Simple Summary
Viruses present a significant challenge to the sustainable growth of the aquaculture sector. Perinereis, a valuable live-feed source in aquaculture, carries the risk of harboring unknown viruses, potentially jeopardizing the biosecurity of aquatic organisms. Despite this risk, there is a notable absence of research investigating novel viruses in Perinereis using meta-transcriptomic sequencing techniques. Therefore, it becomes imperative to explore the viral diversity within Perinereis. In this report, 12 previously unidentified viruses was documented in two distinct Perinereis species through meta-transcriptomic sequencing. These newly identified viruses were classified into four major viral families, shedding light on the intricate viral landscape within Perinereis. By unveiling these novel viruses, this research contributes to a broader knowledge base of the viral ecology in Perinereis, offering valuable insights into the potential risks and impacts associated with Perinereis in aquaculture settings. Such findings also highlight the need for enhancing biosecurity measures and disease management strategies within the aquaculture industry.
Abstract
Perinereis species are essential benthonic animals in coastal ecosystems and have significant roles as live feed in aquaculture, owing to their high-protein and low-fat nutritional profile. Despite their ecological importance, the viral communities associated with these organisms need to be better understood. In this study, we generated 2.6 × 108 reads using meta-transcriptomic sequencing and de novo assembled 5.3 × 103 virus-associated contigs. We identified 12 novel RNA viruses from two species, Perinereis aibuhitensis and P. wilsoni, which were classified into four major viral groups: Picobirnaviridae, Marnaviridae, unclassified Picornavirales, and unclassified Bunyavirales. Our findings revealed the hidden diversity of viruses and genome structures in Perinereis, enriching the RNA virosphere and expanding the host range of Picobirnaviridae, Marnaviridae, and Bunyavirales. This study also highlighted the potential biosecurity risk of the novel viruses carried by Perinereis to aquaculture.
1. Introduction
Nereididae is a group of morphologically diverse marine Annelida polychaeta animals with high species diversity, including more than 45 genera and 719 species [1]. They are extensively distributed in intertidal zones, freshwater areas, and deep-sea environments, and are predominantly found in coastal areas [1]. Perinereis, representing the second most abundant genus within Nereididae, comprises 94 species [2,3,4,5]. Perinereis plays a crucial role in aquaculture hatcheries and recreational fisheries. Perinereis also serves as an effective model organism for absorbing and purifying environmental contaminants [6]. Previous studies have shown that the bioturbation effect of P. aibuhitensis could increase the oxygen distribution in the sediment, reduce ammonia nitrogen, sulfide, and other harmful substances in the water body, and optimize the bottom condition [6,7], playing an important role in improving the aquaculture environment. Furthermore, Perinereis also plays a pivotal role in the natural food chain and serves as an important component of the diet of fish and shorebirds in intertidal zones [8]. Perinereis is believed to be rich in nutrients, including unsaturated fatty acids, proteins, and hormones, which promote oocyte maturation and sperm production, thereby further enhancing larval shrimp production [9,10]. P. aibuhitensis is considered the premier source of unsaturated fatty acids for aquaculture broodstock, owing to its high content of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [8,11]. Consequently, Perinereis is regarded as a premium live feed for broodstock in the aquaculture industry [12,13].
Studies have shown that polychaetes are the vector of shrimp white spot syndrome virus (WSSV) [14,15]. There are many examples of economic losses to the aquaculture industry caused by feeding with virus-carrying Perinereis [16,17,18]. Current research predominantly focuses on the detection of known viruses in economically significant aquaculture species, such as shrimp. Some studies have shown that known high-risk viruses, such as WSSV, decapod iridescent virus 1 (DIV1), and covert mortality nodavirus (CMNV), are detected in Perinereis [19,20,21,22,23,24]. It was also reported that viruses had been previously found in another polychaetes genus, Nereis. For example, Devauchelle reported the discovery of Nereis iridescent virus (NIV) from Nereis diversicolor through electron microscopy [25]. However, the exploration of unknown viruses within the Perinereis genus remains limited [18,19,20,21,22,23,24,25,26,27,28,29]. This gap in knowledge underscores the importance of preemptively identifying and understanding novel Perinereis viruses to mitigate potential economic losses due to disease outbreaks.
The application of high-throughput technology has greatly advanced our understanding of viruses. Compared with the use of traditional molecular biology technology to discover viruses, high-throughput sequencing is faster and more efficient, and plays a pivotal role in the rapid assembly and annotation of the genomes of species, as well as in the discovery of microorganisms within host samples [30,31,32]. Meta-transcriptome, a whole-transcriptome shotgun sequencing technology, can quickly generate a large number of RNA sequences and broadly identify the RNA of all viruses, including known and novel viruses [33]. The employment of high-throughput sequencing has greatly advanced our understanding of virus diversity.
In this study, we employed meta-transcriptomic sequencing to identify novel RNA viruses associated with Perinereis. This study will help us to better understand the hidden world of viruses in Perinereis and the potential impact of Perinereis on aquaculture.
2. Materials and Methods
2.1. Sample Collection and Processing
Specimens for this investigation were obtained from six individuals of the genus Perinereis without obvious clinical signs, which were collected from the same polychaetes farm in Dongfang City, Hainan Province, China, on 21 September 2018. They comprised two species within the Nereididae family, P. aibuhitensis (n = 3) and P. wilsoni (n = 3). Each intact individual sample was surface-rinsed with physiological saline at the time of collection to exclude the influence of environmental contaminants, and then they were thoroughly homogenized and total RNA was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions.
2.2. RNA Library Construction and Meta-Transcriptomic Sequencing
After passing the quality control, the extracted total RNA was used for long non-coding RNA (lncRNA) library construction and meta-transcriptomic sequencing as previously described [34,35]. The Ribo-Zero™ kit (Epicentre, Madison, Wisconsin, USA) was used to remove unnecessary rRNA from total RNA, and RNA libraries were subjected to 150 nt paired-end read sequencing and then performed on the Illumina Hiseq platform (Illumina, San Diego, California, USA). The library construction and sequencing process were performed by Novogene (Beijing, China). Each library corresponded to an individual sample, resulting in three high-throughput libraries for P. aibuhitensis (GW1, GW2, and GW3) and three for P. wilsoni (SW1, SW2, and SW3).
2.3. Sequence Assembly and RNA Virus Discovery
Sequence assembly and RNA virus discovery were conducted for each library, following methods outlined in our prior publications [34,35]. Briefly, we used Fastp (version 0.21.0, HaploX Biotechnology Co., LTD, Shenzhen, China, 2018) [36] to control the quality of the raw data and Trinity to assemble the reads to obtain contigs. They were then compared with the non-redundant nucleotide and protein databases downloaded from GenBank by BLASTn and BLASTx to obtain potential viral contigs. False positives were excluded by the predicted genome structure, ORF prediction, and read mapping. Furthermore, Bowtie2 (version 2.3.5.1, Center for Bioinformatics and Computational Biology, Institute for Advanced Computer Studies, University of Maryland, College Park, Maryland, USA, 2012) [37], Samtools (version 1.9, Sanger Institute, Wellcome Genome Campus, Hinxton, Cambridgeshire, CB10 1SA. UK, 2018), and Geneious Prime (version 2022.0.2, Biomatters Ltd. Auckland, New Zealand, 2022) were used to map and extend target viruses to obtain a near-complete virus sequence. The abundance of viruses in each library was expressed by calculating reads per million mapped reads (RPM) and reads per kilobase of transcript per million mapped reads (RPKM).
