic patients after stimulation with alginate and lipopolysaccharide. Scand. J. Immunol. 1996, 43, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Foss-Freitas, M.C.; Foss, N.T.; Donadi, E.A.; Foss, M.C. In vitro TNF-alpha and IL-6 production by adherent peripheral blood mononuclear cells obtained from type 1 and type 2 diabetic patients evaluated according to the metabolic control. Ann. N. Y. Acad. Sci. 2006, 1079, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Larsson, A.; Vessby, J.; Vessby, B.; Berne, C. Type 1 diabetes is associated with increased cyclooxygenase- and cytokine-mediated inflammation. Diabetes Care 2005, 28, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Fawaz, L.; Elwan, A.E.; Kamel, Y.H.; Farid, T.M.; Kamel, A.; Mohamed, W.A. Value of C-reactive protein and IL-6 measurements in type 1 diabetes mellitus. Arch. Med. Sci. 2009, 5, 383–390. [Google Scholar]
- Devaraj, S.; Dasu, M.R.; Rockwood, J.; Winter, W.; Griffen, S.C.; Jialal, I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: Further evidence of a proinflammatory state. J. Clin. Endocrinol. Metab. 2008, 93, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Delamaire, M.; Maugendre, D.; Moreno, M.; Le Goff, M.C.; Allannic, H.; Genetet, B. Impaired leucocyte functions in diabetic patients. Diabet. Med. 1997, 14, 29–34. [Google Scholar] [CrossRef]
- Kelly, M.; Brown, J.; Thong, Y. Neutrophil and monocyte adherence in diabetes mellitus, alcoholic cirrhosis, uraemia and elderly patients. Int. Arch. Allergy Immunol. 1985, 78, 132–138. [Google Scholar]
- Marhoffer, W.; Stein, M.; Schleinkofer, L.; Federlin, K. Evidence of ex vivo and in vitro impaired neutrophil oxidative burst and phagocytic capacity in type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 1993, 19, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Chanchamroen, S.; Kewcharoenwong, C.; Susaengrat, W.; Ato, M.; Lertmemongkolchai, G. Human polymorphonuclear neutrophil responses to Burkholderia pseudomallei in healthy and diabetic subjects. Infect. Immun. 2009, 77, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Klein, B.; Elian, I.; Fishman, P.; Djaldetti, M. Phagocytotic activity of monocytes from diabetic patients. Diabetes Care 1983, 6, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Tesch, G.H. Role of macrophages in complications of type 2 diabetes. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Foss-Freitas, M.C.; Foss, N.T.; Rassi, D.M.; Donadi, E.A.; Foss, M.C. Evaluation of Cytokine Production from Peripheral Blood Mononuclear Cells of Type 1 Diabetic Patients: Importance of the Methodologic Approach. Ann. N. Y. Acad. Sci. 2008, 1150, 290–296. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Mainous III, A.G.; Buchanan, T.A.; Pearson, W.S. C-reactive protein and glycemic control in adults with diabetes. Diabetes Care 2003, 26, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Wieser, V.; Moschen, A.R.; Tilg, H. Inflammation, cytokines and insulin resistance: A clinical perspective. Arch. Immunol. Ther. Exp. 2013, 61, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Spiegelman, B.M. Tumor necrosis factor alpha: A key component of the obesity-diabetes link. Diabetes 1994, 43, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- DeClue, A.E.; Nickell, J.; Chang, C.H.; Honaker, A. Upregulation of proinflammatory cytokine production in response to bacterial pathogen-associated molecular patterns in dogs with diabetes mellitus undergoing insulin therapy. J. Diabetes Sci. Technol. 2012, 6, 496–502. [Google Scholar] [CrossRef]
- O'Neill, S.; Drobatz, K.; Satyaraj, E.; Hess, R. Evaluation of cytokines and hormones in dogs before and after treatment of diabetic ketoacidosis and in uncomplicated diabetes mellitus. Vet. Immunol. Immunopathol. 2012, 148, 276–283. [Google Scholar] [CrossRef]
