Impact of Mycotoxin Metabolites Deepoxy-Deoxynivalenol and Beta-Zearalenol on Bovine Preimplantation Embryo Development in the Presence of Acetonitrile
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Oocyte Collection
2.3. In Vitro Production of Bovine Embryos
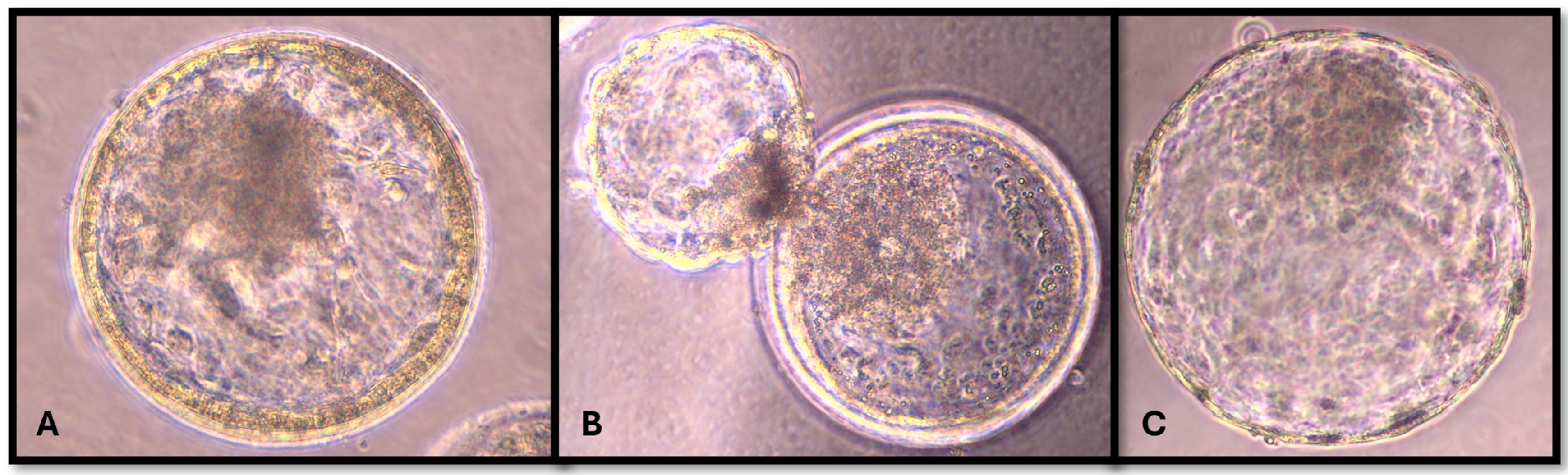
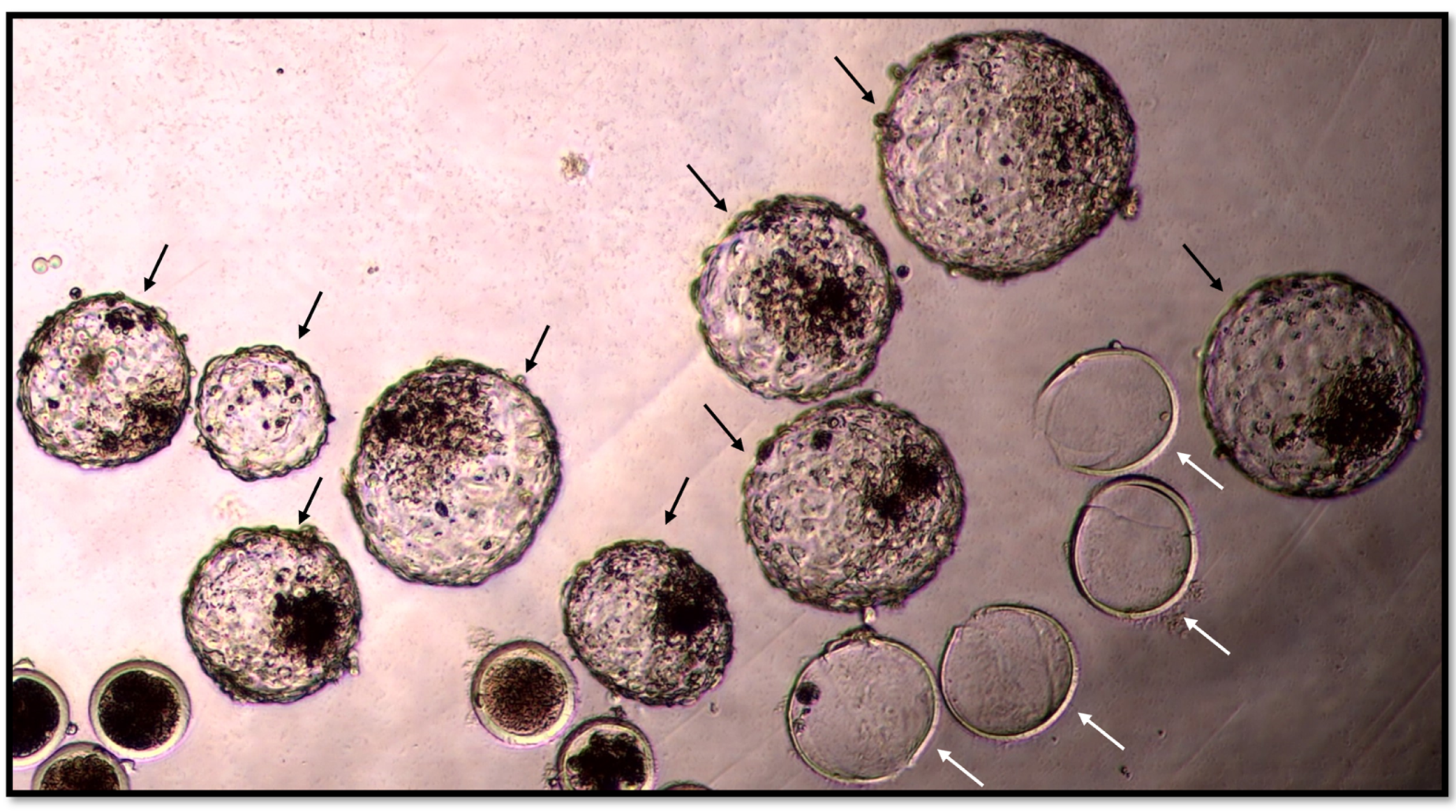
2.4. Morphology and Kinetic Stage of Embryos
2.5. Determination of DOM-1 and β-ZOL Using LC/MS/MS
2.6. Mycotoxin Metabolites
2.6.1. Experiment 1: Effect of Acetonitrile on the In Vitro Development of Bovine Blastocysts
2.6.2. Experiment 2: The Effects of the Individual Mycotoxin Metabolite (β-ZOL or DOM-1 Only) on the In Vitro Development of Bovine Blastocysts
2.6.3. Experiment 3: The Combined Effect of DOM-1 and β- ZOL on the In Vitro Development of Bovine Blastocysts
2.7. Statistical Analyses
3. Results
3.1. Effects of Acetonitrile on the In Vitro Development of Bovine Blastocysts
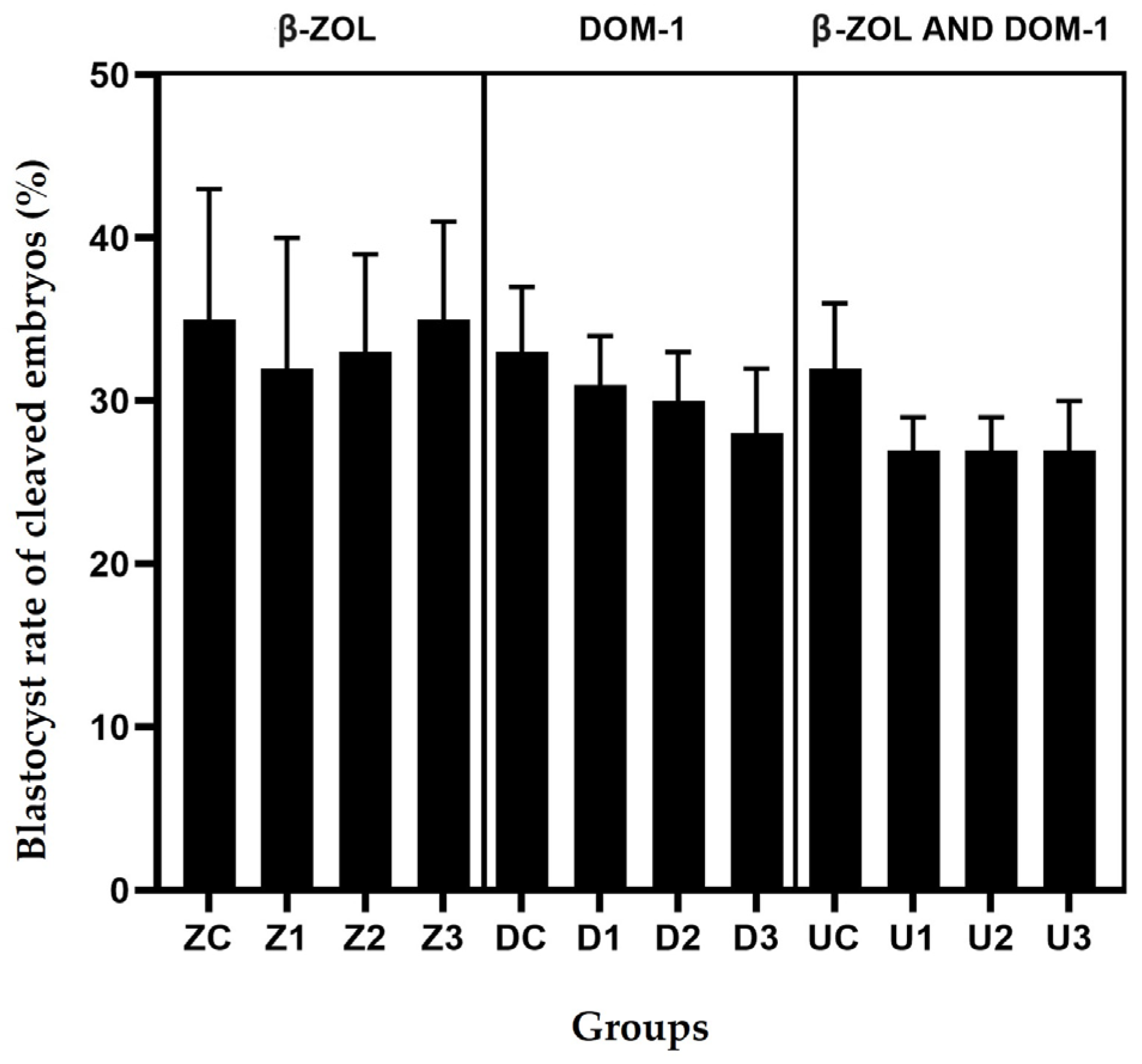
3.2. The Effects of the Individual Mycotoxin Metabolite (Mycotoxin β-ZOL Only and Mycotoxin DOM-1 Only) on the In Vitro Development of Bovine Blastocysts
3.3. The Combined Effect of DOM-1 and β-ZOL on the In Vitro Development of Bovine Blastocysts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar] [CrossRef] [PubMed]
- Jakovac-Strajn, B.; Vengušt, A.; Ujčič-Vrhovnik, I.; Pavšič-Vrtač, K.; Tavčar-Kalcher, G. The natural occurrence of toxigenic moulds and mycotoxins in Slovenian primary grain production. Acta Agric. Slov. 2010, 95, 121–128. [Google Scholar] [CrossRef]
- Babič, J.; Tavčar-Kalcher, G.; Celar, F.A.; Kos, K.; Knific, T.; Jakovac-Strajn, B. Occurrence of Alternaria and Other Toxins in Cereal Grains Intended for Animal Feeding Collected in Slovenia: A Three-Year Study. Toxins 2021, 13, 304. [Google Scholar] [CrossRef]
