Integrated Study of Canine Mammary Tumors Histopathology, Immunohistochemistry, and Cytogenetic Findings
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Tumor Collection
2.3. Histopathology
2.4. Immunohistochemistry
2.5. Evaluation of Immunohistochemical Data
2.6. Follow-Up Data
2.7. Primary Cell Culture and Karyotype Analysis
2.8. Statistical Analysis
3. Results
3.1. Clinicopathological Features
3.2. Immunohistochemistry
3.3. Relationship between Histological Type, Grade, and Immunophenotype
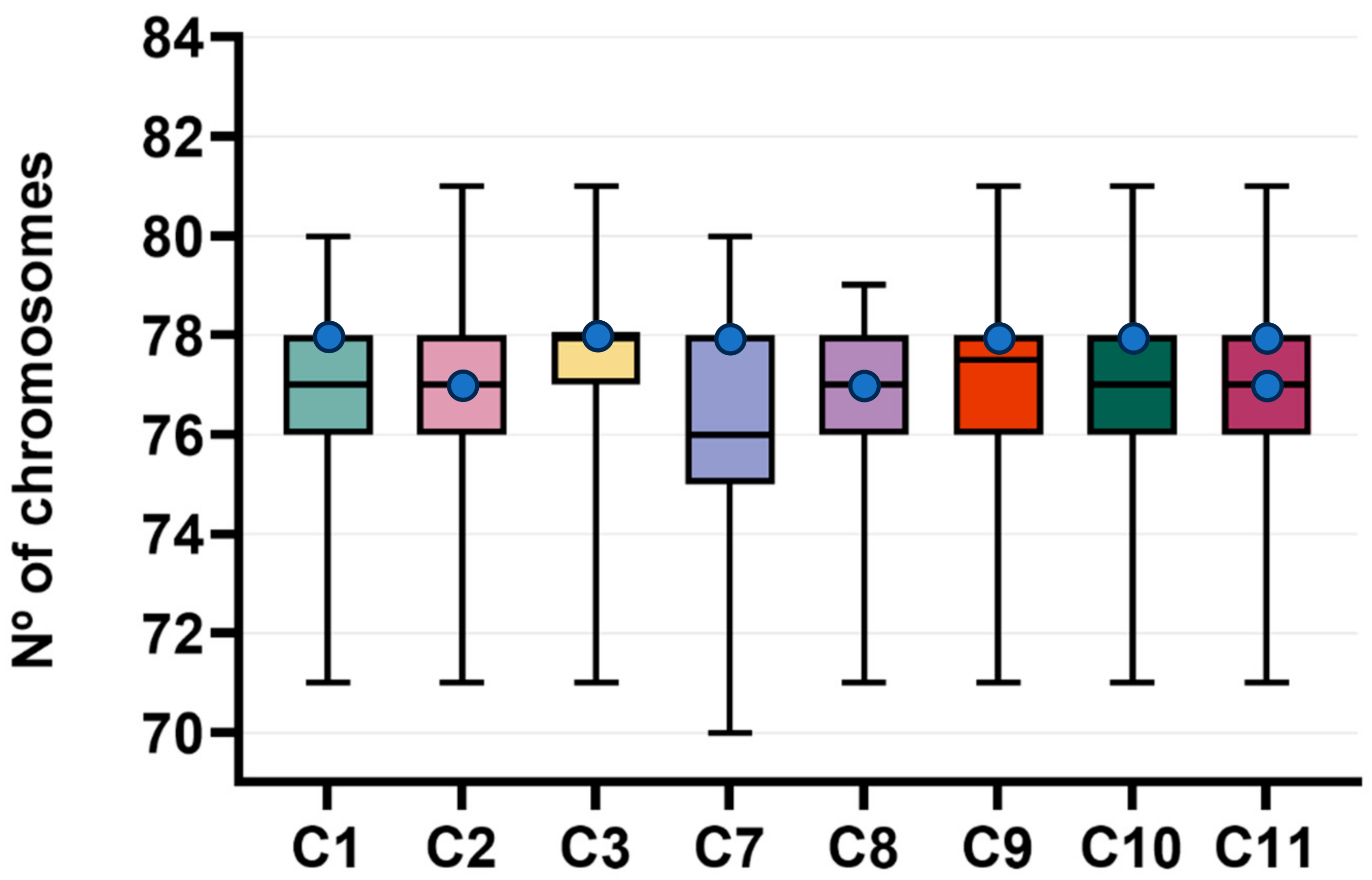
3.4. Karyotype Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ke, C.-H.; Sio, K.-M.; Wang, S.-L.; Kuo, Y.; Huang, W.-H.; Lin, C.-S. The High Expression of Legumain in Canine Neoplasms: A Retrospective Analysis of 100 Cases. Animals 2022, 12, 504. [Google Scholar] [CrossRef]
- Dias-Pereira, P. Morbidity and Mortality in Elderly Dogs—A Model for Human Aging. BMC Veter. Res. 2022, 18, 457. [Google Scholar] [CrossRef]
- Choi, J.; Yoo, M.-J.; Park, S.-Y.; Seol, J.-W. Antitumor Effects of Esculetin, a Natural Coumarin Derivative, against Canine Mammary Gland Tumor Cells by Inducing Cell Cycle Arrest and Apoptosis. Veter. Sci. 2023, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002-2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Santana, Á.; Herráez, P.; Killick, D.R.; De Los Monteros, A.E. Epidemiology of Canine Mammary Tumours on the Canary Archipelago in Spain. BMC Veter. Res. 2022, 18, 268. [Google Scholar] [CrossRef] [PubMed]
- Pastor, N.; Caballé, N.C.; Santella, M.; Ezquerra, L.J.; Tarazona, R.; Duran, E. Epidemiological Study of Canine Mammary Tumors: Age, Breed, Size and Malignancy. Austral. J. Veter. Sci. 2018, 50, 143–147. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Kristiansen, V.M.; Cofone, M.A.; Shofer, F.S.; Breen, A.-M.; Langeland, M.; Mongil, C.M.; Grondahl, A.M.; Teige, J.; Goldschmidt, M.H. Canine Mammary Gland Tumours; a Histological Continuum from Benign to Malignant; Clinical and Histopathological Evidence. Veter. Comp. Oncol. 2009, 7, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Ariyarathna, H.; Aberdein, D.; Thomson, N.; Gibson, I.; Munday, J. Canine Mammary Gland Disease in New Zealand: A Review of Samples from 797 Dogs. N. Z. Veter. J. 2022, 70, 95–100. [Google Scholar] [CrossRef]
- Zheng, H.-H.; Du, C.-T.; Yu, C.; Zhang, Y.-Z.; Huang, R.-L.; Tang, X.-Y.; Xie, G.-H. Epidemiological Investigation of Canine Mammary Tumors in Mainland China Between 2017 and 2021. Front. Veter. Sci. 2022, 9, 843390. [Google Scholar] [CrossRef]
- Saleem, A.; Megha, G.K.; Zehra, A. Novel Promising Serum Biomarkers for Canine Mammary Tumors. Proc. Ind. Natl. Sci. Acad. USA 2021, 87, 302–310. [Google Scholar] [CrossRef]