2.4. Genome Structure Prediction and Phylogenetic Analysis
This study used the ORFfinder [38] to predict potential ORFs, which start with ATG and encode proteins of more than 100 amino acids (aa). CD-search [39], Pfarm [40], and Interpro [41] were used to predict the RNA-dependent RNA polymerase (RdRp) conserved domain of the viral sequence. Through multiple sequence alignments of the aa sequences of complete RdRp coding regions, sequences with identities of less than 90% [42] were considered to be potential novel virus species.
A multiple sequence alignment of the RdRp aa sequences of the Perinereis RNA viruses was performed using MAFFT (version 7.490, Research Institute for Microbial Diseases, Osaka University, Suita City, Osaka Prefecture, Japan, 2013) [43] with the LINS-i algorithm. TrimAl (version 1.2, Comparative Genomics group, Bioinformatics and Genomics Programme, Centre for Genomic Regulation, Barcelona, Spain, 2009) [44] was used to trim the sequences of the multiple sequence alignment. Phylogenetic analyses and the selection of the best-fitting model were performed using IQ-tree (version 2.1.4, Center for Integrative Bioinformatics Vienna, Max F. Perutz Laboratories, University of Vienna, Medical University of Vienna, Vienna, Austria, 2015) [45], with 1000 bootstrap replicates. The best-fitting model for phylogenetic analyses was LG + I + G.
3. Results
3.1. Composition and Diversity of the Perinereis Virome
After quality control, a total of 2.6 × 108 clean reads were obtained from the six high-throughput libraries, which were de novo assembled, and BLASTx identified 5.3 × 103 suspected virus-related contigs. Through the contig length, coverage, and other parameters of these virus sequences, we obtained 24 viral conserved sequences after removing redundant sequences, including 22 complete RdRp coding regions and two viral sequences encoding the N-terminal conserved region of hantavirus glycoprotein Gc (Table S1). Through a multiple sequence alignment of the aa sequences of 22 complete RdRp coding regions, we found that the aa identities of these domains and known viral domains were less than the threshold of 90% [42], and were considered to be potential novel virus species. Finally, a total of 12 viruses were obtained, including a segmented virus. These viruses belonged to four viral families: Picobirnaviridae (n = 1), unclassified Bunyavirales (n = 1), Marnaviridae (n = 7), and unclassified Picornavirales (n = 3) (Table S1).
These twelve viruses were categorized into three groups: ten viruses belong to positive-sense single-stranded RNA viruses, one virus belongs to negative-sense single-stranded RNA viruses, and one virus belongs to double-stranded RNA viruses (Table S1). The coverage of the novel RdRps identified here ranged from 11 to 906, and the abundance of different viruses in the six libraries was calculated based on RPKM and RPM in the ranges of 0.9–98.9 and 1.9–903.4, respectively (Table S1). BLASTp analysis of the complete RdRp protein for each virus revealed that the identities between the 12 novel viruses and the best hit for each virus ranged from 26.7% to 83.9%.
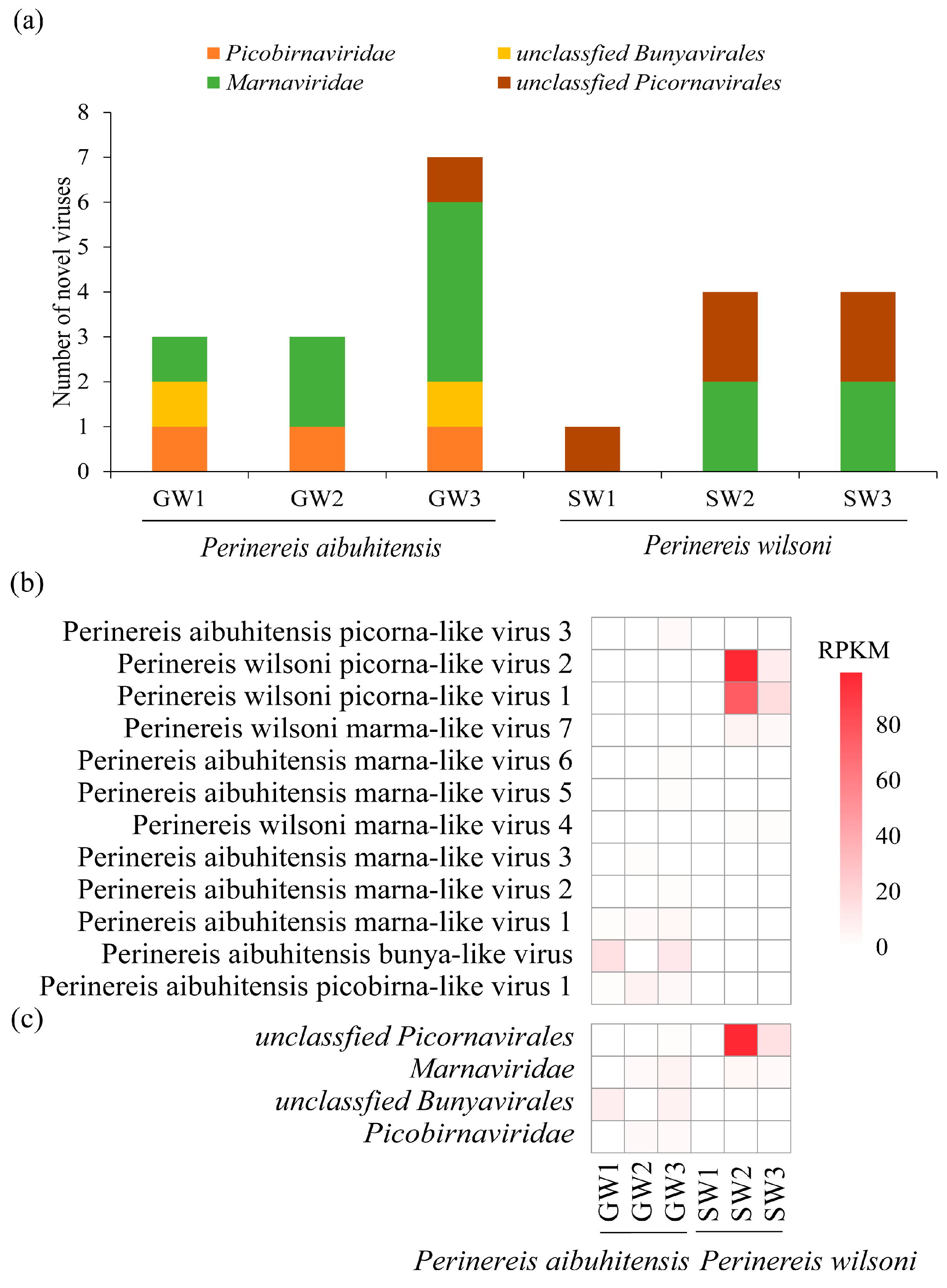
Eight novel viruses were discovered in P. aibuhitensis and four in P. wilsoni. P. aibuhitensis carried viruses from four viral families: Picobirnaviridae, unclassified Bunyavirales, Marnaviridae, and unclassified Picornavirales. The viruses found in P. wilsoni only included two virus categories: Marnaviridae and unclassified Picornavirales. Viruses from Marnaviridae and Picornavirales were presented in both species of Perinereis, with marna-like viruses distributed in two species and five libraries (Figure 1a). Picorna-like viruses have higher viral abundance than viruses from other families identified from Perinereis. Among the 12 viruses we identified, the RPKM and RPM values of Perinereis wilsoni picorna-like virus 1 and Perinereis wilsoni picorna-like virus 2 were relatively high. Perinereis wilsoni picorna-like virus 1 was detected in both the SW2 and SW3 libraries, with its RPKM value reaching up to 75.8 and RPM up to 692.9. Perinereis wilsoni picorna-like virus 2 was detected in three libraries, SW1, SW2, and SW3, with its RPKM values reaching up to 98.9 and RPM up to 903.4 (Figure 1b, Table S1). The abundance of viruses of the Picornavirales lineage (Perinereis wilsoni picorna-like virus 1 and Perinereis wilsoni picorna-like virus 2) carried by P. wilsoni was higher than that of P. aibuhitensis (Figure 1b,c). However, two viruses were found exclusively in P. aibuhitensis: Bunya-like virus in two libraries, and Picobirna-like virus in three libraries (Figure 1a).
