Does Catheter Insertion Site Matter? Contamination of Peripheral Intravenous Catheters during Dental Scaling in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Selection
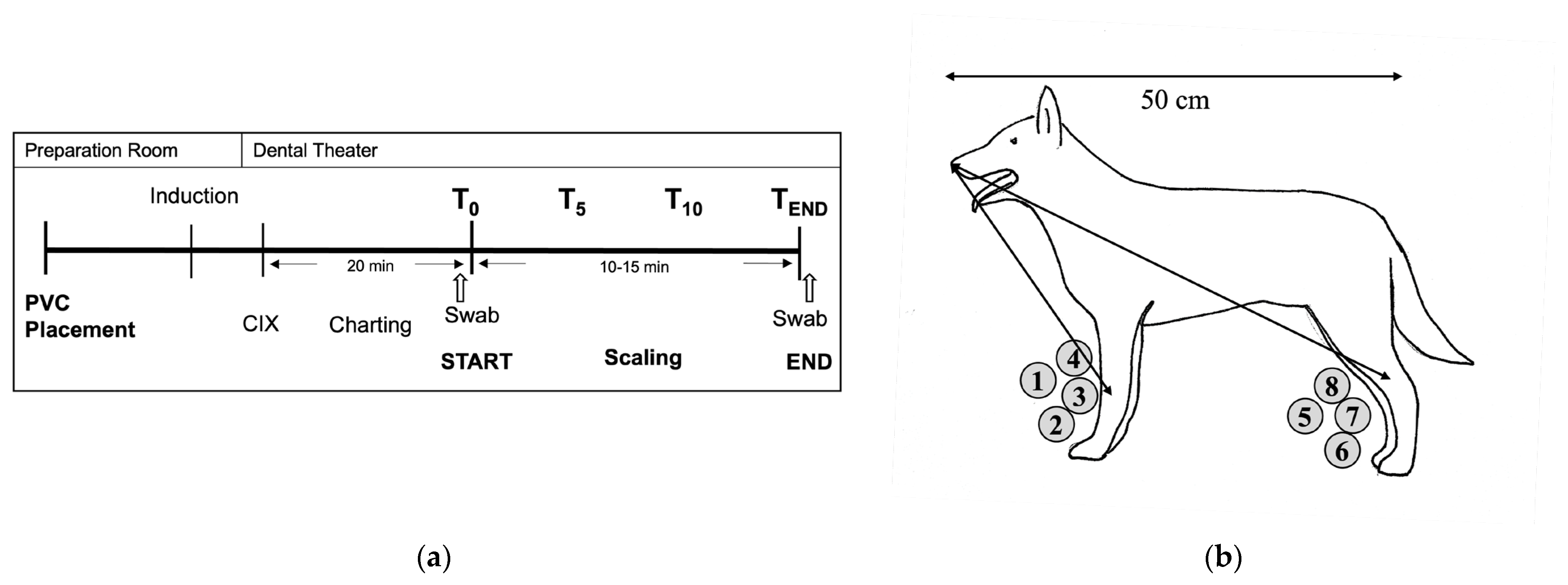
2.2. Study Design and Treatments
PIVC Placement and Anaesthetic and Dental Procedures
2.3. Sampling Procedures
2.3.1. Part A: Sedimentation Plates—Sampling Procedures
2.3.2. Part B: The Dorsal PIVC Injection Port—Sampling Procedures
2.4. Microbiologic Procedures
2.4.1. Part A: Sedimentation Plates in the CV and SV Areas
2.4.2. Part B: The Dorsal PIVC Injection Port
2.5. Statistics
3. Results
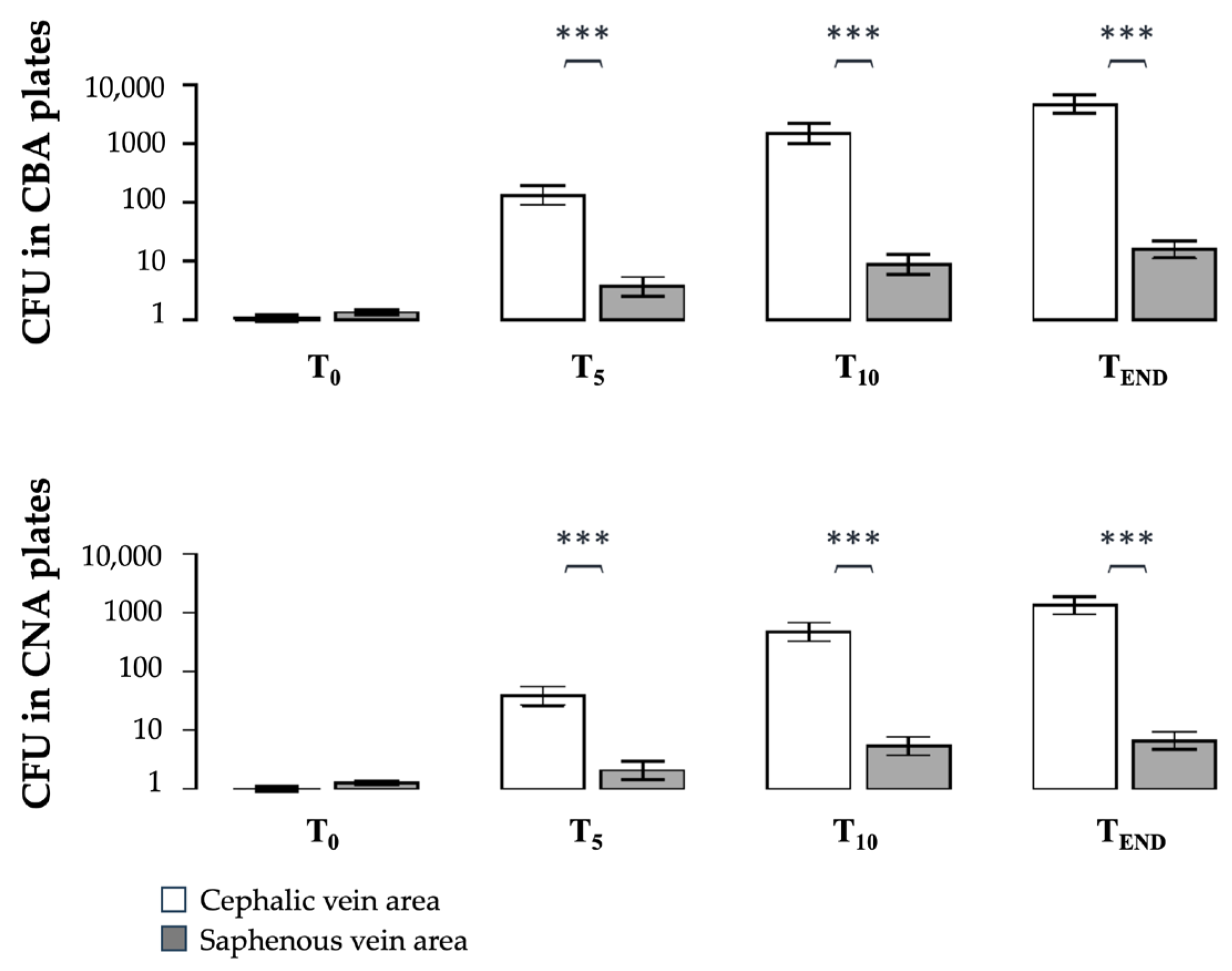
3.1. Part A: Bacterial Contamination in the CV and SV Areas
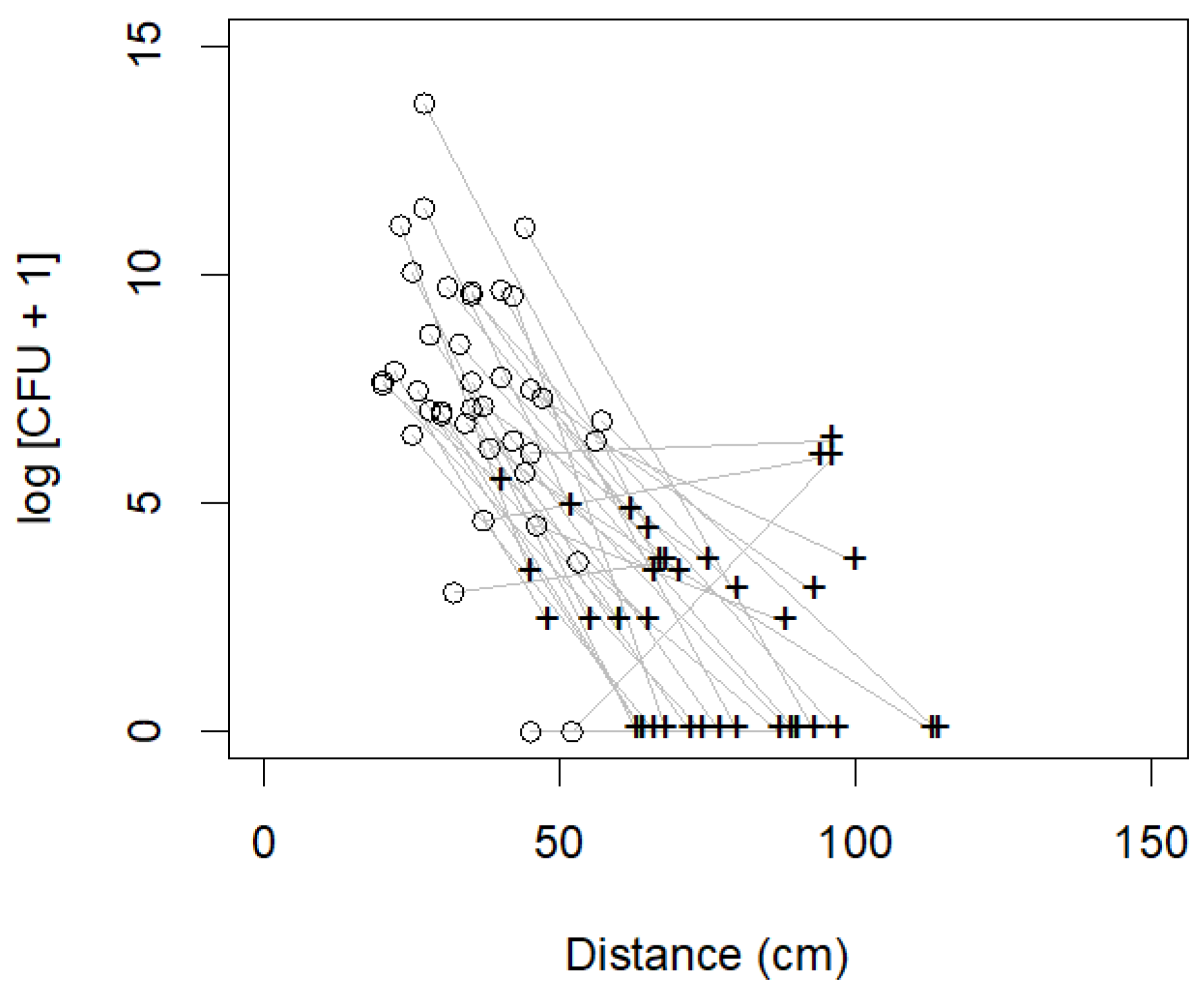
3.2. Part B: Bacterial Contamination and Genera Isolated from the Dorsal PIVC Injection Ports
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmerman, M.F.; Menso, L.; Steinfort, J.; Winkelhoff, A.J.V.; Weijden, G.A.V.D. Atmospheric Contamination during Ultrasonic Scaling. J. Clin. Periodontol. 2004, 31, 458–462. [Google Scholar] [CrossRef]
- Veena, H.R.; Mahantesha, S.; Joseph, P.A.; Patil, S.R.; Patil, S.H. Dissemination of Aerosol and Splatter during Ultrasonic Scaling: A Pilot Study. J. Infect. Public Health 2015, 8, 260–265. [Google Scholar] [CrossRef]
- Leggat, P.A.; Kedjarune, U. Bacterial Aerosols in the Dental Clinic: A Review. Int. Dent. J. 2001, 51, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.D.; Case, A.M.; Stevens, K.B.; Boag, A.; Rycroft, A.N. Factors Contributing to the Contamination of Peripheral Intravenous Catheters in Dogs and Cats. Vet. Rec. 2009, 164, 616. [Google Scholar] [CrossRef]