- Suryawanshi, R.V. Assessment of Efficacy and Toxicity of Cyclophosphamide Chemotherapy in Canines with Malignant Mammary Tumor: A Retrospective Study. Veter. Med. Int. 2021, 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, M.; Capello, K.; Carminato, A.; Zanardello, C.; Baioni, E.; Mutinelli, F. Incidence of Mammary Tumors in the Canine Population Living in the Veneto Region (Northeastern Italy): Risk Factors and Similarities to Human Breast Cancer. Prev. Veter. Med. 2016, 126, 183–189. [Google Scholar] [CrossRef]
- Abdelmegeed, S.; Mohammed, S. Canine Mammary Tumors as a Model for Human Disease (Review). Oncol. Lett. 2018, 15, 8195–8205. [Google Scholar] [CrossRef]
- Ferreira, T.; Gama, A.; Seixas, F.; Faustino-Rocha, A.I.; Lopes, C.; Gaspar, V.M.; Mano, J.F.; Medeiros, R.; Oliveira, P.A. Mammary Glands of Women, Female Dogs and Female Rats: Similarities and Differences to Be Considered in Breast Cancer Research. Veter. Sci. 2023, 10, 379. [Google Scholar] [CrossRef]
- Santos, A.A.; Lopes, C.C.; Ribeiro, J.R.; Martins, L.R.; Santos, J.C.; Amorim, I.F.; Gärtner, F.; Matos, A.J. Identification of Prognostic Factors in Canine Mammary Malignant Tumours: A Multivariable Survival Study. BMC Veter. Res. 2013, 9, 1. [Google Scholar] [CrossRef]
- Gama, A.; Alves, A.; Schmitt, F. Identification of Molecular Phenotypes in Canine Mammary Carcinomas with Clinical Implications: Application of the Human Classification. Virchows Arch. 2008, 453, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V.; Peña, L.; Rasotto, R.; Goldschmidt, M.H.; Gama, A.; Scruggs, J.L.; Kiupel, M. Volume 2: Mammary Tumors. In Surgical Pathology of Tumors of Domestic Animals; Davis-Thompson DVM Foundation: Gurnee, IL, USA, 2019; pp. 1–270. [Google Scholar]
- Peña, L.; Gama, A.; Goldschmidt, M.H.; Abadie, J.; Benazzi, C.; Castagnaro, M.; Díez, L.; Gärtner, F.; Hellmén, E.; Kiupel, M.; et al. Canine Mammary Tumors: A Review and Consensus of Standard Guidelines on Epithelial and Myoepithelial Phenotype Markers, HER2, and Hormone Receptor Assessment Using Immunohistochemistry. Veter. Pathol. 2014, 51, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the Treatment of Women with Early Breast Cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Worley, D.R.; Zapulli, V. Tumors of the Mammary Gland. In Withrow and Macewen’s Small Animal Clinical Oncology; Vail, D.M., Thamm, D.H., Liptak, J.M., Eds.; Elsevier Inc.: Maryland Heights, MO, USA, 2019; pp. 604–625. ISBN 978-0-323-59496-7. [Google Scholar]
- Dobson, J.M. Breed-Predispositions to Cancer in Pedigree Dogs. ISRN Veter. Sci. 2013, 2013, 1–23. [Google Scholar] [CrossRef]
- Tkaczyk-Wlizło, A.; Śmiech, A.; Kowal, K.; Różańska, D.; Ślaska, B. Histopathological Evaluation of Canine Mammary Gland Tumours: A Study of 92 Cases. Med. Weter. 2023, 79, 356–363. [Google Scholar] [CrossRef]
