Suppressive Effects of β-Hydroxybutyrate Administration on Lipopolysaccharide-Induced Inflammation in Broiler Chickens
Abstract
Simple Summary
Abstract
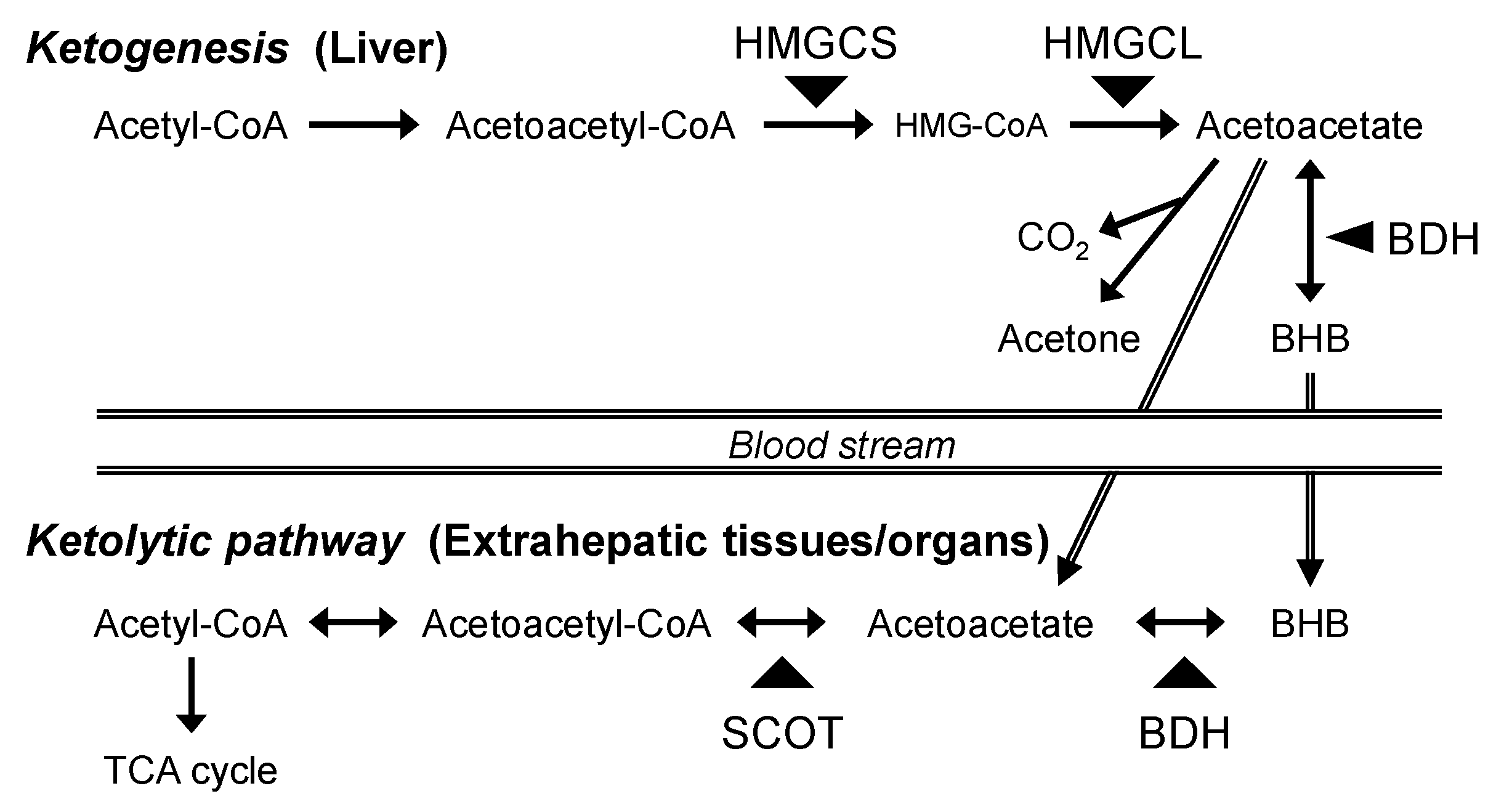
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Analyses of Plasma Inflammation Markers
2.3. Quantification of Gene Expression Levels
2.4. Statistical Analysis
3. Results
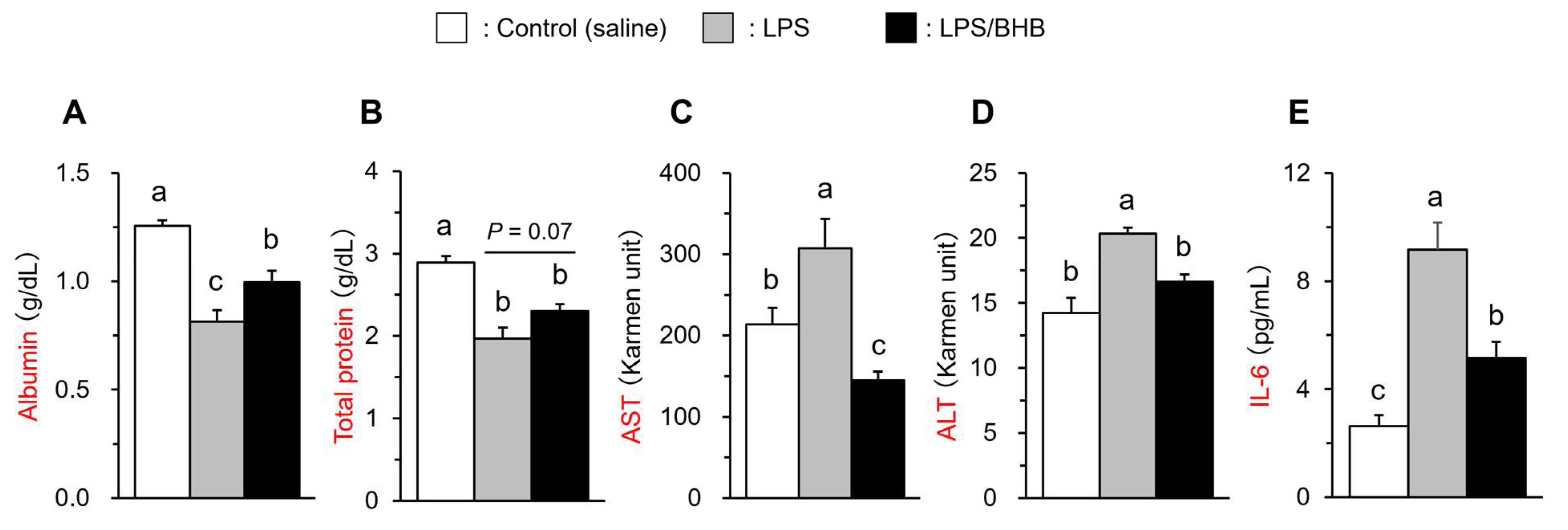
3.1. Plasma Inflammatory Parameters
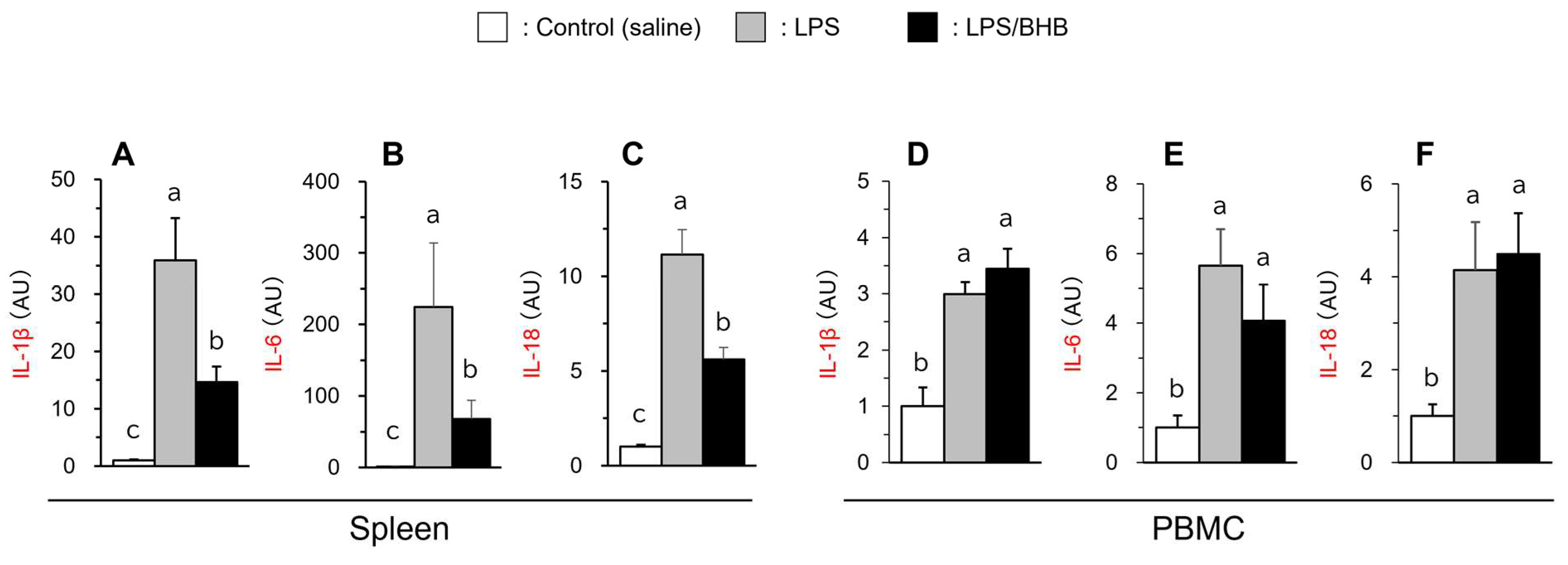
3.2. The Effects of LPS and BHB Administration on Inflammatory Gene Expression
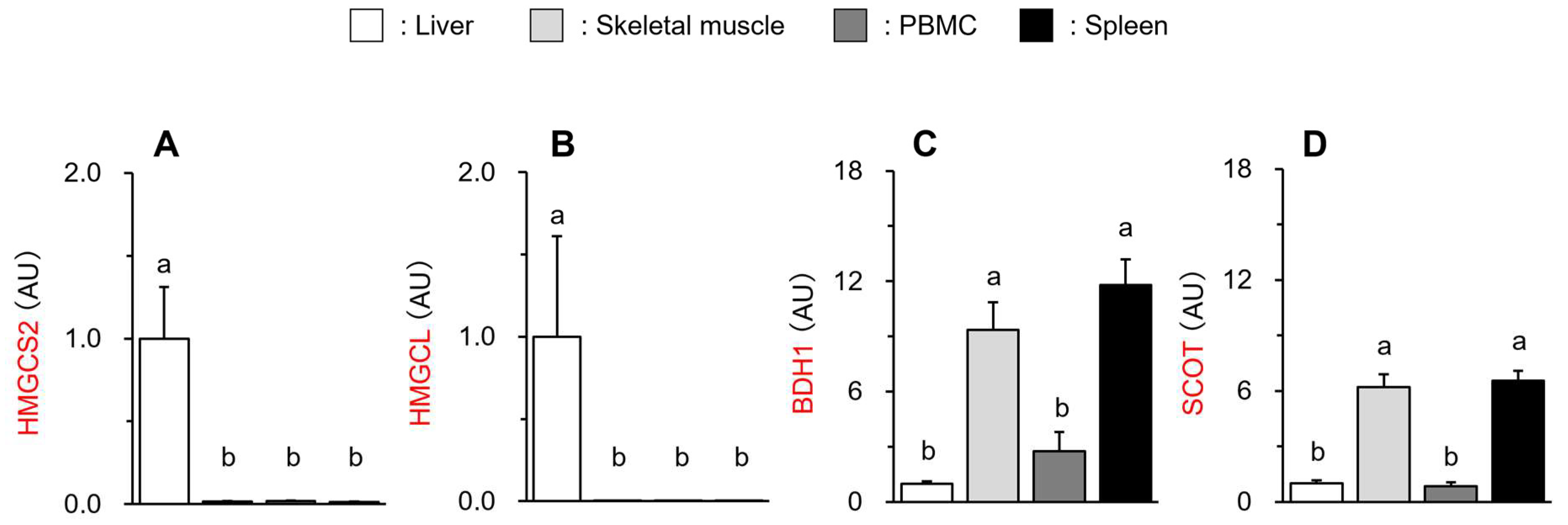
3.3. Different Gene Expression of Ketogenic and Ketolytic Enzymes in Peripheral Tissues/Organs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohtsu, H.; Sato, K.; Nishida, H.; Akiba, Y. High β-hydroxybutyrate concentration in liver and skeletal muscle of newly hatched chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 134, 625–629. [Google Scholar] [CrossRef]
- Mahagna, M.; Nir, I. Comparative development of digestive organs, intestinal disaccharidases and some blood metabolites in broiler and layer-type chicks after hatching. Br. Poult. Sci. 1996, 37, 359–371. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Chen, J.; Guo, X.; Yan, L.; Guo, Y.; Wang, B.; Yuan, J. The duration of food withdrawal affects the intestinal structure, nutrients absorption, and utilization in broiler chicken. FASEB J. 2021, 35, e21178. [Google Scholar] [CrossRef] [PubMed]
- Neudorf, H.; Little, J.P. Impact of fasting & ketogenic interventions on the NLRP3 inflammasome: A narrative review. Biomed. J. 2024, 47, 100677. [Google Scholar] [CrossRef]
