Cytological Features of Inflammatory Mammary Carcinoma in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Cytological Examination
2.3. Data Analysis
3. Results
3.1. Caseload
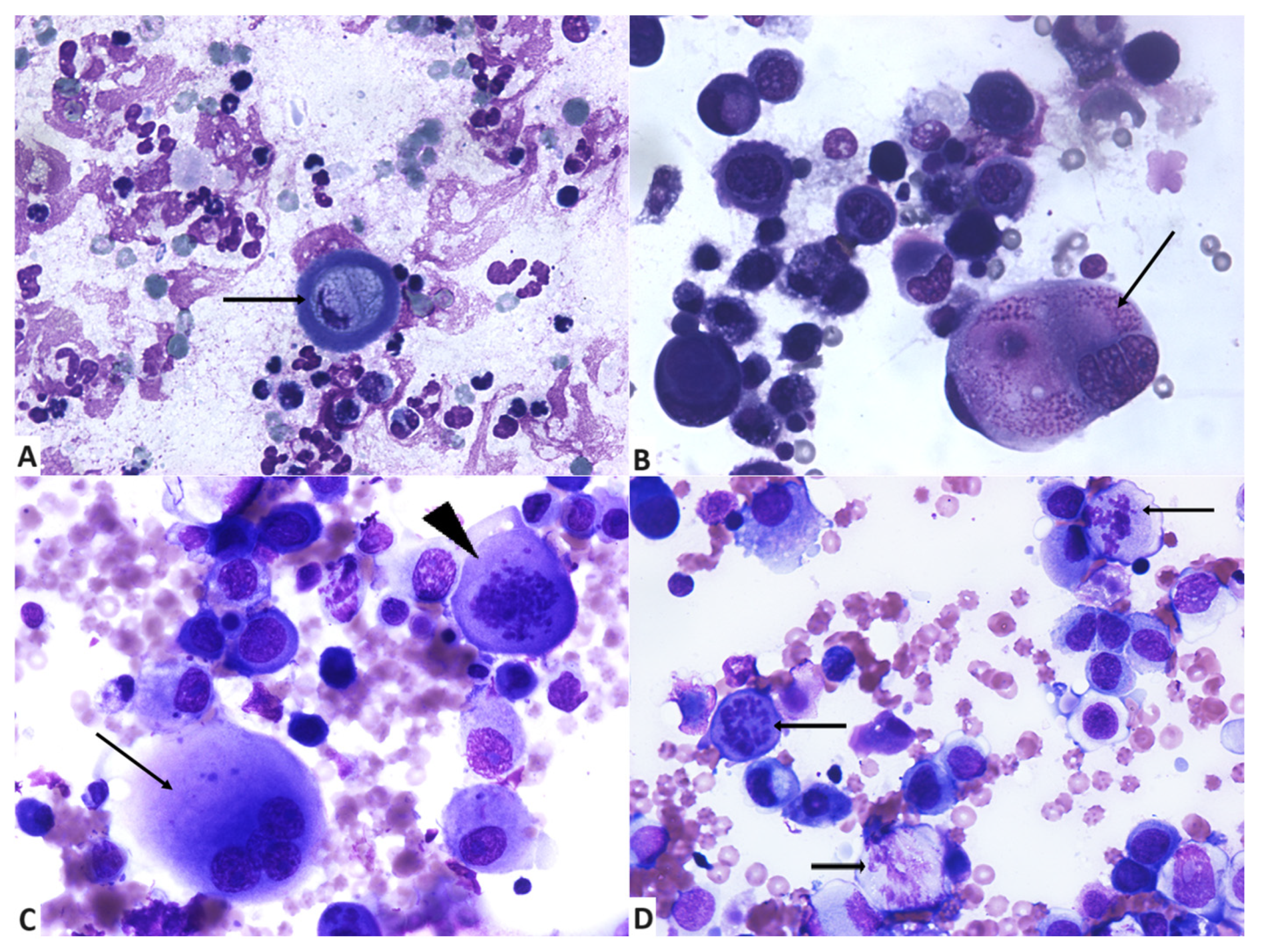
3.2. Cytologycal Smears and Cellular Characteristics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cringanu, D.; Preda, C.; Cringanu, I.; Negreanu, R. Can It May Be a Mixed Neoplasia with the Component of Both Carcinomatous Mastitis and T-Cell Lymphoma with Skin Location—Comparative Study. Sci. Work. 2022, LXVIII, 51–55. [Google Scholar]
- Peña, L.; Perez-Alenza, M.D.; Rodriguez-Bertos, A.; Nieto, A. Canine Inflammatory Mammary Carcinoma: Histopathology, Immunohistochemistry and Clinical Implications of 21 Cases. Breast Cancer Res. Treat. 2003, 78, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alenza, M.D.; Jiménez, A.; Nieto, A.I.; Peña, L. First Description of Feline Inflammatory Mammary Carcinoma: Clinicopathological and Immunohistochemical Characteristics of Three Cases. Breast Cancer Res. 2004, 6, R300–R307. [Google Scholar] [CrossRef]
- Millanta, F.; Verin, R.; Asproni, P.; Giannetti, G.; Poli, A. A Case of Feline Primary Inflammatory Mammary Carcinoma: Clinicopathological and Immunohistochemical Findings. J. Feline Med. Surg. 2012, 14, 420–423. [Google Scholar] [CrossRef]
- Pérez-Alenza, M.; Enrique, T.; Peña, L. Inflammatory Mammary Carcinoma in Dogs: 33 Cases (1995–1999). J. Am. Vet. Med. Assoc. 2001, 219, 1110–1114. [Google Scholar] [CrossRef]
- De Mello Souza, C.; Toledo-Piza, E.; Amorin, R.; Nardi, A.; Tobias, K. Inflammatory Mammary Carcinoma in 12 Dogs: Clinical Features, Cyclooxygenase-2 Expression, and Response to Piroxicam Treatment. Can. Vet. J. 2009, 50, 506–510. [Google Scholar]
- Da Silva, D.M.; Kluthcovsky, L.C.; Jensen de Morais, H.; Pallú, G.M.; Dos Santos, G.C.; Costa Castro, J.L.; Engracia Filho, J.R. Inflammatory Mammary Carcinoma in a Male Dog—Case Report. Top. Companion Anim. Med. 2019, 37, 100357. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.; De Andrés, P.J.; Peña, L.; Perez-Alenza, M.D. Survival Time of Dogs with Inflammatory Mammary Cancer Treated with Palliative Therapy Alone or Palliative Therapy plus Chemotherapy. Vet. Rec. 2009, 165, 78–81. [Google Scholar] [CrossRef]
- Alonso-Miguel, D.; Valdivia, G.; García-San José, P.; Alonso-Diez, Á.; Clares, I.; Portero, M.; Peña, L.; Pérez-Alenza, M.D. Clinical outcome of dogs diagnosed with canine inflammatory mammary cancer treated with metronomic cyclophosphamide, a cyclooxygenase-2 inhibitor and toceranib phosphate. Vet. Comp. Oncol. 2022, 20, 179–188. [Google Scholar] [CrossRef]