- Nguyen, F.; Peña, L.; Ibisch, C.; Loussouarn, D.; Gama, A.; Rieder, N.; Belousov, A.; Campone, M.; Abadie, J. Canine Invasive Mammary Carcinomas as Models of Human Breast Cancer. Part 1: Natural History and Prognostic Factors. Breast Cancer Res. Treat. 2018, 167, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.C.; Campos, C.B.; Teixeira, S.V.; Bertagnolli, A.C.; Lavalle, G.E.; Cassali, G.D. Epidemiological, Clinical and Pathological Evaluation of Overall Survival in Canines with Mammary Neoplasms. Arq. Bras. Med. Veter. Zootec. 2018, 70, 1714–1722. [Google Scholar] [CrossRef]
- Santos, T.R.; Castro, J.R.; Andrade, J.C.; Silva, A.C.R.; Silva, G.M.F.; Ferreira, F.A.; Headley, S.A.; Saut, J.P.E. Risk Factors Associated with Mammary Tumors in Female Dogs. Pesq. Veter. Bras. 2020, 40, 466–473. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Masserdotti, C. Reproductive System. In Canine and Feline Cytopathology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 440–484. ISBN 978-0-323-68368-5. [Google Scholar]
- Peña, L.; Andrés, P.J.D.; Clemente, M.; Cuesta, P.; Pérez-Alenza, M.D. Prognostic Value of Histological Grading in Noninflammatory Canine Mammary Carcinomas in a Prospective Study with Two-Year Follow-Up: Relationship with Clinical and Histological Characteristics. Veter. Pathol. 2013, 50, 94–105. [Google Scholar] [CrossRef]
- Ferreira, E.; Bertagnolli, A.C.; Cavalcanti, M.F.; Schmitt, F.C.; Cassali, G.D. The Relationship between Tumour Size and Expression of Prognostic Markers in Benign and Malignant Canine Mammary Tumours. Veter. Comp. Oncol. 2009, 7, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Dorn, C.R.; Taylor, D.O. Factors Influencing Canine Mammary Cancer Development and Postsurgical Survival. J. Natl. Cancer Inst. 1969, 43, 1249–1261. [Google Scholar]
- Horta, R.D.S.; Lavalle, G.E.; Cunha, R.M.D.C.; Moura, L.L.D.; Araújo, R.B.D.; Cassali, G.D. Influence of Surgical Technique on Overall Survival, Disease Free Interval and New Lesion Development Interval in Dogs with Mammary Tumors. Adv. Breast Cancer Res. 2014, 03, 38–46. [Google Scholar] [CrossRef]
- Zink, M.C.; Farhoody, P.; Elser, S.E.; Ruffini, L.D.; Gibbons, T.A.; Rieger, R.H. Evaluation of the Risk and Age of Onset of Cancer and Behavioral Disorders in Gonadectomized Vizslas. J. Am. Veter. Med. Assoc. 2014, 244, 309–319. [Google Scholar] [CrossRef]
- Torres, C.G.; Iturriaga, M.P.; Cruz, P. Hormonal Carcinogenesis in Canine Mammary Cancer: Molecular Mechanisms of Estradiol Involved in Malignant Progression. Animals 2021, 11, 608. [Google Scholar] [CrossRef]
- Burrai, G.P.; Gabrieli, A.; Moccia, V.; Zappulli, V.; Porcellato, I.; Brachelente, C.; Pirino, S.; Polinas, M.; Antuofermo, E. A Statistical Analysis of Risk Factors and Biological Behavior in Canine Mammary Tumors: A Multicenter Study. Animals 2020, 10, 1687. [Google Scholar] [CrossRef]
