Review of Liquid Vitamin A and E Formulations in Veterinary and Livestock Production: Applications and Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
2. Roles and Nutritional Needs across Animal Species
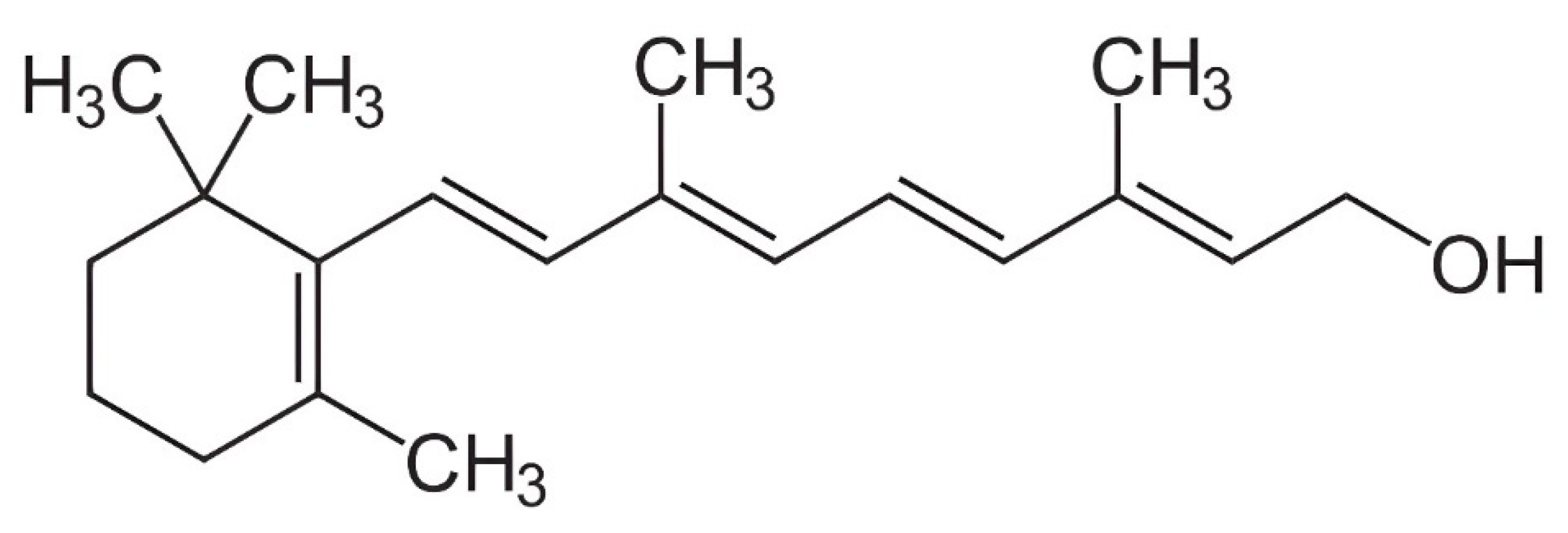
2.1. Vitamin A
- 1 IU of vitamin A is equivalent to
- ○
- 0.3 μg of retinol (vitamin A alcohol);
- ○
- 0.344 μg of retinyl acetate (vitamin A acetate);
- ○
- 0.359 μg of retinyl propionate (vitamin A propionate);
- ○
- 0.55 μg of retinyl palmitate (vitamin A palmitate).