- Mathews, K.A.; Brooks, M.J.; Valliant, A.E. A Prospective Study Of Intravenous Catheter Contamination. J. Vet. Emerg. Crit. Care 1996, 6, 33–43. [Google Scholar] [CrossRef]
- Ramos, P.J.G.; Pérez, C.F.; Santiago, T.A.; Artigao, M.R.B.; Ortiz-Díez, G. Incidence of and Associated Factors for Bacterial Colonization of Intravenous Catheters Removed from Dogs in Response to Clinical Complications. J. Vet. Intern. Med. 2018, 32, 1084–1091. [Google Scholar] [CrossRef]
- Seguela, J.; Pages, J.-P. Bacterial and Fungal Colonisation of Peripheral Intravenous Catheters in Dogs and Cats. J. Small Anim. Pract. 2011, 52, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Marsh-Ng, M.L.; Burney, D.P.; Garcia, J. Surveillance of Infections Associated with Intravenous Catheters in Dogs and Cats in an Intensive Care Unit. J. Am. Anim. Hosp. Assoc. 2014, 43, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Matula, E.; Mastrocco, A.; Prittie, J.; Weltman, J.; Keyserling, C. Microorganism Colonization of Peripheral Venous Catheters in a Small Animal Clinical Setting. J. Vet. Emerg. Crit. Care 2023, 33, 509–519. [Google Scholar] [CrossRef]
- Holmstrom, S.E.; Bellows, J.; Juriga, S.; Knutson, K.; Niemiec, B.A.; Perrone, J.; College, A.V.D. 2013 AAHA Dental Care Guidelines for Dogs and Cats*. J. Am. Anim. Hosp. Assoc. 2013, 49, 75–82. [Google Scholar] [CrossRef]
- Logothetis, D.D.; Martinez-Welles, J.M. Reducing Bacterial Aerosol Contamination with a Chlorhexidine Gluconate Pre-Rinse. J. Am. Dent. Assoc. 1995, 126, 1634–1639. [Google Scholar] [CrossRef]