- Bendridi, N.; Selmi, A.; Balcerczyk, A.; Pirola, L. Ketone bodies as metabolites and signalling molecules at the crossroad between inflammation and epigenetic control of cardiometabolic disorders. Int. J. Mol. Sci. 2022, 23, 14564. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Uchida, Y.; Kadono, K.; Hirao, H.; Kawasoe, J.; Watanabe, T.; Ueda, S.; Okajima, H.; Terajima, H.; Uemoto, S. Up-regulation of FOXO1 and reduced inflammation by β-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc. Natl. Acad. Sci. USA 2019, 116, 13533–13542. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Chau, L.Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002, 8, 240–246. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Hirata, Y.; Shimazaki, S.; Suzuki, S.; Henmi, Y.; Komiyama, H.; Kuwayama, T.; Iwata, H.; Karasawa, T.; Takahashi, M.; Takahashi, H.; et al. β-hydroxybutyrate suppresses NLRP3 inflammasome-mediated placental inflammation and lipopolysaccharide-induced fetal absorption. J. Reprod. Immunol. 2021, 148, 103433. [Google Scholar] [CrossRef]
- Kong, G.; Liu, J.; Li, R.; Lin, J.; Huang, Z.; Yang, Z.; Wu, X.; Zhu, Q. Ketone metabolite β-Hydroxybutyrate ameliorates Inflammation after spinal cord injury by inhibiting the NLRP3 inflammasome. Neurochem. Res. 2021, 46, 213–229. [Google Scholar] [CrossRef]
- Rahman, M.; Muhammad, S.; Khan, M.A.; Chen, H.; Ridder, D.A.; Müller-Fielitz, H.; Pokorná, B.; Vollbrandt, T.; Stölting, I.; Nadrowitz, R.; et al. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014, 5, 3944. [Google Scholar] [CrossRef]