- Torović, L. Fusarium toxins in corn food products: A survey of the Serbian retail market. Food Addit. Contam. Part A 2018, 35, 1596–1609. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Assunção, R.; Twarużek, M.; Kosicki, R.; Grajewski, J.; Viegas, C. Mycotoxins feed contamination in a dairy farm—Potential implications for milk contamination and workers’ exposure in a One Health approach. J. Sci. Food Agric. 2020, 100, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Netro, H.M.; Barreta, M.H.; Costa, E.; Goetten, A.; Dupras, R.; Mills LKoch, J.; Portela, V.M.; Price, C.A.; Chorfi, Y. Effects of the mycotoxin metabolite de-epoxy-deoxynivalenol (DOM-1) on embryo development and sperm motility in cattle. J. Appl. Toxicol. 2020, 41, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Liu, J.Q.; Zou, P.; Jia, L.; Su, Y.T.; Sun, Y.R.; Sun, S.C. Comparison of the toxic effects of different mycotoxins on porcine and mouse oocyte meiosis. PeerJ 2018, 6, e5111. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Kersten, S.; Meyer, U.; Stinshoff, H.; Locher, L.; Rehage, J.; Wrenzycki, C.; Engelhardt, U.H.; Dänicke, S. Diagnostic opportunities for evaluation of the exposure of dairy cows to the mycotoxins deoxynivalenol (DON) and zearalenone (ZEN): Reliability of blood plasma, bile and follicular fluid as indicators. J. Anim. Physiol. Anim. Nutr. 2015, 99, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Gödde, J.; Meyer, U.; Frahm, J.; Westendarp, H.; Dänicke, S. Fusarium toxin-contaminated maize in diets of growing bulls: Effects on performance, slaughtering characteristics, and transfer into physiological liquids. Mycotoxin Res. 2016, 32, 127–135. [Google Scholar] [CrossRef]
- Zhang, G.L.; Feng, Y.L.; Song, J.L.; Zhou, X.S. Zearalenone: A Mycotoxin with Different Toxic Effect in Domestic and Laboratory Animals’ Granulosa Cells. Front. Genet. 2018, 9, 667. [Google Scholar] [CrossRef]
- Keller, L.; Abrunhosa, L.; Keller, K.; Rosa, C.A.; Cavaglieri, L.; Venâncio, A. Zearalenone and Its Derivatives α-Zearalenol and β-Zearalenol Decontamination by Saccharomyces cerevisiae Strains Isolated from Bovine Forage. Toxins 2015, 7, 3297–3308. [Google Scholar] [CrossRef]
- Hartinger, T.; Kröger, I.; Neubauer, V.; Faas, J.; Doupovec, B.; Schatzmayr, D.; Zebeli, Q. Zearalenone and Its Emerging Metabolites Promptly Affect the Rumen Microbiota in Holstein Cows Fed a Forage-Rich Diet. Toxins 2023, 15, 185. [Google Scholar] [CrossRef]