- Matsuda, N.; Matsuda, M.; Notake, S.; Yokokawa, H.; Kawamura, Y.; Hiramatsu, K.; Kikuchi, K. Evaluation of a Simple Protein Extraction Method for Species Identification of Clinically Relevant Staphylococci by Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2012, 50, 3862–3866. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A.; Farr, B.M.; Sherertz, R.J.; Raad, I.I.; O’Grady, N.; Harris, J.S.; Craven, D.E. Guidelines for the Management of Intravascular Catheter-Related Infections. Clin. Infect. Dis. 2001, 32, 1249–1272. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, N.P.; Alexander, M.; Dellinger, E.P.; Gerberding, J.L.; Heard, S.O.; Maki, D.G.; Masur, H.; McCormick, R.D.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the Prevention of Intravascular Catheter–Related Infections. Clin. Infect. Dis. 2002, 35, 1281–1307. [Google Scholar] [CrossRef]
- Tellier, R.; Li, Y.; Cowling, B.J.; Tang, J.W. Recognition of Aerosol Transmission of Infectious Agents: A Commentary. BMC Infect. Dis. 2019, 19, 101. [Google Scholar] [CrossRef]
- Gahlot, R.; Nigam, C.; Kumar, V.; Yadav, G.; Anupurba, S. Catheter-Related Bloodstream Infections. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 162–167. [Google Scholar] [CrossRef]
- Harari, J.; Besser, T.E.; Gustafson, S.B.; Meinkoth, K. Bacterial Isolates from Blood Cultures of Dogs Undergoing Dentistry. Vet. Surg. 1993, 22, 27–30. [Google Scholar] [CrossRef]
- Nieves, M.A.; Hartwig, P.; Kinyon, J.M.; Riedesel, D.H. Bacterial Isolates from Plaque and from Blood During and after Routine Dental Procedures in Dogs. Vet. Surg. 1997, 26, 26–32. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia Associated with Toothbrushing and Dental Extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef]