- Latimer, K.S.; Mahaffey, E.A. Neutrophil adherence and movement in poorly and well-controlled diabetic dogs. Am. J. Vet. Res. 1984, 45, 1498–1500. [Google Scholar]
- Rockett, K.A.; Brookes, R.; Udalova, I.; Vidal, V.; Hill, A.V.; Kwiatkowski, D. 1, 25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect. Immun. 1998, 66, 5314–5321. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Wilkinson, K.A.; Newton, S.M.; Floto, R.A.; Norman, A.W.; Skolimowska, K.; Davidson, R.N.; Sørensen, O.E.; Kampmann, B.; Griffiths, C.J. IFN-γ-and TNF-independent vitamin D-inducible human suppression of mycobacteria: The role of cathelicidin LL-37. J. Immunol. 2007, 178, 7190–7198. [Google Scholar] [CrossRef]
- Motlagh, B.M.; Ahangaran, N.A.; Froushani, S.M.A. Calcitriol modulates the effects of bone marrow-derived mesenchymal stem cells on macrophage functions. Iran. J. Basic Med. Sci. 2015, 18, 672. [Google Scholar] [PubMed]
- Rodriguez-Lecompte, J.; Yitbarek, A.; Cuperus, T.; Echeverry, H.; Van Dijk, A. The immunomodulatory effect of vitamin D in chickens is dose-dependent and influenced by calcium and phosphorus levels. Poult. Sci. 2016, 95, 2547–2556. [Google Scholar] [CrossRef]
- Vieira-Neto, A.; Lima, I.R.P.; Lopes, F., Jr.; Lopera, C.; Zimpel, R.; Sinedino, L.D.P.; Jeong, K.C.; Galvao, K.; Thatcher, W.W.; Nelson, C.D.; et al. Use of calcitriol to maintain postpartum blood calcium and improve immune function in dairy cows. J. Dairy. Sci. 2017, 100, 5805–5823. [Google Scholar] [CrossRef]
- García-Barragán, Á.; Gutiérrez-Pabello, J.A.; Alfonseca-Silva, E. Calcitriol increases nitric oxide production and modulates microbicidal capacity against Mycobacterium bovis in bovine macrophages. Comp. Immunol. Microbiol. Infect. Dis. 2018, 59, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Jaffey, J.A.; Amorim, J.; DeClue, A.E. Effect of calcitriol on in vitro whole blood cytokine production in critically ill dogs. Vet. J. 2018, 236, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Jaffey, J.A.; Amorim, J.; DeClue, A.E. Effects of calcitriol on apoptosis, toll-like receptor 4 expression, and cytokine production of endotoxin-primed canine leukocytes. Am. J. Vet. Res. 2018, 79, 1071–1078. [Google Scholar] [CrossRef]
- Jaffey, J.A.; Amorim, J.; DeClue, A.E. Effects of calcitriol on phagocytic function, toll-like receptor 4 expression, and cytokine production of canine leukocytes. Am. J. Vet. Res. 2018, 79, 1064–1070. [Google Scholar] [CrossRef]
- Greenhagen, R.M.; Frykberg, R.G.; Wukich, D.K. Serum vitamin D and diabetic foot complications. Diabet. Foot Ankle 2019, 10, 1579631. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.; Hassan, M.; Musa, N.; Abdel Atty, S.; Azim, S.A. Vitamin D status in Egyptian children with type 1 diabetes and the role of vitamin D replacement in glycemic control. J. Pediatr. Endocrinol. Metab. 2017, 30, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Savastio, S.; Cadario, F.; Genoni, G.; Bellomo, G.; Bagnati, M.; Secco, G.; Picchi, R.; Giglione, E.; Bona, G. Vitamin D Deficiency and Glycemic Status in Children and Adolescents with Type 1 Diabetes Mellitus. PLoS ONE 2016, 11, e0162554. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Yun, J.M.; Duncan-Staley, C.R.; Jialal, I. Low vitamin D levels correlate with the proinflammatory state in type 1 diabetic subjects with and without microvascular complications. Am. J. Clin. Pathol. 2011, 135, 429–433. [Google Scholar] [CrossRef]
