Japanese Encephalitis Virus and Schizophyllum commune Co-Infection in a Harbor Seal in Japan
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pathological Examination
2.2. Pathogen Detection
2.3. Cells and Viruses
2.4. Virus Isolation
2.5. Plaque Formation
2.6. Mouse Study
2.7. Sequencing
2.8. Phylogenetic Analysis
3. Results
3.1. The Case
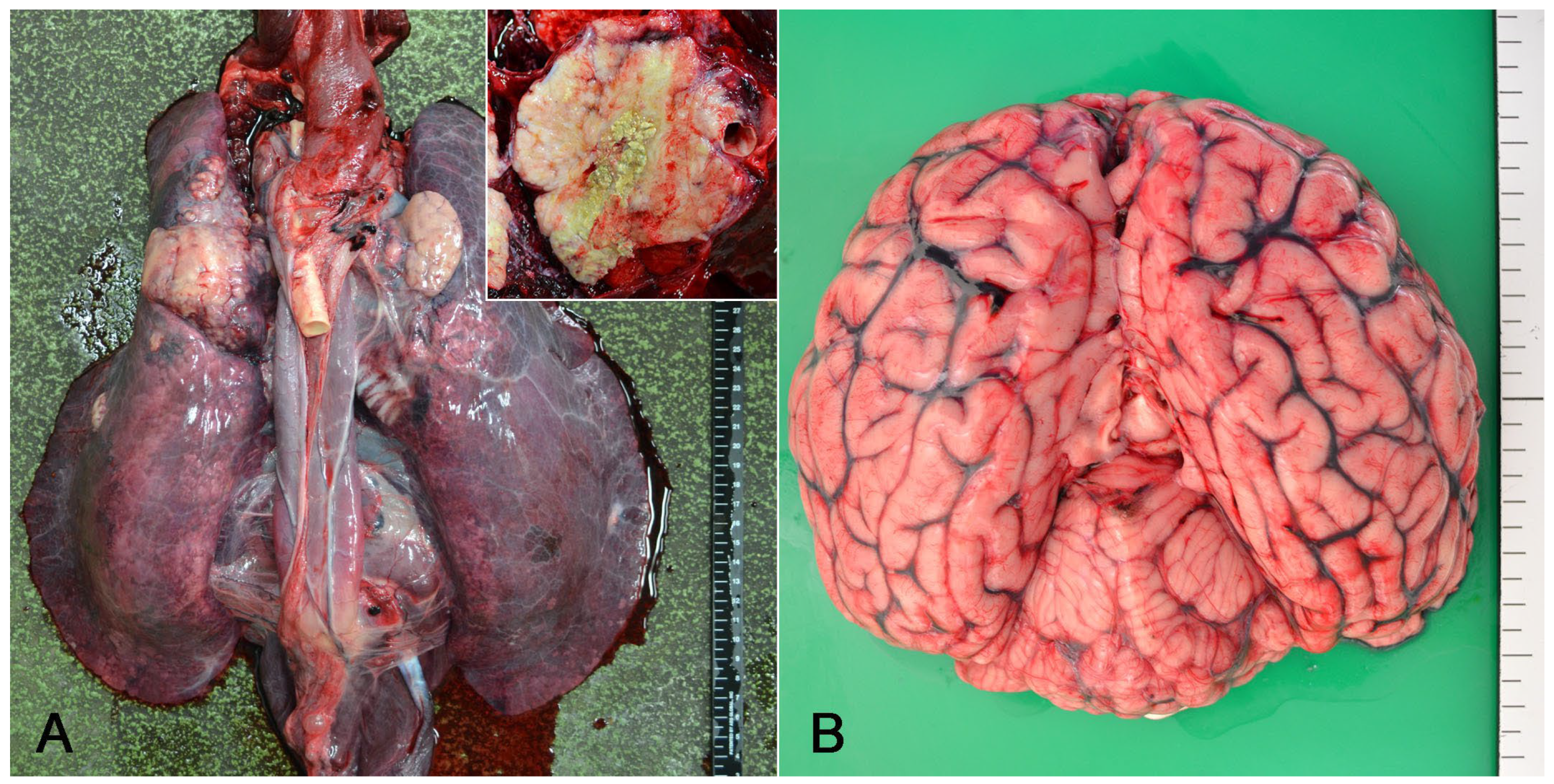
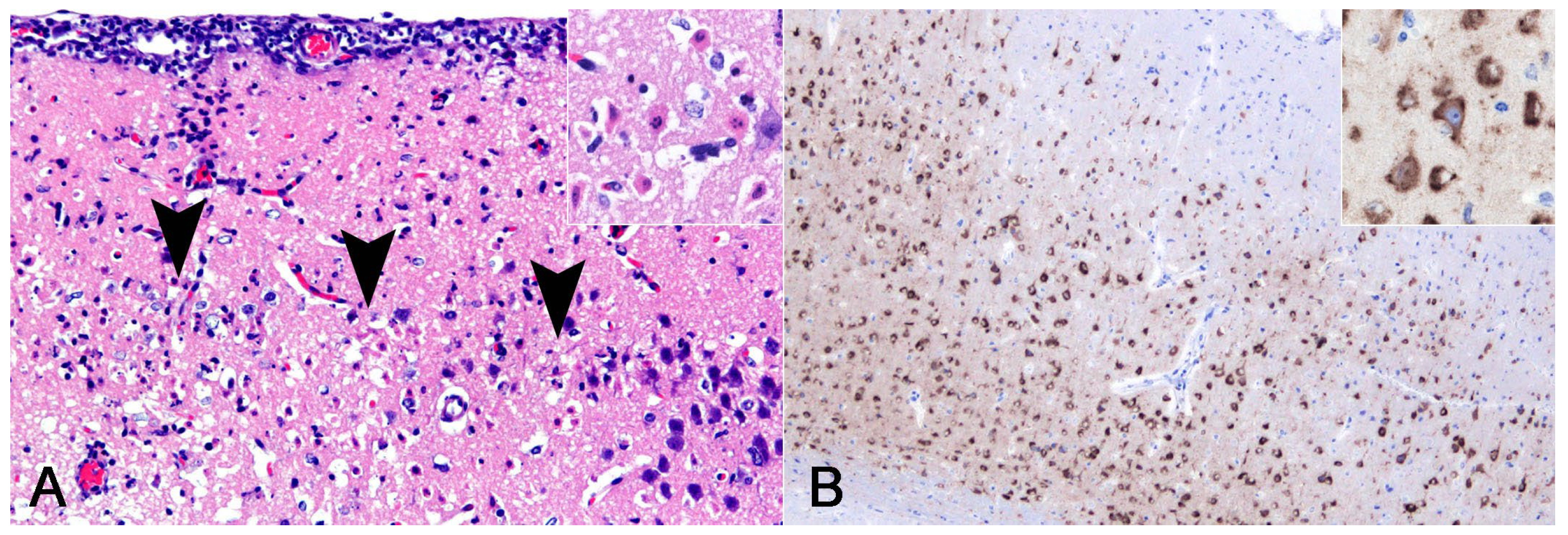
3.2. Pathological Findings
3.3. Molecular Identification of Pathogens
3.4. Virus Isolation
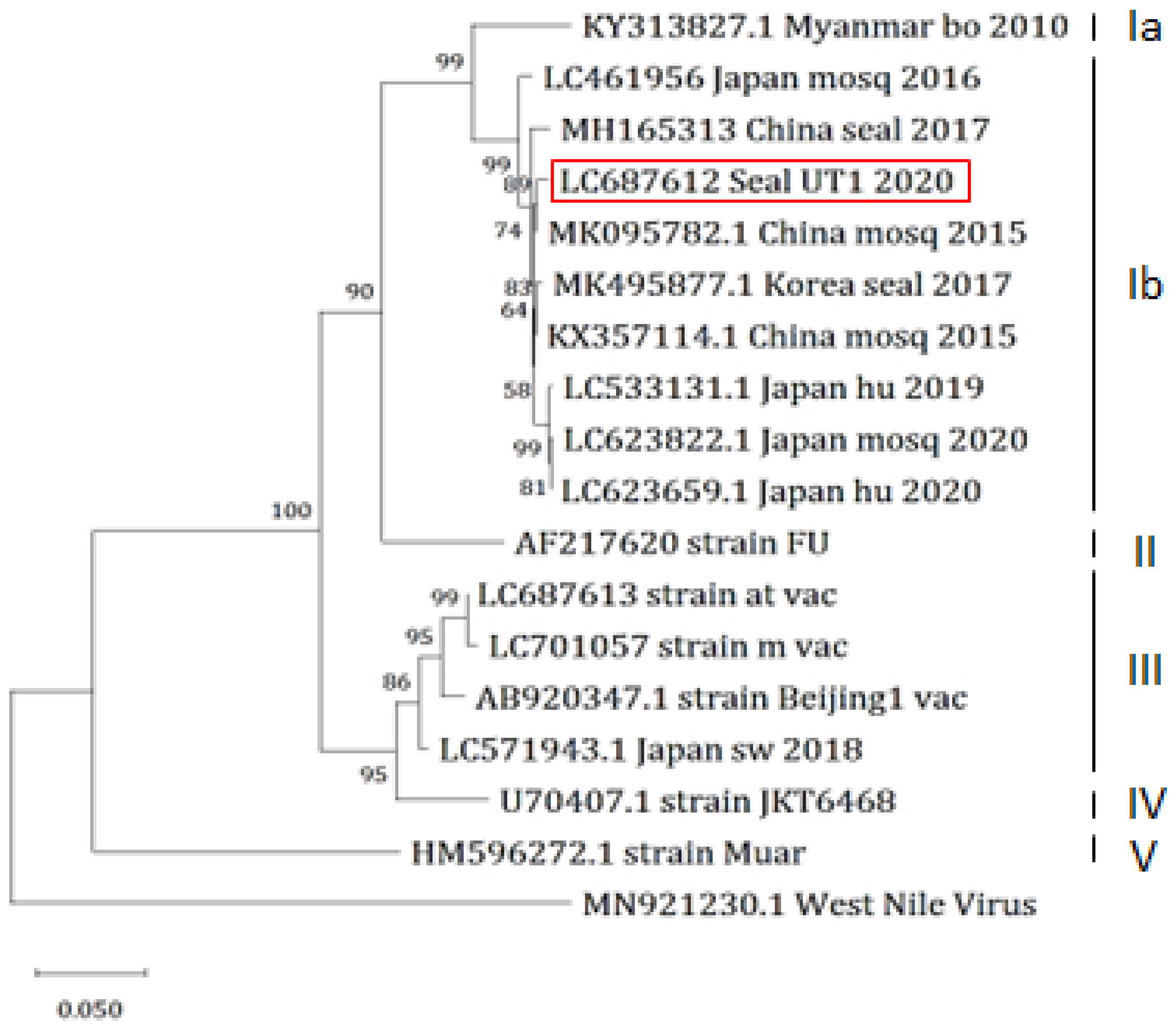
3.5. Sequence Analysis
3.6. Mouse Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.W.; Hoke, C.H. The epidemiology of Japanese encephalitis: Prospects for prevention. Epidemiol. Rev. 1992, 14, 197–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, L.; Sun, S.; Hu, W.; Mu, Q.; Liang, X.; Jin, N.; Dai, T.; Li, H.; Zhuang, G. Long-term neurological sequelae and disease burden of Japanese encephalitis in Gansu province, China. Ann. Glob. Health 2021, 87, 103. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F. New initiatives for the control of Japanese encephalitis by vaccination: Minutes of a WHO/CVI meeting, Bangkok, Thailand, 13–15 October 1998. Vaccine 2000, 18 (Suppl. 2), 1–25. [Google Scholar] [CrossRef]
- Nemeth, N.; Bosco-Lauth, A.; Oesterle, P.; Kohler, D.; Bowen, R. North American birds as potential amplifying hosts of Japanese encephalitis virus. Am. J. Trop. Med. Hyg. 2012, 87, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, M.E.; Garcìa-Nicolàs, O.; Brechbühl, D.; Python, S.; Zumkehr, B.; Posthaus, H.; Oevermann, A.; Summerfield, A. Japanese encephalitis virus tropism in experimentally infected pigs. Vet. Res. 2016, 47, 34. [Google Scholar] [CrossRef] [PubMed]