- Johnson, J.A. Nosocomial Infections. Vet. Clin. N. Am. Small Anim. Pract. 2002, 32, 1101–1126. [Google Scholar] [CrossRef]
- Stull, J.W.; Weese, J.S. Hospital-Acquired Infections Due to Gram-Negative Bacteria. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 217–233. [Google Scholar] [CrossRef]
- McDonald, J.E.; Larsen, N.; Pennington, A.; Connolly, J.; Wallis, C.; Rooks, D.J.; Hall, N.; McCarthy, A.J.; Allison, H.E. Characterising the Canine Oral Microbiome by Direct Sequencing of Reverse-Transcribed RRNA Molecules. PLoS ONE 2016, 11, e0157046. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.R.; Wilson, M.; Buckley, C.M.F.; Spratt, D.A. Cultivable Oral Microbiota of Domestic Dogs. J. Clin. Microbiol. 2005, 43, 5470–5476. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Klein, E.A.; Thompson, E.C.; Blanton, J.M.; Chen, T.; Milella, L.; Buckley, C.M.F.; Davis, I.J.; Bennett, M.-L.; Marshall-Jones, Z.V. The Canine Oral Microbiome. PLoS ONE 2012, 7, e36067. [Google Scholar] [CrossRef]
- Burrows, C. Techniques and Complications of Intravenous and Intraarterial Catheterization in Dogs and Cats. J. Am. Vet. Med. Assoc. 1973, 12, 1357–1363. [Google Scholar]
- Dugdale, A.; Beaumont, G.; Bradbrook, C.; Gurney, M. Veterinary Anaesthesia Principles to Practice, 2nd ed.; Wiley Blackwell: Hoboken, NJ, USA, 2020; ISBN 9781119246770. [Google Scholar]

| Nr. | Breed | Age (Months) | Sex (m/f) | Weight (kg) | ASA |
|---|---|---|---|---|---|
| 1 | Mix | 86 | m | 35.00 | 2 |
| 2 | Labrador Retriever | 109 | m | 37.80 | 2 |