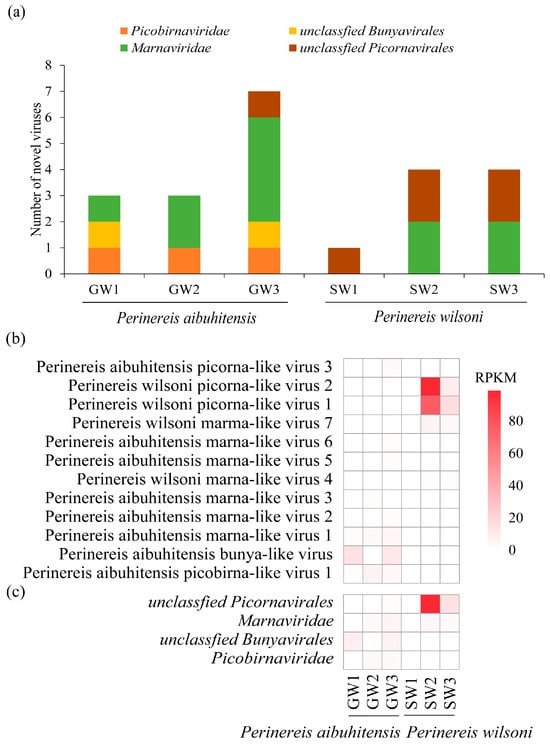
Figure 1.
Virus abundance and distribution in the six high-throughput libraries. (a) The distribution of the 12 viruses identified in this study in the libraries. The x-axis represents the six library names, and the y-axis represents the number of viruses. (b) The abundance of the 12 viruses. (c) The abundance of the four major viral families in the libraries. The red scale on the right side represents RPKM.
3.2. Phylogenetic Analysis of the Novel Viruses in the Order Picornavirales
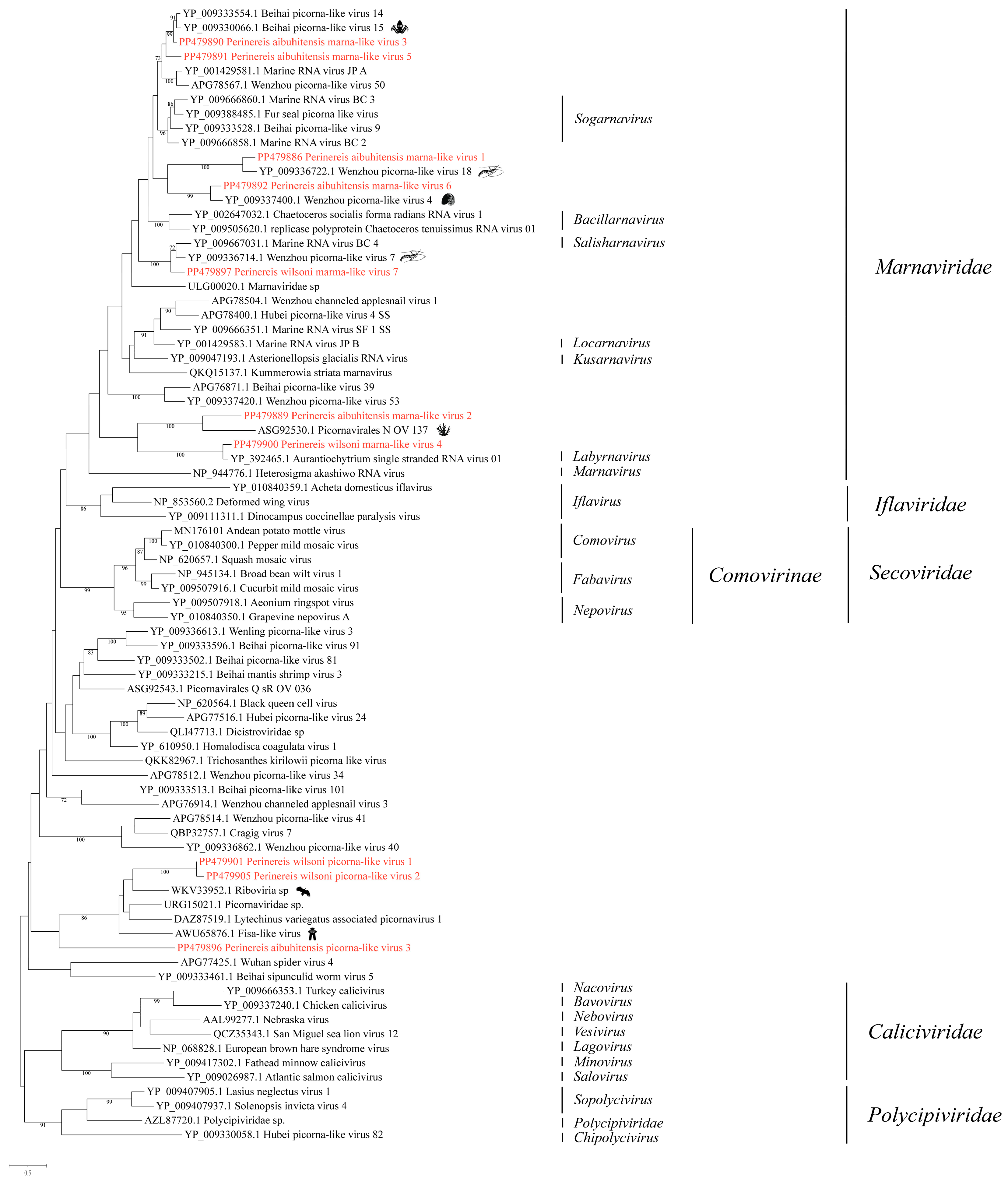
We obtained ten RNA viruses associated with Picornavirales, including seven marna-like viruses and three unclassified picornaviruses (Figure 2).
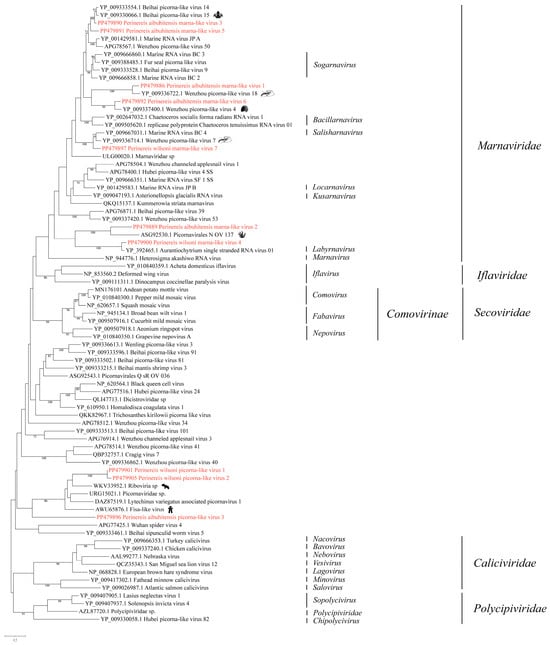
Figure 2.