- Hejazian, S.M.; Hosseiniyan Khatibi, S.M.; Barzegari, A.; Pavon-Djavid, G.; Razi Soofiyani, S.; Hassannejhad, S.; Ahmadian, E.; Ardalan, M.; Zununi Vahed, S. Nrf-2 as a therapeutic target in acute kidney injury. Life Sci. 2021, 264, 118581. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.R.; Teng, F.Y.; Fan, W.; Xu, B.T.; Li, X.Y.; Tan, X.Z.; Guo, M.; Gao, C.L.; Zhang, C.X.; Jiang, Z.Z.; et al. BDH1-mediated βOHB metabolism ameliorates diabetic kidney disease by activation of NRF2-mediated antioxidative pathway. Aging 2023, 15, 13384–13410. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Du, L.; Shao, J.; Qu, Y.; Zhang, L.; Zhang, D.; Cao, L.; Chen, H.; Bi, S. Molecular and metabolic responses to immune stress in the jejunum of broiler chickens: Transcriptomic and metabolomic analysis. Poult. Sci. 2024, 103, 103621. [Google Scholar] [CrossRef]
- Xi, Y.; Li, Y.; Ying, S.; Yan, J.; Shi, Z. Bacterial lipopolysaccharide with different administration routes affects intestinal mucosal morphological, immunological, and microbial barrier functions in goslings. Poult. Sci. 2023, 102, 102599. [Google Scholar] [CrossRef]
- Al-Ogaili, A.S.; Hameed, S.S.; Noori, N. LPS-induced NLRP3 gene expression in chicken. Open Vet. J. 2022, 12, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ji, P.; Hu, Y.; Li, C.; He, J. Study on the hepatoprotective effect mechanism of polysaccharides from charred Angelica sinensis on the layer chickens based on the detection of the intestinal floras and short-chain fatty acids of cecal contents and association analysis. Vet. Sci. 2023, 10, 224. [Google Scholar] [CrossRef]