- Bener, A.; Alsaied, A.; Al-Ali, M.; Al-Kubaisi, A.; Basha, B.; Abraham, A.; Guiter, G.; Mian, M. High prevalence of vitamin D deficiency in type 1 diabetes mellitus and healthy children. Acta Diabetol. 2009, 46, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Giri, D.; Pintus, D.; Burnside, G.; Ghatak, A.; Mehta, F.; Paul, P.; Senniappan, S. Treating vitamin D deficiency in children with type I diabetes could improve their glycaemic control. BMC Res. Notes 2017, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, E.H.; Dehkordi, V.H.; Fatemi, S.M.R.; Zolfaghari, M. Effect of vitamin D supplement therapy on HbA1C and IGF-1 levels in children with type 1 diabetes mellitus and vitamin D deficiency. Electron. J. Gen. Med. 2018, 15, em69. [Google Scholar] [CrossRef]
- Deda, L.; Yeshayahu, Y.; Sud, S.; Cuerden, M.; Cherney, D.Z.; Sochett, E.B.; Mahmud, F.H. Improvements in peripheral vascular function with vitamin D treatment in deficient adolescents with type 1 diabetes. Pediatr. Diabetes 2018, 19, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Cerin, E.; Lamb, K.E.; White, S.R. Modelling count, bounded and skewed continuous outcomes in physical activity research: Beyond linear regression models. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 57. [Google Scholar] [CrossRef]
- Vlassara, H.; Palace, M. Diabetes and advanced glycation endproducts. J. Intern. Med. 2002, 251, 87–101. [Google Scholar] [CrossRef]
- Wada, R.; Yagihashi, S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann. N. Y. Acad. Sci. 2005, 1043, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Comazzi, S.; Bertazzolo, W.; Bonfanti, U.; Spagnolo, V.; Sartorelli, P. Advanced glycation end products and sorbitol in blood from differently compensated diabetic dogs. Res. Vet. Sci. 2008, 84, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Reusch, C.; Liehs, M.; Hoyer, M. Fructosamine. A new parameter for diagnosis and metabolic control in diabetic dogs and cats. J. Vet. Intern. Med. 1993, 7, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Norris, O.; Schermerhorn, T. Relationship between HbA1c, fructosamine and clinical assessment of glycemic control in dogs. PLoS ONE 2022, 17, e0264275. [Google Scholar] [CrossRef] [PubMed]
- Del Baldo, F.; Magna, L.; Dondi, F.; Maramieri, P.; Catrina, O.M.; Corradini, S.; Linari, G.; Golinelli, S.; Tardo, A.M.; Bonfanti, U. Comparison of serum fructosamine and glycated hemoglobin values for assessment of glycemic control in dogs with diabetes mellitus. Am. J. Vet. Res. 2020, 81, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Zeugswetter, F.K.; Beer, R.; Schwendenwein, I. Evaluation of fructosamine concentration as an index marker for glycaemic control in diabetic dogs. Vet. Rec. 2022, 190, e244. [Google Scholar] [CrossRef]
- Cohen-Lahav, M.; Shany, S.; Tobvin, D.; Chaimovitz, C.; Douvdevani, A. Vitamin D decreases NFκB activity by increasing IκBα levels. Nephrol. Dial. Transplant. 2006, 21, 889–897. [Google Scholar] [CrossRef]
- Stio, M.; Martinesi, M.; Bruni, S.; Treves, C.; Mathieu, C.; Verstuyf, A.; d’Albasio, G.; Bagnoli, S.; Bonanomi, A.G. The Vitamin D analogue TX 527 blocks NF-κB activation in peripheral blood mononuclear cells of patients with Crohn's disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 51–60. [Google Scholar] [CrossRef]
- Peng, L.; Malloy, P.J.; Feldman, D. Identification of a functional vitamin D response element in the human insulin-like growth factor binding protein-3 promoter. Mol. Endocrinol. 2004, 18, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C.; Ferruzzi, P.; Caporali, A.; Scaltriti, M.; Bettuzzi, S.; Mancina, R.; Gelmini, S.; Serio, M.; Villari, D.; Vannelli, G. Inhibition of prostate cell growth by BXL-628, a calcitriol analogue selected for a phase II clinical trial in patients with benign prostate hyperplasia. Eur. J. Endocrinol. 2004, 150, 591–603. [Google Scholar] [CrossRef]