- Konno, J.; Endo, K.; Agatsuma, H.; Ishida, N. Cyclic outbreaks of Japanese encephalitis among pigs and humans. Am. J. Epidemiol. 1966, 84, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Endy, T.P.; Nisalak, A. Japanese encephalitis virus: Ecology and epidemiology. Curr. Top. Microbiol. Immunol. 2002, 267, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.I.; Smith, C.E.; Marshall, T.F.; Platt, G.S.; Way, H.J.; Bowen, E.T.; Bright, W.F.; Day, J.; McMahon, D.A.; Hill, M.N.; et al. Arbovirus infections in Sarawak: The role of the domestic pig. Trans. R. Soc. Trop. Med. Hyg. 1976, 70, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Takashima, I.; Watanabe, T.; Ouchi, N.; Hashimoto, N. Ecological studies of Japanese encephalitis virus in Hokkaido: Interepidemic outbreaks of swine abortion and evidence for the virus to overwinter locally. Am. J. Trop. Med. Hyg. 1988, 38, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kawakami, Y.; Fukuhara, S.; Matumoto, M. Experimental stillbirth in pregnant swine infected with Japanese encephalitis virus. Jpn. J. Exp. Med. 1954, 24, 363–375. [Google Scholar] [PubMed]
- Inoue, Y.K. An attenuated mutant of Japanese encephalitis virus. Bull. World Health Organ. 1964, 30, 181–185. [Google Scholar] [PubMed]
- Nah, J.J.; Yang, D.K.; Kim, H.H.; Song, J.Y. The present and future of veterinary vaccines for Japanese encephalitis in Korea. Clin. Exp. Vaccine Res. 2015, 4, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Shimada, K. Seroepizootiological survey of Japanese encephalitis virus and Getah virus in regional horse race tracks from 1991 to 1997 in Japan. J. Vet. Med. Sci. 1999, 61, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Konishi, E.; Shoda, M.; Ajiro, N.; Kondo, T. Development and evaluation of an enzyme-linked immunosorbent assay for quantifying antibodies to Japanese encephalitis virus nonstructural 1 protein to detect subclinical infections in vaccinated horses. J. Clin. Microbiol. 2004, 42, 5087–5093. [Google Scholar] [CrossRef] [PubMed]
- Horimoto, M.; Sakai, T. Maternally derived antibodies to Japanese encephalitis virus in cattle. J. Jap. Assoc. Infect. Dis. 1990, 64, 1205–1208. [Google Scholar] [CrossRef]