- Muroi, H.; Hori, K.; Tokutake, Y.; Hakamata, Y.; Kawabata, F.; Toyomizu, M.; Kikusato, M. Oleuropein suppresses mitochondrial reactive oxygen species generation possibly via an activation of transient receptor potential V1 and sirtuin-1 in cultured chicken muscle cells. Anim. Sci. J. 2021, 93, e13677. [Google Scholar] [CrossRef]
- Kikusato, M.; Nanto, F.; Mukai, K.; Toyomizu, M. Effects of trehalose supplementation on the growth performance and intestinal innate immunity of juvenile chicks. Br. Poult. Sci. 2016, 57, 375–380. [Google Scholar] [CrossRef]
- Liu, J.; Yan, P.; Li, Y.; Yu, J.; Huang, Y.; Bai, R.; Liu, M.; Wang, N.; Liu, L.; Zhu, J.; et al. Gut microbiota and serum metabolome reveal the mechanism by which TCM polysaccharides alleviate salpingitis in laying hens challenged by bacteria. Poult. Sci. 2024, 103, 103288. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, Y.S.; Kim, S.R.; Lee, D.W.; Lee, S.B.; Kim, I.Y. Pre-treatment with β-hydroxybutyrate mitigates cisplatin-induced acute kidney injury. Biochem. Biophys. Res. Commun. 2024, 695, 149482. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, Y.S.; Kim, S.R.; Lee, D.W.; Lee, S.B.; Kim, I.Y. β-hydroxybutyrate ameliorates sepsis-induced acute kidney injury. Mol. Biol. Rep. 2023, 50, 8915–8923. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhuang, Y.; Yu, C.; Wang, Z.; Liu, Y.; Xu, Q.; Liu, K.; Li, Y. D-β-hydroxybutyrate up-regulates Claudin-1 and alleviates the intestinal hyperpermeability in lipopolysaccharide-treated mice. Tissue Cell 2024, 87, 102343. [Google Scholar] [CrossRef]
- Satoh, T. Ketobiotics by poly-3-hydroxybutyrate: A novel prebiotic activation of butyrate-producing bacteria through 3-hydroxybutyrate donation to the microbiota. J. Biotechnol. Biomed. 2023, 5, 158–162. [Google Scholar] [CrossRef]