Phylogenetic analysis of representative viruses in the order Picornavirales. The phylogenetic tree was constructed using MAFFT for sequence alignment, trimAl for sequence trimming, and then IQ-tree to identify the LG + I + G model as the best fit for constructing the Picornavirales phylogenetic tree. Only bootstrap values greater than 70.0% were shown. Novel viruses obtained from Perinereis were marked in red. The host of the viruses most closely related to the newly identified Perinereis viruses was provided as a cartoon.
3.2.1. Phylogenetic Analysis of the Novel Viruses in the Family Marnaviridae
From Perinereis samples, we identified seven RNA viruses related to the Marnaviridae, namely Perinereis aibuhitensis marna-like virus 1, Perinereis aibuhitensis marna-like virus 2, Perinereis aibuhitensis marna-like virus 3, Perinereis wilsoni marna-like virus 4, Perinereis aibuhitensis marna-like virus 5, Perinereis aibuhitensis marna-like virus 6, and Perinereis wilsoni marma-like virus 7. Five viruses were identified in P. aibuhitensis, and two were in P. wilsoni.
The best hit in the BLASTx analysis of the seven viruses revealed that RdRp aa sequence identities ranged from 44.1% to 83.9%. In order to further study the phylogenetic relationship of the seven viruses, phylogenetic analysis was performed using representative RdRp aa sequences from viruses recognized by the International Committee on Taxonomy of Viruses (ICTV) and unclassified Marnaviridae viruses (Figure 2). The seven novel viruses were placed in different branches within the Marnaviridae, thereby further revealing the diversity of the Marnaviridae.
In detail, Perinereis aibuhitensis marna-like virus 1 and Wenzhou picorna-like virus 18 (accession: YP_009336722.1) formed a distinct branch, with the RdRp protein identity of 68.8%. Perinereis wilsoni marma-like virus 7 and Wenzhou picorna-like virus 7 (accession: YP_009336714.1) were closely related, with the aa sequence identity in the RdRp protein reaching 70.5% (Table S1). Perinereis wilsoni marna-like virus 7 was tentatively determined to be within Marnaviridae. The aa sequence identity between Perinereis aibuhitensis marna-like virus 6 and Wenzhou picorna-like virus 4 (accession: YP_009337400.1) in the conserved domain was 65.2% (Figure 2, Table S1).
In addition, we revealed that the first BLASTx hit for Perinereis aibuhitensis marna-like virus 5 and Perinereis aibuhitensis marna-like virus 3 was Beihai picorna-like virus 15 (accession: YP_009330066.1), indicating that the two novel viruses were closely related. The close genetic and phylogenetic relationship further substantiated our conclusion. Phylogenetic analysis revealed that Perinereis aibuhitensis marna-like virus 3, Beihai picorna-like virus 15, and Beihai picorna-like virus 14 formed a distinct branch, which subsequently clustered with Perinereis aibuhitensis marna-like virus 5 (Figure 2). In fact, Perinereis aibuhitensis marna-like virus 3 and Beihai picorna-like virus 15 exhibited a high aa sequence identity of 83.9% in the RdRp conserved domain (Table S1).
Perinereis aibuhitensis marna-like virus 2 and Perinereis wilsoni marna-like virus 4 were evolutionarily distant to the other five marna-like viruses (Figure 2). These two viruses were closely associated with viruses in the unclassified Picornavirales. Perinereis aibuhitensis marna-like virus 2 and Picornavirales N_OV_137 (accession: ASG92530.1), discovered in the diatom, formed a distinct cluster branch. However, the RdRp protein aa sequence identity was only 44.1%. The aa sequence identity between the intact RdRp protein of Perinereis wilsoni marna-like virus 4 and Aurantiochytrium single-stranded RNA virus 01 (accession: YP_392465.1), which infects Schizochytrium sp., a unicellular eukaryote species in the family Thraustochytriaceae, was 81.6% (Figure 2, Table S1).
3.2.2. Phylogenetic Analysis of the Novel Viruses in Unclassified Picornavirales
Our research identified three novel viruses within the unclassified Picornavirales: two from P. wilsoni, named Perinereis wilsoni picorna-like virus 1 and Perinereis wilsoni picorna-like virus 2, and one from P. aibuhitensis, named Perinereis aibuhitensis picorna-like virus 3.
BLASTx analysis of the intact RdRp conserved domain of Perinereis aibuhitensis picorna-like virus 3 showed that the best hit was the Fisa-like virus (accession: AWU65876.1), which was consistent with the results of phylogenetic analysis, though with a low aa sequence identity of 26.7% in the intact RdRp protein. BLASTx analysis of the conserved domains of Perinereis wilsoni picorna-like virus 1 and Perinereis wilsoni picorna-like virus 2 revealed that the most similar strains for both viruses were Riboviria sp. (accession: WKV33952.1) identified from birds. Further phylogenetic analysis revealed that Perinereis wilsoni picorna-like virus 1 and Perinereis wilsoni picorna-like virus 2 clustered together and were subsequently grouped with Riboviria sp. (accession: WKV33952.1). The aa sequence identities of the intact RdRp proteins between the two viruses and Riboviria sp. were 39.7% and 39.1%, respectively (Figure 2, Table S1).
3.3. Phylogenetic Analysis of the Novel Viruses in the Family Picobirnaviridae
One virus identified from P. aibuhitensis fell within the family Picobirnaviridae, which was named Perinereis aibuhitensis picobirna-like virus 1. The best hit of the BLASTx analysis of the conserved domains was scractlig virus 3 (accession: UZT54506.1) from freshwater mussels. Phylogenetic analysis showed that Perinereis aibuhitensis picobirna-like virus 1 and scractlig virus 3 clustered together, sharing an aa sequence identity of 35.3% in the RdRp protein (Figure 3, Table S1).

Figure 3.
Phylogenetic analysis of the novel virus in the family Picobirnaviridae. The phylogenetic tree was constructed using MAFFT for sequence alignment, trimAl for sequence trimming, and then an IQ-tree to identify the LG + I + G model as the best fit for constructing the Picobirnaviridae phylogenetic tree. Only bootstrap values greater than 70.0% were shown. Novel viruses obtained from Perinereis were marked in red. The host of the virus most closely related to the newly identified Perinereis virus was provided as a cartoon.