- Malekinejad, H.; Maas-Bakker, R.F.; Fink-Gremmels, J. Bioactivation of zearalenone by porcine hepatic biotransformation. Vet. Res. 2005, 36, 799–810. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Maes, J.; Verlooy, L.; Buenafe, O.E.; de Witte, P.A.M.; Esguerra, C.V.; Crawford, A.D. Evaluation of 14 Organic Solvents and Carriers for Screening Applications in Zebrafish Embryos and Larvae. PLoS ONE 2012, 7, e43850. [Google Scholar] [CrossRef] [PubMed]
- Alm, H.; Greising, T.; Brüssow, K.P.; Torner, H.; Tiemann, U. The influence of the mycotoxins deoxynivalenol and zearalenol on in vitro maturation of pig oocytes and in vitro culture of pig zygotes. Toxicol. In Vitro 2002, 16, 643–648. [Google Scholar] [CrossRef]
- Sangsritavong, S.; Combs, D.K.; Sartori, R.; Armentano, L.E.; Wiltbank, M.C. High feed intake increases liver blood flow and metabolism of progesterone and estradiol-17beta in dairy cattle. J. Dairy Sci. 2002, 85, 2831–2842. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W.; Williams, E.J.; Evans, A.C.O. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 2011, 123, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Leibfried, L.; First, N.L. Characterization of bovine follicular oocytes and their ability to mature in vitro. J. Anim. Sci. 1979, 48, 76–86. [Google Scholar] [CrossRef]
- Aguila, L.; Treulen, F.; Therrien, J.; Felmer, R.; Valdivia, M.; Smith, L.C. Oocyte Selection for In Vitro Embryo Production in Bovine Species: Noninvasive Approaches for New Challenges of Oocyte Competence. Animals 2020, 10, 2196. [Google Scholar] [CrossRef]
- van der Weijden, V.A.; Chen, S.; Bauersachs, S.; Ulbrich, S.E.; Schoen, J. Gene expression of bovine embryos developing at the air-liquid interface on oviductal epithelial cells (ALI-BOEC). Reprod. Biol. Endocrinol. 2017, 15, 91. [Google Scholar] [CrossRef]