- Wang, C.; Wang, N.; Deng, Y.; Zha, A.; Li, J.; Tan, B.; Qi, M.; Wang, J.; Yin, Y. β-hydroxybutyrate administration improves liver injury and metabolic abnormality in postnatal growth retardation piglets. Front. Vet. Sci. 2023, 10, 1294095. [Google Scholar] [CrossRef]
- Bae, H.R.; Kim, D.H.; Park, M.H.; Lee, B.; Kim, M.J.; Lee, E.K.; Chung, K.W.; Kim, S.M.; Im, D.S.; Chung, H.Y. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget 2016, 7, 66444–66454. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, R.; Jiang, R.; Zhu, M.; Tang, L.; Li, L.; Zhang, L. β-Hydroxybutyrate exacerbates lipopolysaccharide/ d-galactosamine-induced inflammatory response and hepatocyte apoptosis in mice. J. Biochem. Mol. Toxicol. 2019, 33, e22372. [Google Scholar] [CrossRef]
- Shi, X.; Li, X.; Li, D.; Li, Y.; Song, Y.; Deng, Q.; Wang, J.; Zhang, Y.; Ding, H.; Yin, L.; et al. β-Hydroxybutyrate activates the NF-κB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes. Cell. Physiol. Biochem. 2014, 33, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020, 30, 300–314. [Google Scholar] [CrossRef]
- Jesus, K.; Moita, L.F. Ketogenesis favors oxidative phosphorylation to promote disease tolerance. Trends Endocrinol. Metab. 2024, 35, 177–179. [Google Scholar] [CrossRef]
| Gene Symbol | Primer Sequence (5′-3′) Sense (Upper); Antisense (Lower) | Annealing Temperature (°C) | Accession ID | |
|---|---|---|---|---|
| IL-1β | Sense | CTGCCTGCAGAAGAAGCCT | 59 | NP_989855.1 |
| Antisense | ATGTCGAAGGACTGTGAGCG | |||
| IL-6 | Sense | CGACGAGGAGAAATGCCTGA | 51 | NP_989959.1 |
| Antisense | GGGATGACCACTTCATCGGG | |||