- Sarli, G.; Preziosi, R.; Benazzi, C.; Castellani, G.; Marcato, P.S. Prognostic Value of Histologic Stage and Proliferative Activity in Canine Malignant Mammary Tumors. J. Veter. Diagn. Investig. 2002, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, D.A.P.C.; Santana, A.E.; Cury, P.M.; Cordeiro, J.A. Immunocytochemical Study of Ki-67 as a Prognostic Marker in Canine Mammary Neoplasia. Veter. Clin. Pathol. 2004, 33, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Abadie, J.; Nguyen, F.; Loussouarn, D.; Peña, L.; Gama, A.; Rieder, N.; Belousov, A.; Bemelmans, I.; Jaillardon, L.; Ibisch, C.; et al. Canine Invasive Mammary Carcinomas as Models of Human Breast Cancer. Part 2: Immunophenotypes and Prognostic Significance. Breast Cancer Res. Treat. 2018, 167, 459–468. [Google Scholar] [CrossRef]
- Varallo, G.; Gelaleti, G.; Maschio-Signorini, L.; Moschetta, M.; Lopes, J.; De Nardi, A.; Tinucci-Costa, M.; Rocha, R.; de Campos Zuccari, D. Prognostic Phenotypic Classification for Canine Mammary Tumors. Oncol. Lett. 2019, 18, 6545–6553. [Google Scholar] [CrossRef] [PubMed]
- Jaillardon, L.; Abadie, J.; Godard, T.; Campone, M.; Loussouarn, D.; Siliart, B.; Nguyen, F. The Dog as a Naturally-Occurring Model for Insulin-like Growth Factor Type 1 Receptor-Overexpressing Breast Cancer: An Observational Cohort Study. BMC Cancer 2015, 15, 664. [Google Scholar] [CrossRef]
- Kim, H.-W.; Lim, H.-Y.; Shin, J.-I.; Seung, B.-J.; Ju, J.-H.; Sur, J.-H. Breed- and Age-Related Differences in Canine Mammary Tumors. Can. J. Veter. Res. 2016, 80, 146–155. [Google Scholar]
- Ades, F.; Zardavas, D.; Bozovic-Spasojevic, I.; Pugliano, L.; Fumagalli, D.; De Azambuja, E.; Viale, G.; Sotiriou, C.; Piccart, M. Luminal B Breast Cancer: Molecular Characterization, Clinical Management, and Future Perspectives. J. Clin. Oncol. 2014, 32, 2794–2803. [Google Scholar] [CrossRef]
- Cassali, G.; Damasceno, K.; Bertagnolli, A.; Estrela-Lima, A.; Lavalle, G.; Santis, G.; Nardi, A.; Fernandes, C.; Cogliati, B.; Sobral, R.; et al. Consensus Regarding the Diagnosis, Prognosis and Treatment of Canine Mammary Tumors: Benign Mixed Tumors, Carcinomas in Mixed Tumors and Carcinosarcomas. Braz. J. Veter. Pathol. 2017, 10, 87–99. [Google Scholar] [CrossRef]
- Szczerbal, I.; Switonski, M. Clinical Cytogenetics of the Dog: A Review. Animals 2021, 11, 947. [Google Scholar] [CrossRef]
- Cornelisse, C.J.; Rutteman, G.R.; Kuipers-Dijkshoorn, N.J.; Hellmén, E. The Difference in DNA Ploidy Pattern between Some Canine and Human Neoplasms Appears to Be Genuine and a Reflection of Dissimilarities in DNA Aneuploidy Evolution. Anticancer Res. 1994, 14, 1599–1601. [Google Scholar]
- Rutteman, G.R.; Cornelisse, C.J.; Dijkshoorn, N.J.; Poortman, J.; Misdorp, W. Flow Cytometric Analysis of DNA Ploidy in Canine Mammary Tumors. Cancer Res. 1988, 48, 3411–3417. [Google Scholar] [PubMed]