- Rossi, F.; Sabattini, S.; Vascellari, M.; Marconato, L. The impact of toceranib, piroxicam and thalidomide with or without hypofractionated radiation therapy on clinical outcome in dogs with inflammatory mammary carcinoma. Vet. Comp. Oncol. 2018, 16, 497–504. [Google Scholar] [CrossRef]
- Clemente, M.; Perez-Alenza, M.D.; Peña, L. Metastasis of Canine Inflammatory versus Non-Inflammatory Mammary Tumours. J. Comp. Pathol. 2010, 143, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Luiza Silveira, T.; Carneiro, R.; Lavalle, G.; Rodrigues, M.; Cassali, G. Bone Marrow Metastasis in Inflammatory Mammary Carcinoma: A Case Report in Dog. Adv. Anim. Vet. Sci. 2020, 8, 1087–1090. [Google Scholar] [CrossRef]
- Raskin, R.E. Chapter 2—General Categories of Cytologic Interpretation. In Canine and Feline Cytopathology, 4th ed.; Raskin, R.E., Meyer, D.J., Boes, K.M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2023; pp. 15–34. [Google Scholar] [CrossRef]
- Dolka, I.; Czopowicz, M.; Gruk-Jurka, A.; Wojtkowska, A.; Sapierzyński, R.; Jurka, P. Diagnostic Efficacy of Smear Cytology and Robinson’s Cytological Grading of Canine Mammary Tumors with Respect to Histopathology, Cytomorphometry, Metastases and Overall Survival. PLoS ONE 2018, 13, e0191595. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shukla, S.; Shukla, B.P.; Shrivastava, N.; Jatav, G.P.; Patel, B.J.; Raval, S.; Shrivastava, S.; Shrivastava, S.; Narad, A. Comparative Cytology, Histopathology, Immunohistochemistry and Molecular Study of Canine Tumours. Indian J. Vet. Pathol. 2019, 43, 240. [Google Scholar] [CrossRef]
- Lana, S.E.; Rutteman, G.R.; Withrow, S.J. Chapter 26—Tumors of the Mammary Gland. In Withrow & MacEwen’s Small Animal Clinical Oncology, 4th ed.; Withrow, S.J., Vail, D.M., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2007; pp. 619–636. [Google Scholar] [CrossRef]
- Pierini, A.; Millanta, F.; Zanforlin, R.; Vannozzi, I.; Marchetti, V. Usefulness of Cytologic Criteria in Ultrasound-Guided Fine-Needle Aspirates from Subcentimeter Canine Mammary Tumors. J. Vet. Diagn. Investig. 2017, 29, 869–873. [Google Scholar] [CrossRef]
- Bansal, C.; Singh, U.S.; Misra, S.; Sharma, K.L.; Tiwari, V.; Srivastava, A.N. Comparative evaluation of the modified Scarff-Bloom-Richardson grading system on breast carcinoma aspirates and histopathology. Cytojournal 2012, 9, 4. [Google Scholar] [CrossRef]
- White, M.; Rasotto, R.; Monti, P. Use of Cytology for Canine Mammary Masses and Perceived Diagnostic Utility in Four European Countries. J. Small Anim. Pract. 2022, 63, 312–319. [Google Scholar] [CrossRef]
- Militaru, M.; Ciobotaru, E.; Dinescu, G.; Diaconescu, A.; Ionascu, I.; Cranganu, D. Cytological Investigation of Mammary Gland Tumours in the Queen and Bitch. J. Comp. Pathol. 2010, 143, 352. [Google Scholar] [CrossRef]