| IL-18 | Sense | TGATGAGCTGGAATGCGATGCC | 59 | NP_989939.1 |
| Antisense | TGGACGAACCACAAGCAACTGG | |||
| HMGCL | Sense | GGTACTCCCACACTCTGGCG | 58 | NP_001185643.1 |
| Antisense | GGACGCATGAAACATACCCAC | |||
| HMGCS2 | Sense | AGTGGCAAAAAGAGGGGACA | 56 | XM_422225.8 |
| Antisense | CAGTCTTGCCACCGACTTCT | |||
| BDH1 | Sense | GGAGGTCAAAGGGTCGTGTA | 56 | NP_001006547.3 |
| Antisense | CAGGTTGGTGGCAGCTATGA | |||
| SCOT | Sense | TCTACCAGCTGTCATCGCAA | 63 | NP_001006578.2 |
| Antisense | TCCAAAATTGTCAACGCCTGC | |||
| RPS9 | Sense | TGCGAAGTTTTGTGACTGAAACA | 60 | NM_001277757 |
| Antisense | ATTCTTGGAGCATTCAGCCTTTC | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horiuchi, T.; Furukawa, K.; Kikusato, M. Suppressive Effects of β-Hydroxybutyrate Administration on Lipopolysaccharide-Induced Inflammation in Broiler Chickens. Vet. Sci. 2024, 11, 405. https://doi.org/10.3390/vetsci11090405
Horiuchi T, Furukawa K, Kikusato M. Suppressive Effects of β-Hydroxybutyrate Administration on Lipopolysaccharide-Induced Inflammation in Broiler Chickens. Veterinary Sciences. 2024; 11(9):405. https://doi.org/10.3390/vetsci11090405
Chicago/Turabian StyleHoriuchi, Tae, Kyohei Furukawa, and Motoi Kikusato. 2024. "Suppressive Effects of β-Hydroxybutyrate Administration on Lipopolysaccharide-Induced Inflammation in Broiler Chickens" Veterinary Sciences 11, no. 9: 405. https://doi.org/10.3390/vetsci11090405
APA StyleHoriuchi, T., Furukawa, K., & Kikusato, M. (2024). Suppressive Effects of β-Hydroxybutyrate Administration on Lipopolysaccharide-Induced Inflammation in Broiler Chickens. Veterinary Sciences, 11(9), 405. https://doi.org/10.3390/vetsci11090405