3.4. Phylogenetic Analysis of the Novel Viruses in Unclassified Bunyavirales
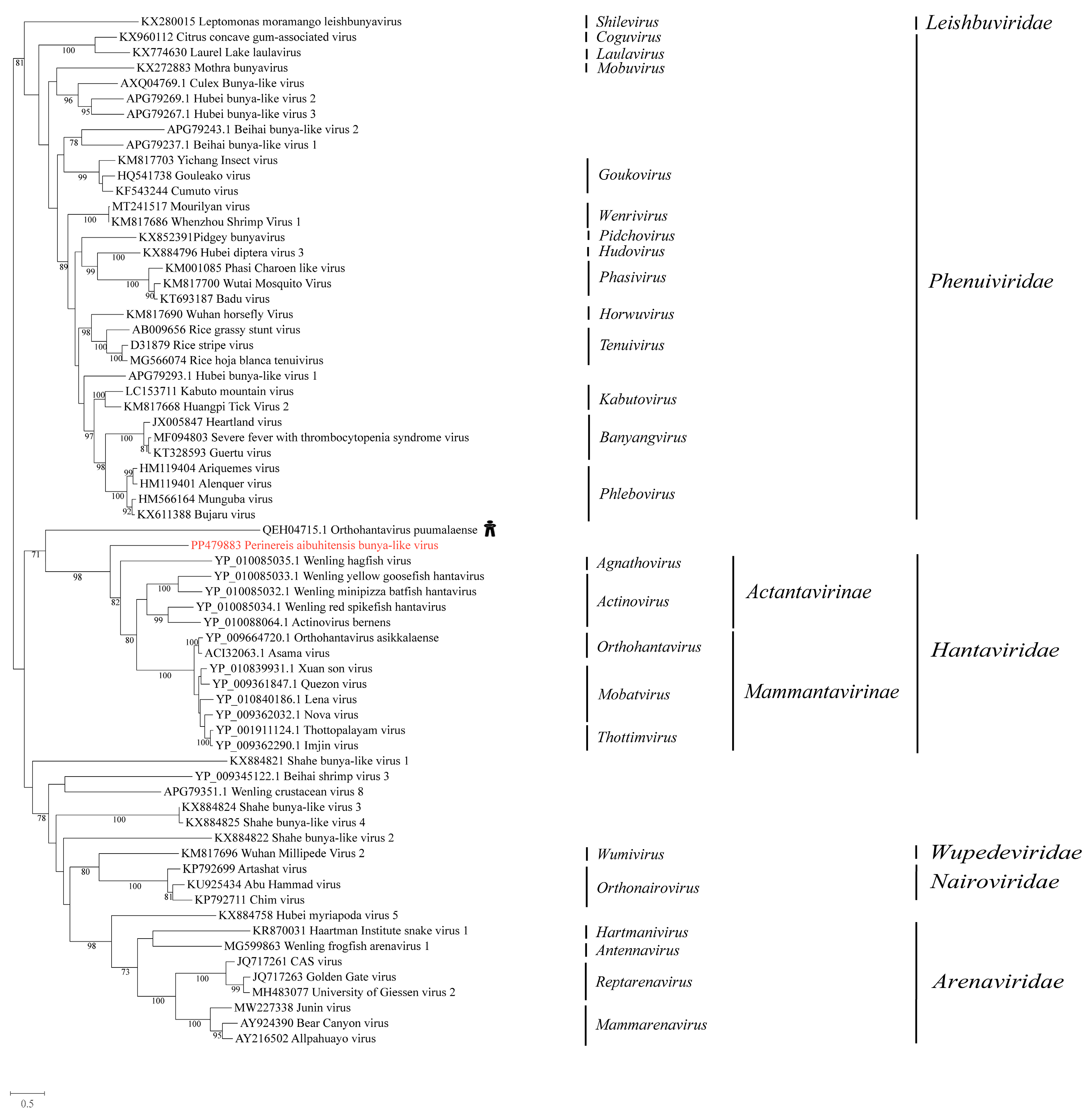
We obtained a bunyavirus from P. aibuhitensis, which was named Perinereis aibuhitensis bunya-like virus. The top hit in the BLASTx search was Orthohantavirus puumalaense (accession: QEH04715.1), a virus initially discovered in humans with human immunodeficiency virus infection. A comparison of the conserved domains between Perinereis aibuhitensis bunya-like virus and Orthohantavirus puumalaense revealed aa identity of 27.7%. A phylogenetic analysis of the RdRp domain indicated that Perinereis aibuhitensis bunya-like virus clustered with viruses of the family Hantaviridae (Figure 4).
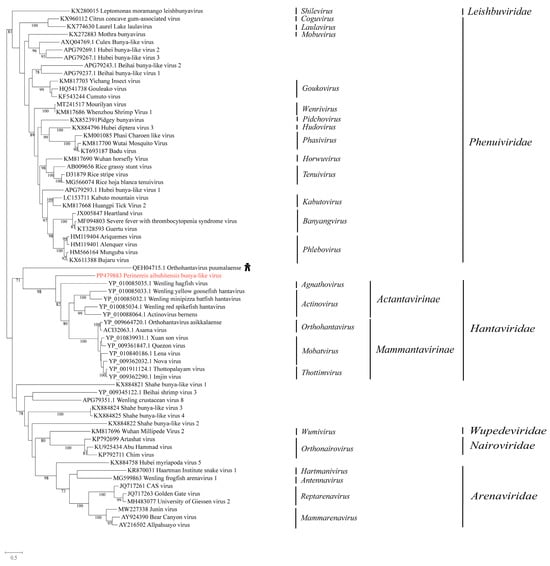
Figure 4.
Phylogenetic analysis of the novel virus in the order Bunyavirales. The phylogenetic tree was constructed using MAFFT for sequence alignment, trimAl for sequence trimming, and then an IQ-tree to identify the LG + I + G model as the best fit for constructing the Bunyavirales phylogenetic tree. Only bootstrap values greater than 70.0% were shown. Novel viruses obtained from Perinereis were marked in red. The host of the virus most closely related to the newly identified Perinereis virus was provided as a cartoon.
3.5. Genome Structures of the Perinereis Viruses
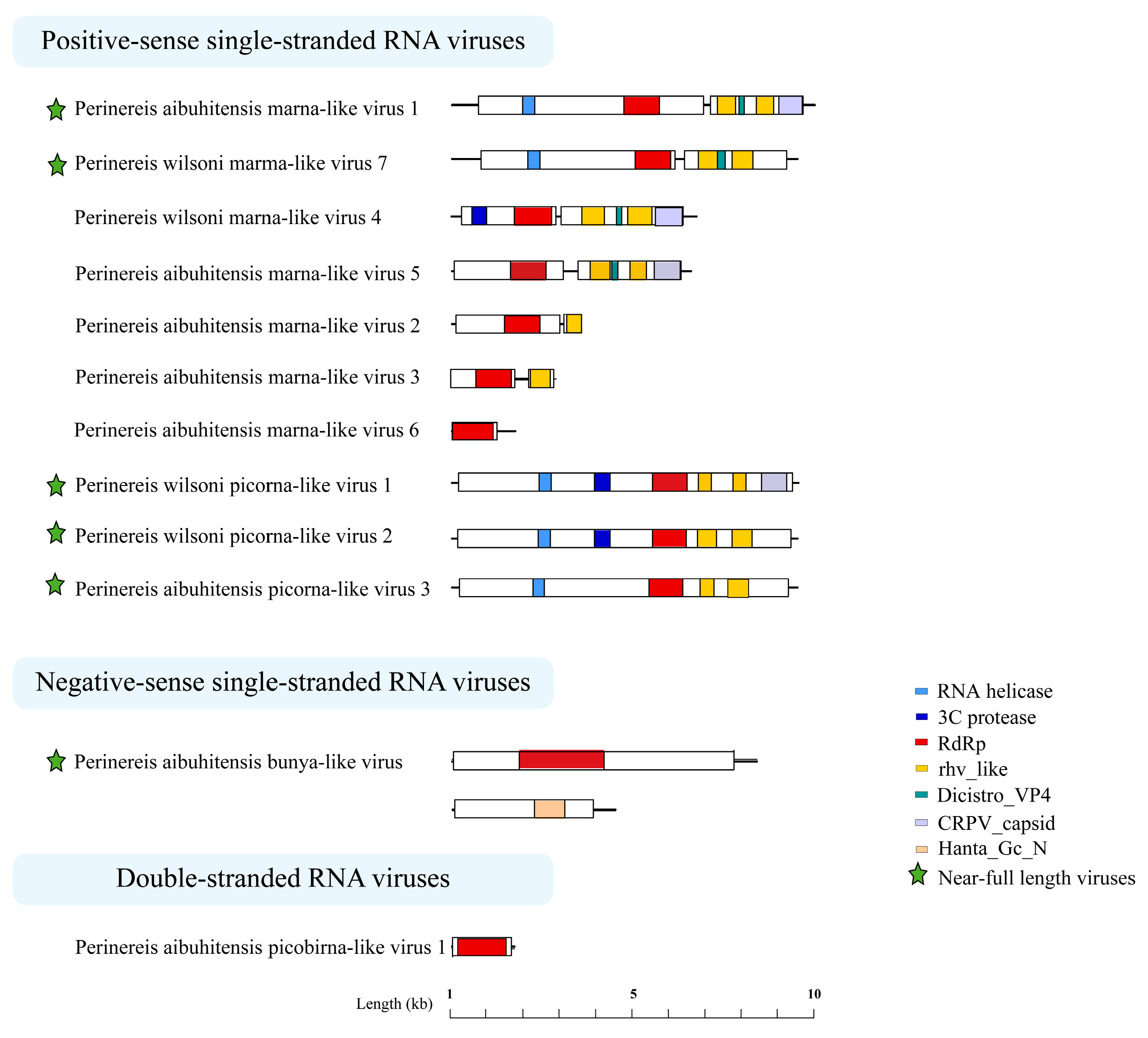
The Perinereis virus genome structures were diverse. Most members of the order Picornavirales have a positive-sense, single-stranded RNA molecule between 7000 and 12,500 nt in length. Five viruses were found in this study that belong to the family Marnaviridae, order Picornavirales. Three of them, including Perinereis aibuhitensis marna-like virus 1, Perinereis wilsoni marna-like virus 4, and Perinereis wilsoni marma-like virus 7, had a near-complete genome sequence between 8000 and 9200 nt in length, consisting of a 5′-Untranslated Region (UTR), ORF1, ORF2, and a 3′-UTR. ORF1 encoded the RNA helicase, 3 C_protease, and non-structural proteins associated with RdRp. ORF2 encoded rhv_like, Dicistrio_VP4, CRPV_capsid, and other structural proteins, which were also possessed by viruses in the family Marnaviridae [46] (Figure 5).