- Hellmén, E.; Lindgren, A. The Accuracy of Cytology in Diagnosis and DNA Analysis of Canine Mammary Tumours. J. Comp. Pathol. 1989, 101, 443–450. [Google Scholar] [CrossRef]
- Cassali, G.D.; Gobbi, H.; Malm, C.; Schmitt, F.C. Evaluation of Accuracy of Fine Needle Aspiration Cytology for Diagnosis of Canine Mammary Tumours: Comparative Features with Human Tumours. Cytopathology 2007, 18, 191–196. [Google Scholar] [CrossRef]
- Haziroglu, R.; Yardimci, B.; Aslan, S.; Yildirim, M.Z.; Yumusak, N.; Beceriklisoy, H.; Agaoglu, R.; Küçükaslan, İ. Cytological Evaluation of Canine Mammary Tumours with Fine Needle Aspiration Biopsy Technique. Revue Méd. Vét. 2010, 161, 212–218. [Google Scholar]
- Allen, S.W.; Prasse, K.W.; Mahaffey, E.A. Cytologie Differentiation of Benign from Malignant Canine Mammary Tumors. Vet. Pathol. 1986, 23, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Colodel, M.; Ferreira, I.; Figueiroa, F.; Rocha, N. Efficacy of Fine Needle Aspiration in the Diagnosis of Spontaneous Mammary Tumors. Vet. Zootec. 2012, 19, 557–563. [Google Scholar]
- Simon, D.; Schoenrock, D.; Nolte, I.; Baumgärtner, W.; Barron, R.; Mischke, R. Cytologic Examination of Fine-Needle Aspirates from Mammary Gland Tumors in the Dog: Diagnostic Accuracy with Comparison to Histopathology and Association with Postoperative Outcome. Vet. Clin. Pathol. 2009, 38, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Sontas, H.; Yuzbasioglu, G.; Toydemir, S.; Arun, S.S.; Ekici, H. Fine-Needle Aspiration Biopsy of Canine Mammary Gland Tumours: A Comparison Between Cytology and Histopathology. Reprod. Domest. Anim. 2012, 47, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Romanelli, G.; Stefanello, D.; Giacoboni, C.; Bonfanti, U.; Bettini, G.; Finotello, R.; Verganti, S.; Valenti, P.; Ciaramella, L.; et al. Prognostic Factors for Dogs with Mammary Inflammatory Carcinoma: 43 Cases (2003–2008). J. Am. Vet. Med. Assoc. 2009, 235, 967–972. [Google Scholar] [CrossRef]
- Gangwar, K.; Yadav, B.K.; Srivastav, A.; Negi, A.; Suresh, C.P.; Pandey, H.; Gangwar, N.; Prabhu, S.N.; Singh, R.; Yadav, K. Epidemiological, Cytological, and Haemato-Serological Analysis of Canine Mammary Gland Tumours. Int. J. Adv. Biochem. Res. 2024, 8, 127–133. [Google Scholar] [CrossRef]
- Miller, M.A.; Lyle, L.T.; Zachary, J.F. Chapter 1—Mechanisms and Morphology of Cellular Injury, Adaptation, and Death. In Pathologic Basis of Veterinary Disease, 7th ed.; Zachary, J.F., Ed.; W.B. Saunders: Saint Louis, MO, USA, 2022; pp. 16–73. [Google Scholar]
- Bratulić, M.; Grabarevic, Z.; Artuković, B.; Capak, D. Number of Nucleoli and Nucleolar Organizer Regions per Nucleus and Nucleolus—Prognostic Value in Canine Mammary Tumors. Vet. Pathol. 1996, 33, 527–532. [Google Scholar] [CrossRef]