- Talmor, Y.; Bernheim, J.; Klein, O.; Green, J.; Rashid, G. Calcitriol blunts pro-atherosclerotic parameters through NFκB and p38 in vitro. Eur. J. Clin. Investig. 2008, 38, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Rostkowska-Nadolska, B.; Sliupkas-Dyrda, E.; Potyka, J.; Kusmierz, D.; Fraczek, M.; Krecicki, T.; Kubik, P.; Zatonski, M.; Latocha, M. Vitamin D derivatives: Calcitriol and tacalcitol inhibits interleukin-6 and interleukin-8 expression in human nasal polyp fibroblast cultures. Adv. Med. Sci. 2010, 55, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Talmor, Y.; Golan, E.; Benchetrit, S.; Bernheim, J.; Klein, O.; Green, J.; Rashid, G. Calcitriol blunts the deleterious impact of advanced glycation end products on endothelial cells. Am. J. Physiol. Ren. Physiol. 2008, 294, F1059–F1064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Allison, L.N.; Jaffey, J.A.; Bradley-Siemens, N.; Tao, Z.; Thompson, M.; Backus, R.C. Immune function and serum vitamin D in shelter dogs: A case-control study. Vet. J. 2020, 261, 105477. [Google Scholar] [CrossRef] [PubMed]
- Jaffey, J.A.; Bessette, M.; Tao, Z.; Bradley-Siemens, N.; Thompson, M. Effects of calcitriol on oxidative burst, phagocytic function, and leukocyte cytokine production in shelter dogs. Canine Med. Genet. 2020, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Borazan, A.; Ustun, H.; Cefle, A.; Sekitmez, N.; Yilmaz, A. Comparative efficacy of oral and intravenous calcitriol treatment in haemodialysis patients: Effects on serum biochemistry and cytokine levels. J. Int. Med. Res. 2003, 31, 489–496. [Google Scholar] [CrossRef]
- Noyola-Martinez, N.; Diaz, L.; Avila, E.; Halhali, A.; Larrea, F.; Barrera, D. Calcitriol downregulates TNF-alpha and IL-6 expression in cultured placental cells from preeclamptic women. Cytokine 2013, 61, 245–250. [Google Scholar] [CrossRef]
- Mao, L.; Ji, F.; Liu, Y.; Zhang, W.; Ma, X. Calcitriol plays a protective role in diabetic nephropathy through anti-inflammatory effects. Int. J. Clin. Exp. Med. 2014, 7, 5437–5444. [Google Scholar] [PubMed]
- O'Brien, B.A.; Huang, Y.; Geng, X.; Dutz, J.P.; Finegood, D.T. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes 2002, 51, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Sabioncello, A.; Rabatic, S.; Kadrnka-Lovrencic, M.; Oberiter, V.; Dekaris, D. Decreased phagocytosis and antibody-dependent cellular cytotoxicity (ADCC) in type-1 diabetes. Biomedicine 1981, 35, 227–229. [Google Scholar] [PubMed]
- Rodriguez-Fernandez, S.; Murillo, M.; Villalba, A.; Perna-Barrull, D.; Cano-Sarabia, M.; Gomez-Munoz, L.; Aguilera, E.; Maspoch, D.; Vazquez, F.; Bel, J.; et al. Impaired Phagocytosis in Dendritic Cells From Pediatric Patients With Type 1 Diabetes Does Not Hamper Their Tolerogenic Potential. Front. Immunol. 2019, 10, 2811. [Google Scholar] [CrossRef] [PubMed]
- Ellger, B.; Debaveye, Y.; Vanhorebeek, I.; Langouche, L.; Giulietti, A.; Van Etten, E.; Herijgers, P.; Mathieu, C.; Van den Berghe, G. Survival benefits of intensive insulin therapy in critical illness: Impact of maintaining normoglycemia versus glycemia-independent actions of insulin. Diabetes 2006, 55, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Weekers, F.; Giulietti, A.-P.; Michalaki, M.; Coopmans, W.; Van Herck, E.; Mathieu, C.; Van den Berghe, G. Metabolic, endocrine, and immune effects of stress hyperglycemia in a rabbit model of prolonged critical illness. Endocrinology 2003, 144, 5329–5338. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, N.; Levy, R.B. 1, 25-dihydroxyvitamin D3 stimulates phagocytosis but suppresses HLA-DR and CD13 antigen expression in human mononuclear phagocytes. Proc. Soc. Exp. Biol. Med. 1996, 211, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Nouari, W.; Ysmail-Dahlouk, L.; Aribi, M. Vitamin D3 enhances bactericidal activity of macrophage against Pseudomonas aeruginosa. Int. Immunopharmacol. 2016, 30, 94–101. [Google Scholar] [CrossRef]