- Lim, S.I.; Kweon, C.H.; Tark, D.S.; Kim, S.H.; Yang, D.K. Sero-survey on Aino, Akabane, Chuzan, bovine ephemeral fever and Japanese encephalitis virus of cattle and swine in Korea. J. Vet. Sci. 2007, 8, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.J.; Byrne, R.J.; Hayes, D.E. Experimental infection of horses with Japanese encephalitis virus. Am. J. Trop. Med. Hyg. 1964, 13, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Ilkal, M.A.; Dhanda, V.; Rao, B.U.; George, S.; Mishra, A.C.; Prasanna, Y.; Gopalkrishna, S.; Pavri, K.M. Absence of viraemia in cattle after experimental infection with Japanese encephalitis virus. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Sato, H.; Suzuki, K.; Yokoyama, M.; Uni, S.; Shibasaki, T.; Sashika, M.; Inokuma, H.; Kai, K.; Maeda, K. Detection of antibodies against Japanese encephalitis virus in raccoons, raccoon dogs and wild boars in Japan. J. Vet. Med. Sci. 2009, 71, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Nidaira, M.; Kyan, H.; Taira, K.; Okano, S.; Oshiro, T.; Kato, T.; Kudo, N.; Azama, Y.; Mahoe, Y.; Kudaka, J.; et al. Survey of Japanese encephalitis virus in pigs and wild boars on Ishigaki and Iriomote Islands in Okinawa, Japan. Epidemiol. Infect. 2014, 142, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Saito, A.; Noguchi, K.; Terada, Y.; Kuwata, R.; Akari, H.; Takasaki, T.; Maeda, K. Seroprevalence of Japanese encephalitis virus infection in captive Japanese macaques (Macaca fuscata). Primates 2014, 55, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Diptyanusa, A.; Herini, E.S.; Indarjulianto, S.; Satoto, T.B.T. Estimation of Japanese encephalitis virus infection prevalence in mosquitoes and bats through nationwide sentinel surveillance in Indonesia. PLoS ONE 2022, 17, e0275647. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiao, M.; Deng, X.; Chen, X.; Sun, S.; Zhang, Q.; Zhang, W.; Tan, F.; Sun, Z.; Chen, X.; et al. Lethal encephalitis in seals with Japanese encephalitis virus infection, China, 2017. Emerg. Infect. Dis. 2019, 25, 1539–1542. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Kim, H.J.; Lee, K.; Choi, J.G.; Kim, Y.H.; Lee, K.K.; Kim, Y.D.; So, B.; Kang, H.E.; Choi, E.J. Co-infection of Dirofilaria immitis and Japanese encephalitis virus in a spotted seal (Phoca largha) in the Republic of Korea. J. Vet. Sci. 2019, 20, e65. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Sengupta, J.; Banerjee, D.; Khetan, A.; Mandal, S.M. Schizophyllum commune: A new organism in eye infection. Mycopathologia 2013, 175, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.N.; Cheng, Y.; Wang, Y.N.; Wu, J.; Liu, C.; An, N. Corneal ulcer possibly caused by the opportunistic pathogen Schizophyllum commune. Int. J. Ophthalmol. 2020, 13, 1164–1166. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Takizawa, K.; Baba, O.; Maeda, T.; Fukushima, K.; Shinya, K. Basidiomycosis: Schizophyllum commune osteomyelitis in a dog. J. Vet. Med. Sci. 2008, 70, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Seki, A.; Kano, R.; Sakai, H.; Nakagawa, M.; Hasegawa, A.; Maruo, K. Mycotic osteomyelitis caused by schizophyllum commune in a dog. Vet. Rec. 2009, 165, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Kano, R.; Oomae, S.; Nakano, Y.; Minami, T.; Sukikara, M.; Nakayama, T.; Hasegawa, A. First report on Schizophyllum commune from a dog. J. Clin. Microbiol. 2002, 40, 3535–3537. [Google Scholar] [CrossRef]
- Hakamata, M.; Kano, R.; Kondo, H.; Watari, T. Canine fungal osteomyelitis. Mycopathologia 2019, 184, 707–708. [Google Scholar] [CrossRef] [PubMed]
- Hanafusa, Y.; Hirano, Y.; Watabe, H.; Hosaka, K.; Ikezawa, M.; Shibahara, T. First isolation of Schizophyllum commune in a harbor seal (Phoca vitulina). Med. Mycol. 2016, 54, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Meason-Smith, C.; Edwards, E.E.; Older, C.E.; Branco, M.; Bryan, L.K.; Lawhon, S.D.; Suchodolski, J.S.; Gomez, G.; Mansell, J.; Hoffmann, A.R. Panfungal polymerase chain reaction for identification of fungal pathogens in formalin-fixed animal tissues. Vet. Pathol. 2017, 54, 640–648. [Google Scholar] [CrossRef]