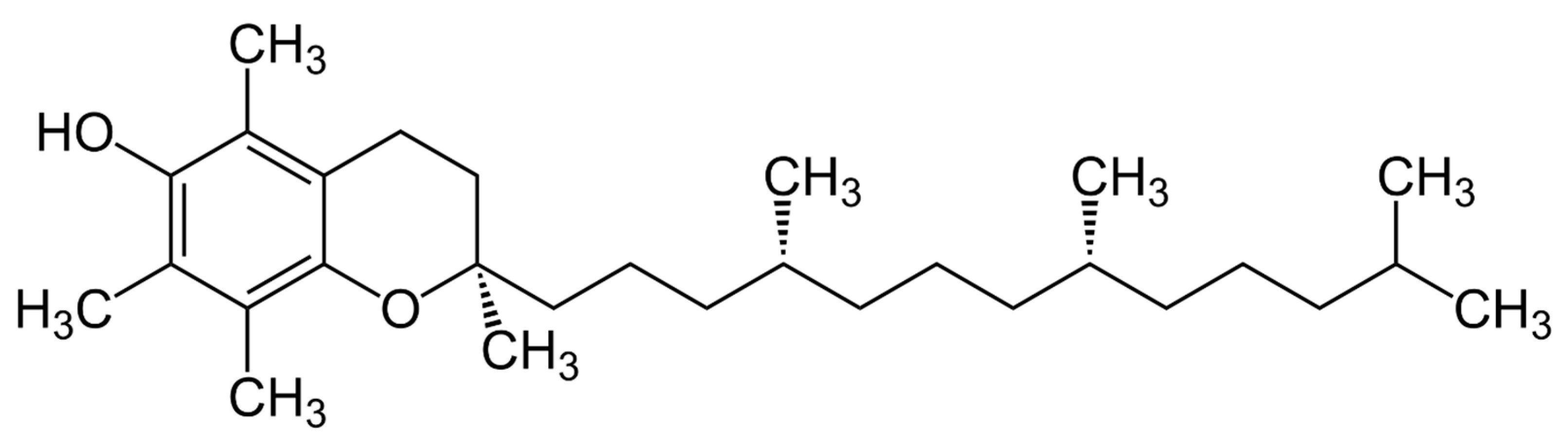
2.2. Vitamin E
- 1 mg of DL-alpha-tocopheryl acetate is equivalent to 1.0 IU of vitamin E;
- 1 mg of D-alpha-tocopheryl acetate is equivalent to 1.36 IU of vitamin E.
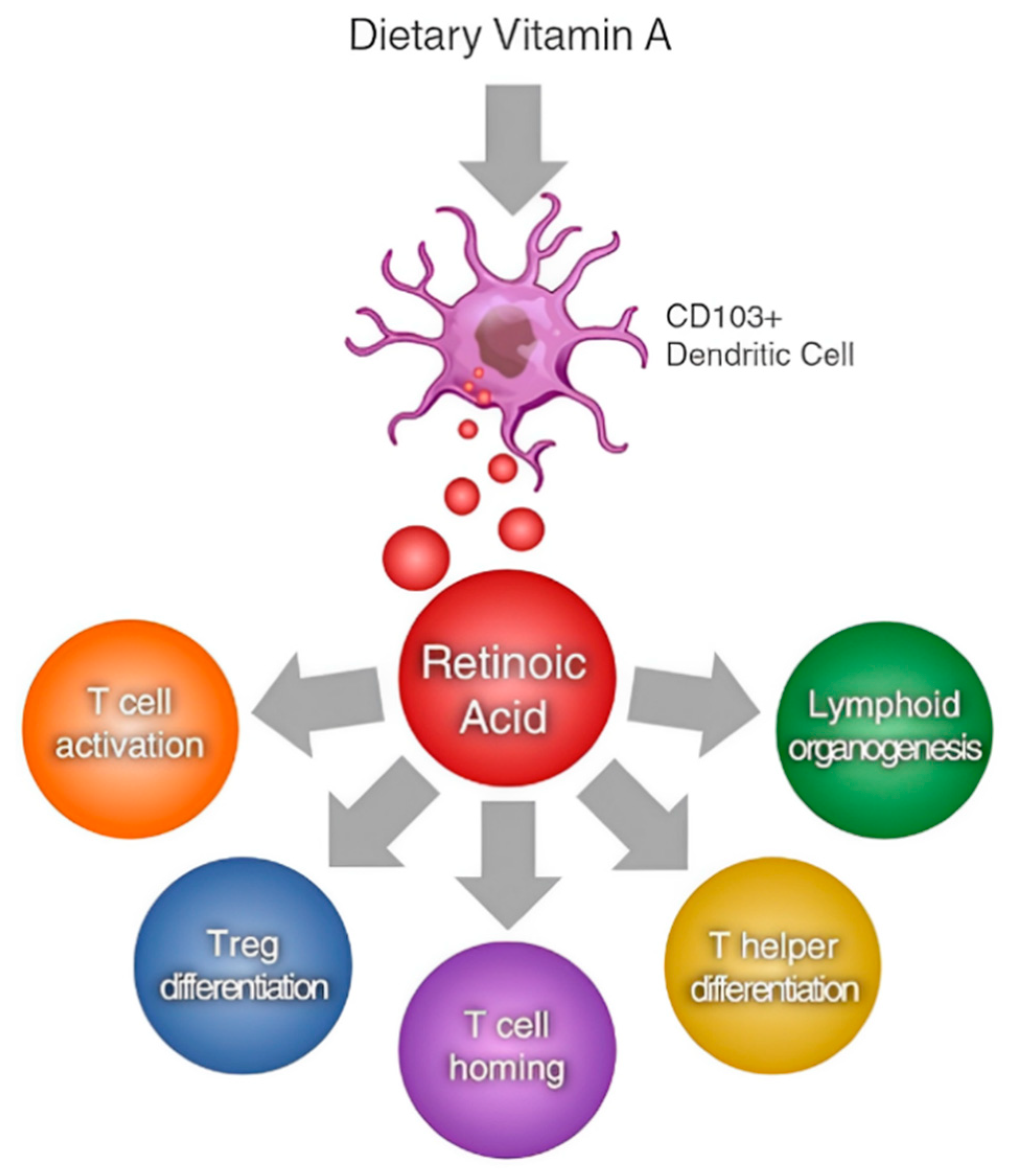
2.3. Interplay between Vitamins A and E
2.4. Nutritional Requirements in Different Animal Species
3. Consequences of Deficiencies in Vitamins A and E
4. Liquid Vitamin A and E Supplements
4.1. Stability and Formulation Strategies
4.2. Liquid Formulations of Vitamins A and E
- Oil-based solutions: Oil-based formulations often use vegetable oils, such as sunflower oil, as carriers for vitamins [111,112]. These formulations may also incorporate hydrophobic solvents like short-chain saturated triglycerides, ethyl stearate, or liquid paraffin [11]. A high-quality oil, especially one rich in α-tocopherol, can enhance the stability of vitamin A by protecting it from oxidation and improving its absorption in the gastrointestinal tract [113]. To further stabilize these formulations, compounds like BHT are frequently added, particularly in products containing retinyl propionate or retinyl palmitate [114]. It is important to note that vitamin A tends to be more stable in oily solutions compared to aqueous ones [11]. In these oil-based products, vitamin E acetate can be included either in its undiluted form or mixed with oils.
- Emulsions: Emulsions are a versatile and widely used drug formulation designed to enhance the solubility and bioavailability of vitamins, particularly for lipophilic vitamins such as A and E. These formulations are especially beneficial in aqueous environments where solubility can be challenging. The effectiveness of emulsions is often