| 3 | Mix | 88 | f | 30.00 | 1 |
| 4 | Mix | 92 | m | 13.30 | 2 |
| 5 | Mix | 152 | f | 24.75 | 2 |
| 6 | Mix | 97 | f | 10.50 | 1 |
| 7 | French Bulldog | 115 | m | 12.60 | 3 |
| 8 | German Shepherd | 93 | m | 34.00 | 2 |
| 9 | Labrador Retriever | 36 | f | 38.50 | 2 |
| 10 | Mix | 202 | m | 14.00 | 3 |
| 11 * | Pug | 73 | m | 7.00 | 2 |
| 12 | Mix | 138 | m | 5.80 | 3 |
| 13 | Mix | 157 | f | 8.55 | 3 |
| 14 | Mix | 132 | m | 16.00 | 1 |
| 15 | Mix | 78 | m | 25.00 | 3 |
| 16 # | Mix | 179 | m | 33.40 | 3 |
| 17 | Mix | 153 | f | 24.00 | 2 |
| 18 | Mix | 122 | f | 11.60 | 2 |
| 19 | Mix | 36 | f | 13.30 | 2 |
| 20 | Border Terrier | 159 | m | 12.10 | 3 |
| 21 | Labrador Retriever | 12 | f | 32.80 | 1 |
| 22 | Havanese | 124 | f | 5.50 | 2 |
| 23 | Havanese | 96 | m | 3.90 | 3 |
| 24 | Boxer | 75 | f | 28.00 | 1 |
| 25 * | Boxer | 75 | m | 30.00 | 1 |
| 26 | English Cocker Spaniel | 155 | f | 11.20 | 2 |
| 27 # | Jack Russell Terrier | 96 | f | 5.20 | 2 |
| 28 * | Mix | 85 | m | 9.8 | 2 |
| 29 * | Pug | 87 | m | 9.40 | 2 |
| 30 | Mix | 156 | m | 8.30 | 2 |
| 31 | Mix | 104 | f | 15.50 | 2 |
| 32 # | Australian Shepherd | 89 | f | 22.20 | 1 |
| 33 | Cavalier King Charles Spaniel | 105 | m | 9.00 | 3 |
| 34 | Mix | 109 | f | 29.20 | 2 |
| 35 | Yorkshire Terrier | 81 | f | 3.70 | 2 |
| 36 | Mix | 142 | f | 9.50 | 2 |
| 37 # | Poodle Dog | 121 | m | 10.00 | 2 |
| 38 | Parson Russell Terrier | 156 | f | 7.30 | 3 |
| 39 # | Collie | 124 | f | 22.00 | 2 |
| 40 | Beagle | 106 | w | 13.20 | 1 |
| 41 | Spitz Dog | 73 | w | 7.40 | 2 |
| 42 | Pitbull | 136 | w | 28.00 | 2 |
| 43 | Mix | 126 | w | 25.00 | 1 |
| Pairwise Tests | CFU in CBA | CFU in CNA | ||