- Razza, E.M.; Pedersen, H.S.; Stroebech, L.; Fontes, P.K.; Kadarmideen, H.N.; Callesen, H.; Pihl, M.; Nogueira, M.F.G.; Hyttel, P. Simulated physiological oocyte maturation has side effects on bovine oocytes and embryos. J. Assist. Reprod. Genet. 2019, 36, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, Q.; Pu, L.; Ahmed Wadood, A.; Waqas, M.; Xie, L.; Shekhar Pareek, C.; Xu, H.; Liang, X.; Lu, Y. Proteomics Analysis Reveals that Warburg Effect along with Modification in Lipid Metabolism Improves In Vitro Embryo Development under Low Oxygen. Int. J. Mol. Sci. 2020, 21, 1996. [Google Scholar] [CrossRef] [PubMed]
- Fields, S.D.; Hansen, P.J.; Ealy, A.D. Fibroblast growth factor requirements for in vitro development of bovine embryos. Theriogenology 2011, 75, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Saeki, K.; Hoshi, M.; Kainuma, H. Effects of oxygen concentration and oviductal epithelial tissue on the development of in vitro matured and fertilized bovine oocytes cultured in protein-free medium. Theriogenology 1994, 41, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Barfield, J.; Demetrio, D. Considerations for Evaluating In Vitro-Produced Bovine Embryos—IETS Manual, 5th ed.; IETS: Champaign, IL, USA, 2022. [Google Scholar]
- Cortinovis, C.; Caloni, F.; Schreiber, N.B.; Spicer, L.J. Effects of fumonisin B1 alone and combined with deoxynivalenol or zearalenone on porcine granulosa cell proliferation and steroid production. Theriogenology 2014, 81, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- SigmaPlot, Version 12.5; Systat Software, Inc.: San Jose, CA, USA, 2013.
- Saillenfait, A.M.; Sabaté, J.P. Comparative Developmental Toxicities of Aliphatic Nitriles: In Vivo and in Vitro Observations. Toxicol. Appl. Pharmacol. 2000, 163, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, J.; Berg, D.K.; Pfeffer, P.L. Morphological and Gene Expression Changes in Cattle Embryos from Hatched Blastocyst to Early Gastrulation Stages after Transfer of In Vitro Produced Embryos. PLoS ONE 2015, 10, e0129787. [Google Scholar] [CrossRef]