due to the creation of transparent microemulsions, which significantly improve the solubility and stability of these vitamins [110,115].These emulsions are particularly advantageous for species with less efficient fat digestion or in scenarios where rapid vitamin absorption is required. For instance, a standard emulsion product may contain 50,000 IU/mL of vitamin A, emulsified with lecithin and polysorbate 80 [11]. This combination creates a stable and homogeneous mixture, which ensures effective delivery and absorption of the vitamin. Polysorbate 80, a non-ionic solubilizer commonly used in both oral and topical pharmaceuticals, has hydrophobic and hydrophilic components. The primary hydrophobic part in polysorbate 80 is polyethylene glycol-20 sorbitan oleate, which plays a crucial role in the solubilization process [116].Another example of an emulsion is a vitamin A/D/E preparation for injection, which includes 23.0 g of retinyl propionate, 0.2 g of cholecalciferol, 5.5 g of DL-α-tocopheryl acetate, 15.0 g of PEG-15 hydroxystearate (as a solubilizer), 0.5 g of butylated hydroxytoluene, 1.0 g of benzyl alcohol, and water to make up 100 mL [11].A specialized type of emulsion is the microemulsion, where retinyl esters, for example, are emulsified in an aqueous solution containing substances such as gelatin and sugar [104]. Microemulsions consist of droplets of the oily phase, approximately 1 μm in diameter, and remain stable within a temperature range of 50–60 °C [115]. Additionally, various types of fat-soluble vitamin emulsions can be formulated, including oil-in-water, water-in-oil, and oil-in-water-in-oil emulsions, offering a range of options depending on the desired application [117].