- Borge, K.S.; Nord, S.; Van Loo, P.; Lingjærde, O.C.; Gunnes, G.; Alnæs, G.I.G.; Solvang, H.K.; Lüders, T.; Kristensen, V.N.; Børresen-Dale, A.-L.; et al. Canine Mammary Tumours Are Affected by Frequent Copy Number Aberrations, Including Amplification of MYC and Loss of PTEN. PLoS ONE 2015, 10, e0126371. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Ye, S.; Zheng, J.; Li, J.; Chen, Z.; Zhang, Y.; Li, S. Establishment and Characterization of a HER2-Enriched Canine Mammary Cancerous Myoepithelial Cell Line. BMC Veter. Res. 2023, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- de Faria Lainetti, P.; Brandi, A.; Leis Filho, A.F.; Prado, M.C.M.; Kobayashi, P.E.; Laufer-Amorim, R.; Fonseca-Alves, C.E. Establishment and Characterization of Canine Mammary Gland Carcinoma Cell Lines With Vasculogenic Mimicry Ability in Vitro and in Vivo. Front. Veter. Sci. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Zhang, H.; Pei, S.; Zhou, B.; Wang, H.; Du, H.; Zhang, D.; Lin, D. Establishment and Characterization of a New Triple-Negative Canine Mammary Cancer Cell Line. Tissue Cell 2018, 54, 10–19. [Google Scholar] [CrossRef]
- Caceres, S.; Peña, L.; de Andres, P.J.; Illera, M.J.; Lopez, M.S.; Woodward, W.A.; Reuben, J.M.; Illera, J.C. Establishment and Characterization of a New Cell Line of Canine Inflammatory Mammary Cancer: IPC-366. PLoS ONE 2015, 10, e0122277. [Google Scholar] [CrossRef]
- Smid, M.; Hoes, M.; Sieuwerts, A.M.; Sleijfer, S.; Zhang, Y.; Wang, Y.; Foekens, J.A.; Martens, J.W.M. Patterns and Incidence of Chromosomal Instability and Their Prognostic Relevance in Breast Cancer Subtypes. Breast Cancer Res. Treat. 2011, 128, 23–30. [Google Scholar] [CrossRef]
| Antibody | Clone (Code Number) | Origin | Dilution | Pre-Treatment | Incubation |
|---|---|---|---|---|---|
| ERα | C-311 (sc-787) | Santa Cruz Biotechnology, Dallas, TX, USA | 1:100 | Microwave | Overnight |
| PR | NCL-L-PGR (312) | Novocastra, UK | 1:40 | Microwave | Overnight |
| HER2 | Polyclonal (A0485) | Dako, Denmark | 1:40 | Microwave | Overnight |
| Ki-67 | MIB-1 (M7240) | Dako, Denmark | 1:50 | Microwave | Overnight |
| N | % | |
|---|---|---|
| Breed (n = 12) | ||
| Mixed | 6 | 50.0 |
| Labrador Retriever | 2 | 16.8 |
| Boxer | 1 | 8.3 |
| French Bulldog | 1 | 8.3 |
| German Shepherd | 1 | 8.3 |
| Pinscher | 1 | 8.3 |
| Age (years; n = 12) | ||
| 7 years | 8 | 66.7 |
| >7 years | 4 | 33.3 |
| Weight (kg; n = 10) a | ||
| <10 kg | 4 | 40.0 |
| 10–23 kg | 1 | 10.0 |
| >23 kg | 5 | 50.0 |
| Ovariohysterectomy (n = 12) | ||
| No | 7 | 58.3 |
| Yes, prior to tumor development | 2 | 16.7 |
| Yes, performed with mastectomy | 3 | 25.0 |
| Contraception (n = 12) | ||
| No | 11 | 91.7 |
| Yes | 1 | 8.3 |
| Parity (n = 12) | ||
| Nulliparous | 4 | 33.3 |