- Xu, H.; Soruri, A.; Gieseler, R.; Peters, J. 1, 25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand. J. Immunol. 1993, 38, 535–540. [Google Scholar] [CrossRef]
- Spittler, A.; Willheim, M.; Leutmezer, F.; Oehler, R.; Krugluger, W.; Reissner, C.; Lucas, T.; Brodowicz, T.; Roth, E.; BOLTZ-NITULESCU, G. Effects of 1α, 25-dihydroxyvitamin D3 and cytokines on the expression of MHC antigens, complement receptors and other antigens on human blood monocytes and U937 cells: Role in cell differentiation, activation and phagocytosis. Immunology 1997, 90, 286–293. [Google Scholar] [CrossRef]
| Variable | NODM | Control | p-Value |
|---|---|---|---|
| Number of dogs | 20 | 20 | --- |
| Age (years) a | 9.3 (1.9) | 9.5 (1.9) | 0.78 c |
| Weight (kgs) b | 7.6 (6.4) | 8.4 (18.1) | 0.59 d |
| BCS b | 5 (1) | 5 (1) | 0.64 d |
| Sex (female, male) | 9, 11 | 9, 11 | 1.0 e |
| Neutered (yes, no) | 19, 1 | 19, 1 | 1.0 e |
| Breeds | Chihuahua (n = 8), Labrador Retriever mix (n = 4), Bichon Frise (n = 2), Miniature Pinscher (n = 2), Pomeranian (n = 2), Rottweiler (n = 2), Poodle mix (n = 2), Havanese (n = 2), Australian Shepherd mix (n = 2), Labrador retriever (n = 2), Jack Russel Terrier mix (n = 2), Miniature Pinscher mix (n = 2), Maltese–Poodle mix (n = 2), Rat Terrier (n = 2), Australian Cattle Dog (n = 2), Yorkshire Terrier (n = 2) | --- | |
| Variable | β | % Change | Robust Std. Error | z | p | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | NODM | 1.2 | 216% | 0.17 | 6.8 | <0.001 | 0.8 | 127% | 1.5 | 340% |
| Calcitriol | 0.3 | 42% | 0.13 | 2.8 | 0.005 | 0.1 | 11% | 0.6 | 81% | |
| LPS | 1.5 | 365% | 0.13 | 11.7 | <0.001 | 1.3 | 259% | 1.8 | 502% | |
| LTA | 1.3 | 274% | 0.14 | 9.2 | <0.001 | 1.0 | 182% | 1.6 | 395% | |
| IL-8 | NODM | 0.7 | 95% | 0.17 | 3.9 | <0.001 | 0.3 | 40% | 1.0 | 172% |
| Calcitriol | −0.1 | −7% | 0.04 | −2.0 | 0.04 | −0.1 | −14% | 0.0 | 0% | |
| LPS | 0.5 | 61% | 0.09 | 5.2 | <0.001 | 0.3 | 35% | 0.7 | 92% | |
| LTA | 0.5 | 58% | 0.09 | 4.9 | <0.001 | 0.3 | 32% | 0.6 | 90% | |
| IL-10 | NODM | 0.5 | 60% | 0.19 | 2.5 | 0.01 | 0.1 | 10% | 0.8 | 133% |
| Calcitriol | 0.1 | 13% | 0.08 | 1.6 | 0.11 | 0.0 | −3% | 0.3 | 32% | |
| LPS | 2.5 | 1078% | 0.15 | 17.0 | <0.001 | 2.2 | 786% | 2.8 | 1466% | |
| LTA | 2.2 | 780% | 0.15 | 14.1 | <0.001 | 1.9 | 550% | 2.5 | 1091% | |
| TNF-α | NODM | 0.4 | 50% | 0.21 | 1.9 | 0.06 | 0.0 | −1% | 0.8 | 127% |
| Calcitriol | −0.4 | −33% | 0.09 | −4.5 | <0.001 | −0.6 | −44% | −0.2 | −20% | |
| LPS | 2.2 | 760% | 0.12 | 18.5 | <0.001 | 1.9 | 585% | 2.4 | 980% | |
| LTA | 1.8 | 533% | 0.12 | 15.7 | <0.001 | 1.6 | 403% | 2.1 | 697% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaffey, J.A.; Kreisler, R.; Graves, T.K.; Al-Nakkash, L.; Backus, R.C.; Allison, L. Ex Vivo Immune Function and Modulatory Effects of Calcitriol in Dogs with Naturally Occurring Diabetes Mellitus. Vet. Sci. 2024, 11, 193. https://doi.org/10.3390/vetsci11050193
Jaffey JA, Kreisler R, Graves TK, Al-Nakkash L, Backus RC, Allison L. Ex Vivo Immune Function and Modulatory Effects of Calcitriol in Dogs with Naturally Occurring Diabetes Mellitus. Veterinary Sciences. 2024; 11(5):193. https://doi.org/10.3390/vetsci11050193
Chicago/Turabian StyleJaffey, Jared A., Rachael Kreisler, Thomas K. Graves, Layla Al-Nakkash, Robert C. Backus, and Lauren Allison. 2024. "Ex Vivo Immune Function and Modulatory Effects of Calcitriol in Dogs with Naturally Occurring Diabetes Mellitus" Veterinary Sciences 11, no. 5: 193. https://doi.org/10.3390/vetsci11050193
APA StyleJaffey, J. A., Kreisler, R., Graves, T. K., Al-Nakkash, L., Backus, R. C., & Allison, L. (2024). Ex Vivo Immune Function and Modulatory Effects of Calcitriol in Dogs with Naturally Occurring Diabetes Mellitus. Veterinary Sciences, 11(5), 193. https://doi.org/10.3390/vetsci11050193