- Hamdoun, A.; Epel, D. Embryo stability and vulnerability in an always changing world. Proc. Natl. Acad. Sci. USA 2007, 104, 1745–1750. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. Mycotoxins in cattle feeds and carry-over to dairy milk: A review. Food Addit. Contam. Part A 2008, 25, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Moros-Nicolás, C.; Chevret, P.; Jiménez-Movilla, M.; Algarra, B.; Cots-Rodríguez, P.; González-Brusi, L.; Avilés, M.; Izquierdo-Rico, M.J. New Insights into the Mammalian Egg Zona Pellucida. Int. J. Mol. Sci. 2021, 22, 3276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, C.; Liang, J.; Fan, L.; Liu, D.; Zhang, X.; Liu, F. A comparison of the clinical effects of thinning and drilling on laser-assisted hatching. Lasers Med. Sci. 2022, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Production Variable | Control | Acetonitrile Group e |
|---|---|---|
| Cleavage rate | 87.50% (70/80) | 83.80% (67/80) |
| Blastocysts | 11.43% (8/70) | 4.48% (3/67) |
| Expanded blastocysts | 11.43% (8/70) | 8.96% (6/67) |
| Hatching or hatched blastocysts | 12.86% (9/70) | 2.99% (2/67) |
| Total blastocysts | 31% a ± 5.1 (25/80) | 16% b ± 4.2 (13/80) |
| Total blastocysts/cleaved | 36% c ± 9.7 (25/70) | 19% d ± 3.9 (13/67) |
| Groups | Conc. ± SEM of Mycotoxin Metabolites on Day 8 (ng/mL) | Blastocyst Rate | Blastocyst Rate of Cleaved Embryos |
|---|---|---|---|
| Proportion ± SEM (N) | Proportion ± SEM (N) | ||
| β-ZOL | |||
| CONTROL GROUP 1 | - | 26% ± 5.7 (23/89) | 35% ± 7.9 (23/65) |
| Z1 2 | 0.125 ± 0.003 | 25% ± 6.8 (21/85) | 32% ± 8.3 (21/65) |
| Z2 3 | 0.245 ± 0.006 | 26% ± 5.2 (21/82) | 33% ± 6.2 (21/63) |
| Z3 4 | 0.370 ± 0.008 | 27% ± 5.3 (24/90) | 35% ± 6.1 (24/68) |
| DOM-1 | |||
| CONTROL GROUP 1 | - | 25% ± 2.8 (86/340) | 33% ± 3.5 (86/260) |
| D1 5 | 76.5 ± 4.3 | 24% ± 2.7 (83/344) | 31% ± 3.4 (83/266) |
| D2 6 | 99.7 ± 5.5 | 24% ± 2.5 (81/343) | 30% ± 3.1 (81/269) |
| D3 7 | 126.2 ± 6.8 | 21% ± 3.4 (74/348) | 28% ± 4.2 (74/265) |
| β-ZOL AND DOM-1 | |||
| CONTROL GROUP 1 | - | 24% ± 3.2 (95/396) | 32% ± 3.8 (95/295) |
| U1 8 | DOM1: 76.0 ± 2.5 | 20% ± 1.7 (77/381) | 27% ± 2.1 (77/285) |
| β-ZOL: 0.126 ± 0.005 | |||
| U2 9 | DOM1: 99.1 ± 3.2 | 19% ± 1.8 (75/388) | 27% ± 2.2 (75/279) |