| Primiparous | 2 | 16.7 |
| Multiparous | 1 | 8.3 |
| Unknown | 5 | 41.7 |
| Multicentricity (n = 17) | ||
| Single | 6 | 50.0 |
| Multiple | 6 | 50.0 |
| Tumor location (n = 17) | ||
| Thoracic glands (M1–M2) | 4 | 23.5 |
| Abdominal glands (M3–M4) | 6 | 35.3 |
| Inguinal glands (M5) | 7 | 41.2 |
| Mammary chain (n = 17) | ||
| Left | 10 | 58.8 |
| Right | 7 | 41.2 |
| Tumor size (n = 17) | ||
| <3 cm | 11 | 64.7 |
| 3–5 cm | 2 | 11.8 |
| >5 cm | 4 | 23.5 |
| Surgical procedure (n = 12) | ||
| Single mastectomy | 3 | 25.0 |
| Regional mastectomy | 9 | 75.0 |
| N | % | ||
|---|---|---|---|
| Benign epithelial neoplasms | |||
| Simple benign tumors | Adenoma—simple | 1 | 4.2 |
| Non-simple benign tumors | Complex adenoma | 3 | 12.5 |
| Ductal associated benign tumors | Intraductal papillary adenoma | 2 | 8.3 |
| Ductal adenoma | 1 | 4.2 | |
| Malignant epithelial neoplasms | |||
| Simple carcinoma | Tubulopapillary carcinoma | 8 | 33.3 |
| Solid carcinoma | 1 | 4.2 | |
| Non-simple carcinoma | Carcinoma arising in a complex adenoma/benign mixed tumor | 2 | 8.3 |
| Complex carcinoma | 2 | 8.3 | |
| Carcinoma and malignant myoepithelioma | 1 | 4.2 | |
| Mixed carcinoma | 2 | 8.3 | |
| Special Type | Adenosquamous carcinoma | 1 | 4.2 |
| Total | 24 | 100 | |
| N | % | |
|---|---|---|
| Necrosis (n = 24) | ||
| Absent | 19 | 79.2 |
| Present | 5 | 20.8 |
| Inflammatory infiltrate (n = 24) | ||
| Absent | 13 | 54.1 |
| Slight | 0 | 0.0 |
| Moderate | 4 | 16.7 |
| Marked | 7 | 29.2 |
| Vascular invasion (n = 17) | ||
| Absent | 14 | 82.4% |
| Present | 3 | 17.6% |
| Lymph node metastasis (n = 7) a | ||
| Absent | 4 | 57.1 |
| Present | 3 | 42.9 |
| Histological grade (n = 17) | ||
| Grade I | 7 | 41.2 |
| Grade II | 7 | 41.2 |
| Grade III | 3 | 17.6 |
| N | % | |
|---|---|---|
| ERα (n = 12) | ||
| ER+ | 12 | 100 |
| ER− | 0 | 0 |
| PR (n = 12) | ||
| PR+ | 7 | 58.3 |
| PR− | 5 | 41.7 |
| HER2 (n = 12) | ||
| 0 | 4 | 33.3 |
| 1+ | 6 | 50.0 |
| 2+ | 2 | 16.7 |
| 3+ | 0 | 0.0 |
| Ki-67 (n = 12) | ||
| Ki-67 low (≤20.9%) | 6 | 50.0 |
| Ki-67 high (>20.9%) | 6 | 50.0 |
| Subtype (n = 12) | ||
| Luminal A-like (Ki-67 index ≤ 20.9%) | 6 | 50.0 |
| Luminal B-like (HER2-negative) (Ki-67 index > 20.9%) | 6 | 50.0 |
| Luminal B-like (HER2-positive) | 0 | 0.0 |
| HER2-positive | 0 | 0.0 |
| Triple-negative | 0 | 0.0 |
| Case # | Breed | Gender | Age | Histology | Tumor Grade | Molecular Subtype |
|---|---|---|---|---|---|---|
| 1 | Mixed | ♀ | 10 | Complex carcinoma | I | Luminal B-like (HER2-negative) |
| 2 | French Bulldog | ♀ | 4 | Carcinoma arising in a complex adenoma | I | Luminal A-like |
| 3 | Mixed | ♀ | 7 | Carcinoma-and-malignant myoepithelioma | II | Luminal A-like |
| 4 | Mixed | ♀ | 15 | Solid carcinoma | III | Luminal B-like (HER2-negative) |