- Aqueous Solutions: To effectively produce aqueous solutions of lipophilic vitamins such as A, D, E, and K, the use of solubilizers is essential. Key solubilizers include Polysorbate 80, PEG glyceryl trihydroxystearate, PEG glyceryl triricinoleate, and PEG hydroxystearate, all of which are known to form micelles that encapsulate these lipophilic vitamins, thereby facilitating their dissolution in water [11]. The quantity of solubilizer required can vary considerably depending on the specific vitamin. For instance, the solubilizer needed for vitamin E acetate differs from that required for vitamin A.Specific formulations can be used to prepare clear aqueous solutions, as detailed by Buehler [11]. For example, to produce unstabilized vitamin A drops (50,000 IU/mL), the formulation may include 3.0 g of retinyl palmitate, 10.0 g of PEG 40 glyceryl trihydroxystearate, 5.0 g of polyethylene glycol 400, and 100 mL of water. A vitamin E acetate solution (20 mg/mL) might consist of 2.0 g of DL-α-tocopheryl acetate, 8.0 g of polysorbate 80, and 100 mL of water. Additionally, a formulation for combined vitamin A and E drops (825,000 IU + 50 mg per ml, respectively) could use 1.50 g of retinyl palmitate, 5.0 g of DL-α-tocopheryl acetate, and 20.0 g of PEG 40 glyceryl trihydroxystearate, along with an appropriate amount of antioxidant, preservative, and flavoring, all dissolved in 100 mL of water. It is important to note that when using solubilizers, the effectiveness of preservatives must be carefully evaluated, as solubilizers can potentially diminish their efficacy.
5. Comparative Bioavailability
6. Applications in Veterinary Medicine and Livestock Production
6.1. Use of Injectable Forms for Enhancing Reproductive Performance
6.2. Use of Injectable Forms in Enhancing Animal Growth and Performance
6.3. Incorporating Liquid Vitamin A and E into Veterinary Practices via Water Administration
6.4. Usage of Liquid Vitamin A and E Drops in Veterinary Practice
6.5. Regulatory Guidance
7. Challenges in Applying Liquid Formulations across Animal Species
7.1. Cattle
7.2. Swine
7.3. Poultry
7.4. Pets
8. Perspectives and Future Directions
9. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Serine-threonine kinase B |
| ATRA | All-trans-retinoic acid |
| BHA | Butylhydroxyanisole |
| BHT | Butylhydroxytoluene |
| BW | Body weight |
| DM | Dry matter |
| EFSA | European Food Safety Authority |
| EMA | European Medicines Agency |
| FEEDAP | The Panel on Additives and Products or Substances used in Animal Feed |
| FRAP | Ferric Reducing Ability of Plasma |
| i.m. | Intramuscular |
| IBV | Infectious bronchitis virus |
| ID3 | DNA-binding inhibitor 3 |
| IgG | Immunoglobulin G |
| IU | International Unit |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| mTOR | Mammalian Target for Rapamycin |
| MYF5 | Myogenic Factor 5 (a protein coding gene) |
| MYOD | Myoblast Determination Protein |
| MYOG | Myogenin |
| NASEM | The National Academies of Sciences, Engineering, and Medicine |
| NFκB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NRF2 | Nuclear Factor Erythroid 2–related factor 2 |
| PAX7 | Paired Box 7 (a protein-coding gene) |
| PEG | Polyethylene glycol |
| RFM | Retained fetal membranes |
| s.c. | Subcutaneous |
| UV | Ultraviolet |
| 40BHLHE40 | Class E basic helix-loop-helix protein |
References
- Mordor Intelligence. Feed Vitamins Market Size & Share Analysis-Growth Trends & Forecasts up to 2029. 2024. Available online: https://www.mordorintelligence.com/industry-reports/global-feed-vitamins-market-industry/market-size (accessed on 5 July 2024).