- Ayers, M.; Adachi, D.; Johnson, G.; Andonova, M.; Drebot, M.; Tellier, R. A single tube RT-PCR assay for the detection of mosquito-borne flaviviruses. J. Virol. Methods. 2006, 135, 235239. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Nisseiken Japanese Encephalitis Live Vaccine. Available online: https://www.jp-nisseiken.co.jp/en/products/pdf/pig/JEL_en.pdf (accessed on 7 May 2024).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yan, J.Y.; He, H.Q.; Yan, R.; Sun, Y.; Tang, X.-W.; Zhou, Y.; Pan, J.-H.; Mao, H.-Y.; Zhang, Y.-J.; et al. Serological and molecular epidemiology of Japanese encephalitis in Zhejiang, China, 2015-2018. PLoS Negl. Trop. Dis. 2020, 14, e0008574. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wu, Z.; Chen, H.; Li, C.; Guo, X.; Liu, R.; Wang, G.; Zhou, M.; Zhao, T. Japanese encephalitis virus infection rate and detection of genotype I from Culex tritaeniorhynchus collected from Jiangsu, China. Vector-Borne Zoonotic Dis. 2017, 17, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zheng, H.; Tong, W.; Li, G.; Wang, T.; Li, L.; Gao, F.; Shan, T.; Yu, H.; Zhou, Y.; et al. Acidity/alkalinity of Japanese encephalitis virus E protein residue 138 alters neurovirulence in mice. J. Virol. 2018, 92, e00108-18. [Google Scholar] [CrossRef]
- Reisen, W.K.; Aslam, Y.; Siddiqui, T.F.; Khan, A.Q. A mark-release-recapture experiment with Culex tritaeniorhynchus Giles. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 167–177. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, P.; Liu, B.; Shi, G.; Wang, H.; Liu, L.; Guo, X.; Ren, H.; Gong, M. Measure post-bloodmeal dispersal of mosquitoes and duration of radioactivity by using the isotope ³²P. J Insect Sci. 2014, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Kawai, S.; Oda, T.; Miyagi, I.; Suenaga, O.; Nishigaki, J.; Omori, N. Dispersal experiment of Culex tritaeniorhynchus in Nagasaki area (Preliminary report). Trop. Med. 1969, 11, 37–44. [Google Scholar]