| 5 | Labrador retriever | ♀ | 7 | Tubulopapillary carcinoma | II | Luminal A-like |
| 6 | Mixed | ♀ | 6 | Tubulopapillary carcinoma | I | Luminal A-like |
| 7 | Mixed | ♀/n | 11 | Tubulopapillary carcinoma | III | Luminal B-like (HER2-negative) |
| 8 | Mixed | ♀ | 6 | Tubulopapillary carcinoma | II | Luminal B-like (HER2-negative) |
| 9 | Pinscher | ♀ | 6 | Complex carcinoma | II | Luminal A-like |
| 10 | Boxer | ♀ | 7 | Tubulopapillary carcinoma | I | Luminal A-like |
| 11 | Labrador | ♀/n | 7 | Mixed carcinoma | II | Luminal B-like (HER2-negative) |
| 12 | German shepherd | ♀ | 8 | Mixed carcinoma | I | Luminal B-like (HER2-negative) |
| Histological Type | Histological Grade | p | |
|---|---|---|---|
| Grade I | Grade II/III | ||
| Simple carcinoma | 2 (13.3%) | 7 (46.7%) | 0.329 |
| Non-simple carcinoma | 3 (20.0%) | 3 (20.0%) | |
| Total | 5 (33.35) | 10 (66.7%) | 15 (100.0%) |
| Histological Type | Immunophenotype | p | |
|---|---|---|---|
| Luminal A-Like | Luminal B-Like (HER2-Negative) | ||
| Simple carcinoma | 3 (25.0%) | 3 (25.0%) | 0.999 |
| Non-simple carcinoma | 3 (25.0%) | 3 (25.0%) | |
| Total | 6 (50.0%) | 6 (50.0%) | 12 (100.0%) |
| Case # | Tumor Grade | Diploidy (%) | Aneuploidy (%) (%Hypoploidy + %Hyperploidy) | Number of Polyploidy |
|---|---|---|---|---|
| 1 | I | 34.0 | 66.0 (58.0 + 8.0) | – |
| 2 | I | 28.0 | 72.0 (56.0 + 16.0) | 2 |
| 3 | II | 44.0 | 56.0 (40.0 + 16.0) | – |
| 7 | III | 26.0 | 74.0 (72.0 + 2.0) | 1 |
| 8 | II | 24.0 | 76.0 (68.0 + 8.0) | 2 |
| 9 | II | 38.0 | 62.0 (50.0 + 12.0) | 2 |
| 10 | I | 36.0 | 64.0 (52.0 + 12.0) | – |
| 11 | II | 22.0 | 78.0 (60.0 + 18.0) | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, T.; Miranda, M.; Pinto-Leite, R.; Mano, J.F.; Medeiros, R.; Oliveira, P.A.; Gama, A. Integrated Study of Canine Mammary Tumors Histopathology, Immunohistochemistry, and Cytogenetic Findings. Vet. Sci. 2024, 11, 409. https://doi.org/10.3390/vetsci11090409
Ferreira T, Miranda M, Pinto-Leite R, Mano JF, Medeiros R, Oliveira PA, Gama A. Integrated Study of Canine Mammary Tumors Histopathology, Immunohistochemistry, and Cytogenetic Findings. Veterinary Sciences. 2024; 11(9):409. https://doi.org/10.3390/vetsci11090409
Chicago/Turabian StyleFerreira, Tiago, Maria Miranda, Rosário Pinto-Leite, João F. Mano, Rui Medeiros, Paula A. Oliveira, and Adelina Gama. 2024. "Integrated Study of Canine Mammary Tumors Histopathology, Immunohistochemistry, and Cytogenetic Findings" Veterinary Sciences 11, no. 9: 409. https://doi.org/10.3390/vetsci11090409
APA StyleFerreira, T., Miranda, M., Pinto-Leite, R., Mano, J. F., Medeiros, R., Oliveira, P. A., & Gama, A. (2024). Integrated Study of Canine Mammary Tumors Histopathology, Immunohistochemistry, and Cytogenetic Findings. Veterinary Sciences, 11(9), 409. https://doi.org/10.3390/vetsci11090409