| β-ZOL: 0.246 ± 0.003 | |||
| U3 10 | DOM1: 125.4 ± 4.0 | 21% ± 2.6 (83/393) | 27% ± 2.9 (83/304) |
| β-ZOL: 0.382 ± 0.02 |
| Group | Blastocyst | Expanded/Expanding Blastocyst | Hatched/Hatching Blastocyst |
|---|---|---|---|
| β-ZOL | |||
| CONTROL GROUP 1 | 8% ± 2.7 | 7% ± 3.2 | 8% ± 3.5 |
| Z1 2 | 10% ± 2.7 | 3% ± 2.2 | 12% ± 4.8 |
| Z2 3 | 7% ± 2.4 | 9% ± 3.5 | 6% ± 2.2 |
| Z3 4 | 6% ± 2.1 | 8% ± 2.3 | 10% ± 3.6 |
| DOM-1 | |||
| CONTROL GROUP 1 | 6% ± 1.4 | 7% ± 1.7 | 11% ± 2.1 |
| D1 5 | 5% ± 1.1 | 13% ± 2.0 | 6% ± 1.2 |
| D2 6 | 6% ± 1.7 | 10% ± 1.8 | 7% ± 1.4 |
| D3 7 | 5% ± 1.4 | 7% ± 1.6 | 8% ± 1.5 |
| β-ZOL AND DOM-1 | |||
| CONTROL GROUP 1 | 9% ± 2.2 | 10% ± 2.0 | 6% ± 1.4 |
| U1 8 | 6% ± 1.1 | 8% ± 1.6 | 7% ± 1.1 |
| U2 9 | 5% ± 1.0 | 9% ± 1.4 | 6% ± 1.7 |
| U3 10 | 4% ± 1.0 | 8% ± 1.8 | 9% ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gačnikar, J.; Mrkun, J.; Babič, J.; Sterniša, M.; Zakošek Pipan, M. Impact of Mycotoxin Metabolites Deepoxy-Deoxynivalenol and Beta-Zearalenol on Bovine Preimplantation Embryo Development in the Presence of Acetonitrile. Vet. Sci. 2024, 11, 267. https://doi.org/10.3390/vetsci11060267
Gačnikar J, Mrkun J, Babič J, Sterniša M, Zakošek Pipan M. Impact of Mycotoxin Metabolites Deepoxy-Deoxynivalenol and Beta-Zearalenol on Bovine Preimplantation Embryo Development in the Presence of Acetonitrile. Veterinary Sciences. 2024; 11(6):267. https://doi.org/10.3390/vetsci11060267
Chicago/Turabian StyleGačnikar, J., J. Mrkun, J. Babič, M. Sterniša, and M. Zakošek Pipan. 2024. "Impact of Mycotoxin Metabolites Deepoxy-Deoxynivalenol and Beta-Zearalenol on Bovine Preimplantation Embryo Development in the Presence of Acetonitrile" Veterinary Sciences 11, no. 6: 267. https://doi.org/10.3390/vetsci11060267
APA StyleGačnikar, J., Mrkun, J., Babič, J., Sterniša, M., & Zakošek Pipan, M. (2024). Impact of Mycotoxin Metabolites Deepoxy-Deoxynivalenol and Beta-Zearalenol on Bovine Preimplantation Embryo Development in the Presence of Acetonitrile. Veterinary Sciences, 11(6), 267. https://doi.org/10.3390/vetsci11060267