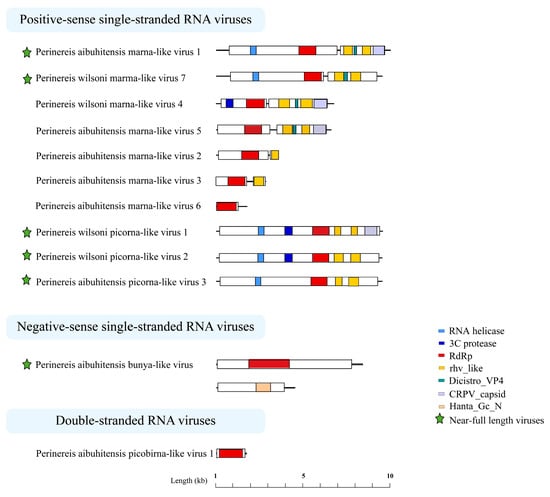
Figure 5.
Predicted genome structures of the 12 novel viruses identified from Perinereis spp. Seven conserved domains were predicted from 12 novel viruses obtained from Perinereis spp., annotated with seven colors. Each conserved domain corresponds to a color. RdRp: RNA-dependent RNA polymerase.
At the same time, we identified three viruses belonging to unclassified Picornavirales with near-complete genome structures, ranging from 9100 nt to 9200 nt in length. These viruses exhibited genome structures similar to those of viruses of the order Picornavirales [47], yet with slight differences. The ORFs of Perinereis aibuhitensis picorna-like virus 3 encoded RNA helicase, RdRp, and two rhv_like proteins, sharing the same genome structure as the Fisa-like virus (accession: AWU65876.1) found in human feces [48]. Perinereis wilsoni picorna-like virus 2 and Perinereis aibuhitensis picorna-like virus 3 were similar in overall genome structure. The difference was that the former encoded an additional 3C_protease domain, besides an RNA helicase, an RdRp, and two rhv_like proteins (Figure 5). Of particular note, Perinereis wilsoni picorna-like virus 2 had the highest abundance among the 12 viruses we identified, with a RPKM value of 98.9. Perinereis wilsoni picorna-like virus 1 also had a high abundance, with a RPKM value of 75.8. Compared with the structure of Perinereis aibuhitensis picorna-like virus 3, this virus encoded two more domains, i.e., 3C_protease and CRPV_capsid. Similarly, compared with Perinereis wilsoni picorna-like virus 2, it encoded one additional CRPV_capsid domain. These results indicated that the picorna-like viruses obtained from Perinereis had distinct genome structures.
In addition, we identified two segments from Perinereis aibuhitensis bunya-like virus, each encoding a distinct ORF. The L segment with a length of 8066 nt encoded the RdRp conserved domain between positions 1772 and 4006 nt; similarly, the M segment with a length of 4327 nt encoded the N-terminal conserved region of hantavirus glycoprotein Gc between positions 2178 and 2929 nt (Figure 5). This was consistent with the virus structure of the family Hantaviridae, published by ICTV [49].
4. Discussion
The field of marine virology is experiencing a rapid expansion, drawing increased attention due to its critical implications for marine industry safety and the health standards of aquatic products. The introduction of high-throughput sequencing technologies has revolutionized the discovery and characterization of novel marine viruses, marking a significant advancement in our understanding of marine virology [31,33,50]. Perinereis, commonly used as live feed in aquaculture, has emerged as a potential vector for virus transmission [8,11,12,13]. Research has established connections between polychaetes and viruses such as the WSSV, highlighting the intricate relationships within marine ecosystems [14,15]. The presence of Perinereis carrying - virus, such as that of WSSV, DIV1, and CMNV, has been associated with economic losses in aquaculture, underscoring the importance of studying virus transmission dynamics in marine environments [16,17,18,19,20,21,22,23,24]. While current research predominantly focuses on known viruses affecting aquaculture species [18,19,20,21,22,23,24], there remains a significant gap in the exploration of unknown Perinereis viruses. This underscores the urgent need to identify and comprehend these viruses to mitigate the risk of disease outbreaks and subsequent economic losses in the aquaculture industry. Transcriptomic approaches have emerged as powerful and efficient tools for virus discovery, offering a promising avenue for studying Perinereis diseases. However, their application in this context, particularly in the realm of bioinformatics, has been underutilized. Further exploration and utilization of transcriptomic tools hold great potential for enhancing our understanding of marine virology and improving disease management strategies in aquaculture settings.
In this study, we leveraged meta-transcriptomic sequencing to unearth a suite of novel viruses within Perinereis, enriching our understanding of their virome and providing crucial data to preemptively address potential biosecurity risks. The identification of 12 novel RNA viruses within Perinereis spp. significantly broadens the scope of the known virome associated with these annelids. Although there is no clear pathogenic risk among the 12 novel viruses identified, the detection of these viruses in both P. aibuhitensis and P. wilsoni raises the specter of interspecies transmission and necessitates further investigation into their pathogenic potential and implications for aquaculture. In addition, combined with the previous paper [51] and private communication between the authors, we further analyzed of the correlation between the RPM value of RNA viruses and copies resulting from the fluorescence quantitative PCR Ct value, and regressed a trend relationship between RPM and virus copies (VCs), which is VCs (copies/ng-RNA) = 0.7401 × RPM0.8621. Considering that higher viral loads in the host are more likely caused by infection, we speculated that a virus with an RPM value greater than 300 may be equivalent to a virus load above 100 copies/ng-RNA in the tissue, of which the presence of the virus may be considered an infection but not a contamination. In our present study, the RPM values of two viruses, i.e., Perinereis wilsoni picorna-like virus 1 and Perinereis wilsoni picorna-like virus 2, were above 600, which suggested that these two viruses might be highly copied in the samples. However, more experiments are needed to confirm the activity of these viruses and to further assess the risk.