| Examined Criteria | Score | No. of Samples (%) |
|---|---|---|
| Cell size | Small | 0 (0%) |
| Medium | 6 (24%) | |
| Large | 19 (76%) | |
| Squamous metaplasia | − | 12 (48%) |
| + | 10 (40%) | |
| + + | 2 (8%) | |
| + + + | 1 (4%) | |
| Cellular cohesiveness | Low | 16 (64%) |
| Moderate | 4 (16%) | |
| High | 5 (20%) |
| Cytological Features | Samples without Squamous Metaplasia | Samples with Squamous Metaplasia | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Cellularity | Poor | Moderate | High | Poor | Moderate | Good | 0.163 |
| 1 | 2 | 9 | 3 | 3 | 7 | ||
| Cohesiveness | Low | Moderate | High | Low | Moderate | High | 0.032 * |
| 5 | 3 | 4 | 11 | 1 | 1 | ||
| Examined Criteria | Score | No. of Samples (%) |
|---|---|---|
| Anisokaryosis | − | 0 (0%) |
| + | 5 (20%) | |
| + + | 6 (24%) | |
| + + + | 14 (56%) | |
| Abnormal nuclear shape | − | 1 (4%) |
| + | 7 (28%) | |
| + + | 10 (40%) | |
| + + + | 7 (28%) | |
| Nuclear moulding | − | 15 (60%) |
| + | 3 (12%) | |
| + + | 5 (20%) | |
| + + + | 2 (8%) | |
| Multinucleation | − | 1 (4%) |
| + | 5 (20%) | |
| + + | 7 (28%) | |
| + + + | 12 (48%) | |
| Abnormal mitotic figures | Absent | 13 (52%) |
| Low numbers | 7 (28%) | |
| Moderate numbers | 1 (4%) | |
| High numbers | 4 (16%) |
| Examined Criteria | Score | No. of Samples (%) |
|---|---|---|
| Presence of nucleoli | Not evident | 4 (16%) |
| Occasionally evident | 17 (68%) | |
| Evident | 4 (16%) | |
| No. of nucleoli | 0 | 4 (16%) |
| <3 | 14 (64%) | |
| 3–5 | 3 (12%) | |
| >5 | 4 (28%) | |
| Abnormal nucleolar shapes | − | 9 (36%) |
| + | 11 (44%) | |
| + + | 3 (12%) | |
| + + + | 2 (8%) | |
| Macronucleoli | − | 7 (28%) |
| + | 7 (28%) | |
| + + | 3 (12%) | |
| + + + | 8 (32%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pîrvu, A.-M.; Caniatti, M.; Pieri, M.; Roccabianca, P.; Militaru, M. Cytological Features of Inflammatory Mammary Carcinoma in Dogs. Vet. Sci. 2024, 11, 389. https://doi.org/10.3390/vetsci11090389
Pîrvu A-M, Caniatti M, Pieri M, Roccabianca P, Militaru M. Cytological Features of Inflammatory Mammary Carcinoma in Dogs. Veterinary Sciences. 2024; 11(9):389. https://doi.org/10.3390/vetsci11090389
Chicago/Turabian StylePîrvu, Adina-Mihaela, Mario Caniatti, Marta Pieri, Paola Roccabianca, and Manuella Militaru. 2024. "Cytological Features of Inflammatory Mammary Carcinoma in Dogs" Veterinary Sciences 11, no. 9: 389. https://doi.org/10.3390/vetsci11090389
APA StylePîrvu, A.-M., Caniatti, M., Pieri, M., Roccabianca, P., & Militaru, M. (2024). Cytological Features of Inflammatory Mammary Carcinoma in Dogs. Veterinary Sciences, 11(9), 389. https://doi.org/10.3390/vetsci11090389