|---|---|---|---|---|
| Time Point | CV Area | SV Area | CV Area | SV Area |
| T0–T5 | 2.09 (***) | 0.43 (*) | 1.58 (***) | 0.21 (-) |
| T0–T10 | 3.15 (***) | 0.80 (***) | 2.67 (***) | 0.62 (**) |
| T0–TEND | 3.63 (***) | 1.07 (***) | 3.12 (***) | 0.72 (***) |
| T5–T10 | 1.06 (***) | 0.37 (-) | 1.09 (***) | 0.41 (.) |
| T5–TEND | 1.54 (***) | 0.63 (***) | 1.54 (***) | 0.51 (*) |
| T10–TEND | 0.49 (-) | 0.26 (-) | 0.45 (-) | 0.10 (-) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calice, I.; Ballas, P.; Vogl, C.; Purwin, S.; Ehling-Schulz, M.; Rocchi, A. Does Catheter Insertion Site Matter? Contamination of Peripheral Intravenous Catheters during Dental Scaling in Dogs. Vet. Sci. 2024, 11, 407. https://doi.org/10.3390/vetsci11090407
Calice I, Ballas P, Vogl C, Purwin S, Ehling-Schulz M, Rocchi A. Does Catheter Insertion Site Matter? Contamination of Peripheral Intravenous Catheters during Dental Scaling in Dogs. Veterinary Sciences. 2024; 11(9):407. https://doi.org/10.3390/vetsci11090407
Chicago/Turabian StyleCalice, Ivana, Panagiotis Ballas, Claus Vogl, Sandra Purwin, Monika Ehling-Schulz, and Attilio Rocchi. 2024. "Does Catheter Insertion Site Matter? Contamination of Peripheral Intravenous Catheters during Dental Scaling in Dogs" Veterinary Sciences 11, no. 9: 407. https://doi.org/10.3390/vetsci11090407
APA StyleCalice, I., Ballas, P., Vogl, C., Purwin, S., Ehling-Schulz, M., & Rocchi, A. (2024). Does Catheter Insertion Site Matter? Contamination of Peripheral Intravenous Catheters during Dental Scaling in Dogs. Veterinary Sciences, 11(9), 407. https://doi.org/10.3390/vetsci11090407