The wide geographical distribution of Perinereis spp. spans the Indo-Pacific to the southeastern Atlantic [52,53]. The presence of diverse RNA viruses in P. aibuhitensis, a dominant species in intertidal zones, hints at the potential for widespread viral dissemination through the food chain, facilitated by ocean currents. Moreover, the increasing reliance on aquaculture and the trade of aquatic products, including the use of Perinereis as a live feed, may inadvertently amplify the risk of viruses spread across international borders [16,54]. The limited current understanding of Perinereis viruses serves as a reminder of the vast, uncharted virome that awaits discovery and characterization. This study not only contributes to the foundational knowledge of Perinereis virology, but also emphasizes the need for a proactive approach to managing the health of our marine ecosystems and the sustainability of our aquaculture industries.
5. Conclusions
In this study, we identified and characterized 12 novel RNA viruses from Perinereis, spanning four major viral families: Picobirnaviridae, Marnaviridae, and unclassified members of Picornavirales and Bunyavirales. Our research uncovered the tip of the iceberg of the RNA virome diversity within Perinereis. The discovery of these novel viruses provides critical insights into the biosecurity risks of using Perinereis in aquaculture.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/vetsci11060273/s1, Table S1: Diversity of viruses in Perinereis.
Author Contributions
Conceptualization, X.D. and J.L.; methodology, X.D., J.L., F.Z., and F.M.; software, J.L.; validation, F.Z. and C.Z.; formal analysis, J.L., F.Z., X.D., and J.H.; investigation, X.D., L.Q., J.L., and G.W.; resources, X.D. and J.H.; data curation, J.L., F.Z., C.Z., and F.M.; writing—original draft preparation, X.D. and J.L.; writing—review and editing, X.D., W.S., and J.H.; visualization, J.L., F.Z., C.Z., X.D., and W.S.; supervision, X.D. and J.H.; project administration, X.D. and J.H.; funding acquisition, X.D. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2023YFD2402200), Qingdao Municipal Natural Science Foundation (23-2-1-181-zyyd-jch), Central Public Interest Scientific Institution Basal Research Fund, CAFS (NO. 2023TD42), and China Agriculture Research Systems (CARS-48).
Institutional Review Board Statement
The Ethics Committee of the Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, reviewed and approved this animal study. ID Number: YSFRI-2019007.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data obtained from the meta-transcriptomic sequencing conducted in this study can be accessed at the NCBI Sequence Read Archive (SRA) database with the BioProject accession number PRJNA1088991. The viral sequences derived from this study have been deposited in GenBank with the accession numbers PP479882-PP479905 (Table S1).
Acknowledgments
We thank Ting Li for her help with the sampling.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wilson, R.S.; Glasby, C.J.; Bakken, T. The Nereididae (Annelida)—Diagnoses, descriptions, and a key to the genera. ZooKeys 2023, 1182, 35. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Guerrero, T.F. Redescription of ttwo overlooked species of the Perinereis Nuntia complex and morphological delimitation of P. Nuntia (Savigny in Lamarck, 1818) from the Red Sea (Annelida, Nereididae). Zoosystema 2019, 41, 465–496. [Google Scholar] [CrossRef]
- Elgetany, A.H.; Struck, T.H.; Glasby, C.J. Three new species of the genus Perinereis (Annelida, Nereididae) from egyptian coasts. ZooKeys 2022, 1132, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Guerrero, T.F.; Park, T.; Idris, I. Review of some Perinereis Kinberg, 1865 (Annelida: Nereididae) species of group 2 sensu Hutchings, Reid & Wilson, 1991 from the eastern and south-eastern Asian seas. J. Mar. Biol. Assoc. UK 2021, 101, 279–307. [Google Scholar] [CrossRef]
- Conde-Vela, V.M. Reinstatement of Perinereis Bairdii (Webster, 1884) and description of P. Websteri Sp. Nov. from Bermuda, including the reproductive morphology of two Atlantic Perinereis Species (Annelida: Errantia: Phyllodocida). Eur. J. Taxon. 2021, 787, 104–145. [Google Scholar] [CrossRef]
- Sakurai, T.; Kobayashi, J.; Ito, N.; Serizawa, S.; Shiraishi, H.; Yabe, T.; Ishii, Y.; Suzuki, N. Respiratory uptake and depuration kinetics of perfluorooctanesulfonate (PFOS) in a marine sandworm species. Bull. Environ. Contam. Toxicol. 2017, 99, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, J.; Jiang, Z.; Du, M.; Liu, Y.; Mao, Y.; Gao, Y.; Fang, J. Environmental remediation potential of Perinereis aibuhitensis (Polychaeta) based on the effects of temperature and feed types on its carbon and nitrogen budgets. Mar. Biol. Res. 2016, 12, 583–594. [Google Scholar] [CrossRef]
- Wouters, R.; Lavens, P.; Nieto, J.; Sorgeloos, P. Penaeid shrimp broodstock nutrition: An updated review on research and development. Aquaculture 2001, 202, 1–21. [Google Scholar] [CrossRef]
- Moussa Dorgham, M.; Hamdy, R.; Hassan, H.; Rasheedy, A.; Mohamed Atta, M. Biochemical composition of the nereidid Perinereis cultrifera from the Egyptian Mediterranean coast. Rev. Biol. Mar. Oceanogr. 2015, 50, 535–543. [Google Scholar] [CrossRef]
- Techaprempreecha, S.; Khongchareonporn, N.; Chaicharoenpong, C.; Aranyakananda, P.; Chunhabundit, S.; Petsom, A. Nutritional composition of farmed and wild sandworms, Perinereis Nuntia. Fuel Energy Abstr. 2011, 169, 265–269. [Google Scholar] [CrossRef]
- Lytle, J.S.; Lytle, T.F.; Ogle, J.T. Polyunsaturated fatty acid profiles as a comparative tool in assessing maturation diets of Penaeus Vannamei. Aquaculture 1990, 89, 287–299. [Google Scholar] [CrossRef]
- Lim, J.A.; Loo, P.L.; Tan, K.S.; Ng, N.K. Fish culture waste improves growth and fatty acid composition of the Polychaete Namalycastis sp. (Nereididae) and its potential use as feed for mud crabs. Aquac. Res. 2021, 52, 2622–2639. [Google Scholar] [CrossRef]
- Olive, P. Polychaeta as a World Resource: A review of patterns of exploitation as sea angling baits and the potential for aquaculture based production. Mem. Mus. Natn. Hist. Nat. 1994, 162, 23–27. [Google Scholar]
- Vijayan, K.; Raj, V.S.; Balasubramanian, C.; Alavandi, S.; Sekhar, V.T.; Santiago, T. Polychaete Worms—A vector for white spot syndrome virus (WSSV). Dis. Aquat. Organ. 2005, 63, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Desrina, H. On the Role of the Polychaete Dendronereis Spp. in the Transmission of White Spot Syndrome Virus in Shrimp Ponds; Wageningen University: Wageningen, The Netherlands, 2014. [Google Scholar]
- Flegel, T.; Fegan, D.F. Strategies for preventing the spread of fish and shellfish diseases. Fish. Sci. 2002, 68, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Flegel, T.W. RNA-Seq data analysis in galaxy. ScienceAsia 2006, 32, 215. [Google Scholar] [CrossRef]
- Thitamadee, S.; Prachumwat, A.; Srisala, J.; Jaroenlak, P.; Salachan, P.V.; Sritunyalucksana, K.; Flegel, T.W.; Itsathitphaisarn, O. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 2016, 452, 69–87. [Google Scholar] [CrossRef]
- WOAH. Report of the Meeting of WOAH Aquatic Animal Health Standards Commission. Available online: https://www.woah.org/app/uploads/2022/11/a-aac-sep-2022-report.pdf (accessed on 22 May 2024).