- Uchil, P.D.; Satchidanandam, V. Phylogenetic analysis of Japanese encephalitis virus: Envelope gene based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent. Am. J. Trop. Med. Hyg. 2001, 65, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Ni, H.; Beasley, D.W.C.; Ekkelenkamp, M.; Cardosa, M.J.; Barrett, A.D.T. Origin and evolution of Japanese encephalitis virus in Southeast Asia. J. Virol. 2003, 77, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, T.; Loan, H.T.K.; Inoue, S.; Sumiyoshi, M.; Haruta, Y.; Nga, P.T.; Huoung, V.T.Q.; Parquet, M.D.C.; Hasebe, F.; Morita, K. Evidence of frequent introductions of Japanese encephalitis virus from south-east Asia and continental east Asia to Japan. J. Gen. Virol. 2009, 90, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Nitatpattana, N.; Dubot-Pérès, A.; Ar Gouilh, M.; Souris, M.; Barbazan, P.; Yoksan, S.; de Lamballerie, X.; Gonzalez, J.-P. Change in Japanese encephalitis virus distribution, Thailand. Emerg. Infect. Dis. 2008, 14, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Nga, P.T.; del Carmen Parquet, M.; Cuong, V.D.; Ma, S.-P.; Hasebe, F.; Inoue, S.; Makino, Y.; Takagi, M.; Nam, V.S.; Morita, K. Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: Implications for frequent introductions of JEV from Southeast Asia to East Asia. J. Gen. Virol. 2004, 85, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Su, C.L.; Yang, C.F.; Teng, H.J.; Lu, L.-C.; Lin, C.; Tsai, K.-H.; Chen, Y.-Y.; Chen, L.-Y.; Chang, S.-F.; Shu, P.-Y. Molecular epidemiology of Japanese encephalitis virus in mosquitoes in Taiwan during 2005–2012. PLoS Negl. Trop. Dis. 2014, 8, e3122. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, H.; Li, X.; Fu, S.; Cao, L.; Shao, N.; Zhang, W.; Wang, Q.; Lu, Z.; Lei, W.; et al. Changing geographic distribution of Japanese encephalitis virus genotypes, 1935-2017. Vector-Borne Zoonotic Dis. 2019, 19, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Schuh, A.J.; Ward, M.J.; Leigh Brown, A.J.; Barrett, A.D.T. Dynamics of the emergence and establishment of a newly dominant genotype of Japanese encephalitis virus throughout Asia. J. Virol. 2014, 88, 4522–4532. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.C.; Chen, Y.Y.; Chen, J.M.; Huang, C.; Huang, M.; Chiou, S.S. Effectiveness of live-attenuated genotype III Japanese encephalitis viral vaccine against circulating genotype I viruses in swine. Viruses 2022, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, X.; Zhang, J.; Guo, S.; Pang, L.; Shi, K.; Liu, K.; Shao, D.; Qiu, Y.; Liu, L.; et al. Partial cross-protection between Japanese encephalitis virus genotype I and III in mice. PLoS Negl. Trop. Dis. 2019, 13, e0007601. [Google Scholar] [CrossRef] [PubMed]


| Antigen | Type | Dilution | Antigen Retrieval | Catalog No. | Source |
|---|---|---|---|---|---|
| JEV envelope protein | Rabbit, polyclonal | 1:200 | Heat, pH 9.0 | GTX125867 | GeneTex, Irvine, CA, USA |
| Myeloperoxidase | Rabbit, polyclonal | 1:400 | None | A0398 | Dako, Santa Clara, CA, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, M.; Ito, S.; Katsumata, E.; Chambers, J.K.; Matsugo, H.; Takenaka-Uema, A.; Murakami, S.; Uchida, K.; Horimoto, T. Japanese Encephalitis Virus and Schizophyllum commune Co-Infection in a Harbor Seal in Japan. Vet. Sci. 2024, 11, 215. https://doi.org/10.3390/vetsci11050215
Fujii M, Ito S, Katsumata E, Chambers JK, Matsugo H, Takenaka-Uema A, Murakami S, Uchida K, Horimoto T. Japanese Encephalitis Virus and Schizophyllum commune Co-Infection in a Harbor Seal in Japan. Veterinary Sciences. 2024; 11(5):215. https://doi.org/10.3390/vetsci11050215
Chicago/Turabian StyleFujii, Marina, Soma Ito, Etsuko Katsumata, James K. Chambers, Hiromichi Matsugo, Akiko Takenaka-Uema, Shin Murakami, Kazuyuki Uchida, and Taisuke Horimoto. 2024. "Japanese Encephalitis Virus and Schizophyllum commune Co-Infection in a Harbor Seal in Japan" Veterinary Sciences 11, no. 5: 215. https://doi.org/10.3390/vetsci11050215
APA StyleFujii, M., Ito, S., Katsumata, E., Chambers, J. K., Matsugo, H., Takenaka-Uema, A., Murakami, S., Uchida, K., & Horimoto, T. (2024). Japanese Encephalitis Virus and Schizophyllum commune Co-Infection in a Harbor Seal in Japan. Veterinary Sciences, 11(5), 215. https://doi.org/10.3390/vetsci11050215