- Haryadi, D.; Verreth, J.a.J.; Verdegem, M.C.J.; Vlak, J.M. Transmission of white spot syndrome sirus (WSSV) from Dendronereis Spp. (Peters) (Nereididae) to Penaeid shrimp. J. Fish Dis. 2015, 38, 419–428. [Google Scholar] [CrossRef] [PubMed]
- NACA. Disease Advisory: Decapod Iridescent Virus 1 (DIV1): An Emerging Threat to the Shrimp Industry. Available online: https://enaca.org/?id=1098&title=decapod-iridescent-virus-1-an-emerging-threat-to-the-shrimpindustry (accessed on 22 May 2024).
- Qiu, L.; Chen, M.M.; Wan, X.Y.; Li, C.; Zhang, Q.L.; Wang, R.Y.; Cheng, D.Y.; Dong, X.; Yang, B.; Wang, X.H.; et al. Characterization of a new member of Iridoviridae, shrimp hemocyte iridescent virus (SHIV), found in white leg shrimp (Litopenaeus vannamei). Sci. Rep. 2017, 7, 11834. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, M.M.; Wan, X.Y.; Zhang, Q.L.; Li, C.; Dong, X.; Yang, B.; Huang, J. Detection and quantification of shrimp hemocyte iridescent virus by TaqMan probe based real-time PCR. J. Invertebr. Pathol. 2018, 154, 95–101. [Google Scholar] [CrossRef]
- Zhao, W.X.; Wan, X.Y.; Xia, J.T.; Yao, L.; Xv, R.D.; Wang, W.; Yu, X.T.; Zhang, Q.L. Investigation of the prevalence of covert mortality nodavirus (CMNV) in shrimp from 2021 to 2022. Prog. Fish. Sci. 2024, 45, 1–10. [Google Scholar] [CrossRef]
- Devauchelle, G. Ultrastructural characterization of an iridovirus from the marine worm Nereis diversicolor (O.F. Müller). Virology 1977, 81, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo E Costa, P.; Gil, J.; Passos, A.M.; Pereira, P.; Melo, P.; Batista, F.; Cancela Da Fonseca, L. The market features of imported non-indigenous Polychaetes in Portugal and consequent ecological concerns. Sci. Mar. 2006, 70, 287–292. [Google Scholar] [CrossRef]
- Haditomo, A.H.C.; Chilmawati, D. The white sport syndrome virus (WSSV) load in Dendronereis spp. 2012. [Google Scholar]
- Prastowo, B.W.; Ariawan, K.; Nur, E.M.; Rahardianti, R. Identification of Polychaete worm, Nereis Sp. as a white spot syndrome virus (WSSV) vector in their habitat and challenge test in the laboratory. J. Fish. Sci. 2009, 11, 183–191. [Google Scholar]
- Yang, M.; Zeng, C.; Xia, J.; Liu, Q.; Fang, J.; Zhang, Q. Disinfection of Perinereis aibuhitensis eggs with peroxymonosulfate to eliminate covert mortality nodavirus (CMNV). Aquaculture 2023, 572, 739539. [Google Scholar] [CrossRef]
- Palacios, G.; Druce, J.; Du, L.; Tran, T.; Birch, C.; Briese, T.; Conlan, S.; Quan, P.-L.; Hui, J.; Marshall, J.; et al. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 2008, 358, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.D.; Tian, J.H.; Chen, L.J.; Chen, X.; Li, C.X.; Qin, X.C.; Li, J.; Cao, J.P.; Eden, J.S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Bikel, S.; Valdez-Lara, A.; Cornejo-Granados, F.; Rico, K.; Canizales-Quinteros, S.; Soberón, X.; Del Pozo-Yauner, L.; Ochoa-Leyva, A. Combining metagenomics, metatranscriptomics and viromics to explore novel Microbial Interactions: Towards a Systems-Level Understanding of Human Microbiome. Comput. Struct. Biotechnol. J. 2015, 13, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, Y.Z.; Holmes, E.C. Meta-transcriptomics and the evolutionary biology of RNA viruses. Virus Res. 2018, 243, 83–90. [Google Scholar] [CrossRef]
- Dong, X.; Hu, T.; Liu, Q.; Li, C.; Sun, Y.; Wang, Y. A novel hepe-like virus from farmed giant freshwater prawn Macrobrachium Rosenbergii. Viruses 2020, 3, 323. [Google Scholar] [CrossRef]
- Dong, X.; Li, C.; Wang, Y.; Hu, T.; Zhang, F.; Meng, F.; Gao, M.; Han, X.; Wang, G.; Qin, J.; et al. Diversity and connectedness of brine shrimp viruses in global hypersaline ecosystems. Sci. China Life Sci. 2024, 67, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- NCBI. ORFfinder Home. Available online: https://www.ncbi.nlm.nih.gov/orffinder (accessed on 23 March 2024).
- NCBI. Conserved Domain Search. Available online: https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi (accessed on 23 March 2024).
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- InterPro. Available online: https://www.ebi.ac.uk/interpro/ (accessed on 23 March 2024).
- Edgar, R.C.; Taylor, B.; Lin, V.; Altman, T.; Barbera, P.; Meleshko, D.; Lohr, D.; Novakovsky, G.; Buchfink, B.; Al-Shayeb, B.; et al. Petabase-scale sequence alignment catalyses viral discovery. Nature 2022, 602, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale Phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.S.; Vlok, M.; Culley, A.I.; Suttle, C.A.; Takao, Y.; Tomaru, Y. ICTV report consortium ICTV virus taxonomy profile: Marnaviridae 2021. J. Gen. Virol. 2021, 102, 001633. [Google Scholar] [CrossRef]
- Picornavirales. ICTV. Available online: https://ictv.global/report_9th/RNApos/Picornavirales (accessed on 1 February 2024).
- Siqueira, J.D.; Dominguez-Bello, M.G.; Contreras, M.; Lander, O.; Caballero-Arias, H.; Xutao, D.; Noya-Alarcon, O.; Delwart, E. Complex virome in feces from Amerindian children in isolated Amazonian villages. Nat. Commun. 2018, 9, 4270. [Google Scholar] [CrossRef]
- Laenen, L.; Vergote, V.; Calisher, C.; Klempa, B.; Klingström, J.; Kuhn, J.; Maes, P. Hantaviridae: Current classification and future perspectives. Viruses 2019, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A. Marine viruses—Major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, W.; Tian, X.; Hou, X.; Cui, X.; Xiao, Y.; Jiao, Q.; Zhou, P.; Liu, L.; Shi, W.; et al. A total infectome approach to understand the etiology of infectious disease in pigs. Microbiome 2022, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- WoRMS. World Register of Marine Species—Perinereis Wilsoni Glasby & Hsieh, 2006. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=390131 (accessed on 18 March 2024).
- WoRMS. World Register of Marine Species—Perinereis Aibuhitensis (Grube, 1878). Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=716016 (accessed on 18 March 2024).
- CRAB. Live Saltwater Bait and the Introduction of Non-Native Species into California. Available online: https://www.opc.ca.gov/webmaster/ftp/project_pages/AIS/AIS_LiveBait.pdf (accessed on 18 March 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).