- Shastak, Y.; Pelletier, W. The role of vitamin A in non-ruminant immunology. Front. Anim. Sci. 2023, 4, 1197802. [Google Scholar] [CrossRef]
- Shastak, Y.; Obermueller-Jevic, U.; Pelletier, W. A Century of Vitamin E: Early Milestones and Future Directions in Animal Nutrition. Agriculture 2023, 13, 1526. [Google Scholar] [CrossRef]
- Mishra, P.; Paital, B.; Jena, S.; Swain, S.S.; Kumar, S.; Yadav, M.K.; Chainy, G.B.N.; Samanta, L. Possible activation of NRF2 by Vitamin E/Curcumin against altered thyroid hormone induced oxidative stress via NFĸB/AKT/mTOR/KEAP1 signalling in rat heart. Sci. Rep. 2019, 9, 7408. [Google Scholar] [CrossRef]
- Subudhi, U.; Das, K.; Paital, B.; Bhanja, S.; Chainy, G.B. Supplementation of curcumin and vitamin E enhances oxidative stress, but restores hepatic histoarchitecture in hypothyroid rats. Life Sci. 2009, 84, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Yan, S.; Shi, B.; Bao, H.; Gong, J.; Guo, X.; Li, J. Effects of vitamin A on the milk performance, antioxidant functions and immune functions of dairy cows. Anim. Feed. Sci. Technol. 2014, 192, 15–23. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Su, G.; Shi, B.; Shan, A. High concentration of vitamin E supplementation in sow diet during the last week of gestation and lactation affects the immunological variables and antioxidative parameters in piglets. J. Dairy Res. 2017, 84, 8–13. [Google Scholar] [CrossRef]
- Idamokoro, E.M.; Falowo, A.B.; Oyeagu, C.E.; Afolayan, A.J. Multifunctional activity of vitamin E in animal and animal products: A review. Anim. Sci. J. 2020, 91, e13352. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Delving into Vitamin A Supplementation in Poultry Nutrition: Current Knowledge, Functional Effects, and Practical Implications. Worlds Poult. Sci. J. 2023, 79, 17. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Vitamin A supply in swine production: A review of current science and practical considerations. Appl. Anim. Sci. 2023, 39, 289–305. [Google Scholar] [CrossRef]
- Buehler, V. Vademecum for Vitamin Formulations, 2nd ed.; Medpharm Gmbh Scientific Publisher: Bahnhofsweg, Germany, 2001; 144p. [Google Scholar]
- Duque, P.; Vieira, C.P.; Bastos, B.; Vieira, J. The evolution of vitamin C biosynthesis and transport in animals. BMC Ecol. Evol. 2022, 22, 84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McDowell, L.R. Vitamin Nutrition of Livestock Animals: Overview from VitaminDiscovery to Today. Can. J. Anim. Sci. 2006, 86, 171–179. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Nutritional Balance Matters: Assessing the Ramifications of Vitamin A Deficiency on Poultry Health and Productivity. Poultry 2023, 2, 37. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Balancing Vitamin A Supply for Cattle: A Review of the Current Knowledge. In Advances in Animal Science and Zoology 21; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2023; Chapter 2; Available online: https://novapublishers.com/wp-content/uploads/2023/11/Advances-in-Animal-Science-and-Zoology.-Volume-21-Chapter-2.pdf (accessed on 14 December 2023).
- Shastak, Y.; Pelletier, W.; Kuntz, A. Insights into Analytical Precision: Understanding the Factors Influencing Accurate Vitamin A Determination in Various Samples. Analytica 2024, 5, 4. [Google Scholar] [CrossRef]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Nutrient Requirements of Dairy Cattle; The National Academies Press: Washington, DC, USA, 2021. [Google Scholar]
- Baker, D.H. Bioavailability of minerals and vitamins. In Swine Nutrition, 2nd ed.; Lewisand, A.J., Southern, L.L., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2001; pp. 357–379. [Google Scholar]
- Schmidt, M. Quick Nutritional Support through Drinking Water. 2019. Available online: https://www.pig333.com/articles/quick-nutritional-support-through-drinking-water_15166/ (accessed on 5 June 2024).
- Lindemann, M.D.; Brendemuhl, J.H.; Chiba, L.I.; Darroch, C.S.; Dove, C.R.; Estienne, M.J.; Harper, A.F. A regional evaluation of injections of high levels of vitamin A on reproductive performance of sows. J. Anim. Sci. 2008, 86, 333–338. [Google Scholar] [CrossRef]
- Mahmoud, G.B.; Abdel-Raheem, S.M.; Hussein, H.A. Effect of combination of vitamin E and selenium injections on reproductive performance and blood parameters of Ossimi rams. Small Rumin. Res. 2013, 113, 103–108. [Google Scholar] [CrossRef]
- Pontes, G.C.; Monteiro, P.L., Jr.; Prata, A.B.; Guardieiro, M.M.; Pinto, D.A.; Fernandes, G.O.; Wiltbank, M.C.; Santos, J.E.; Sartori, R. Effect of injectable vitamin E on incidence of retained fetal membranes and reproductive performance of dairy cows. J. Dairy Sci. 2015, 98, 2437–2449. [Google Scholar] [CrossRef]
- Wang, B.; Nie, W.; Fu, X.; de Avila, J.M.; Ma, Y.; Zhu, M.J.; Maquivar, M.; Parish, S.M.; Busboom, J.R.; Nelson, M.L.; et al. Neonatal vitamin A injection promotes cattle muscle growth and increases oxidative muscle fibers. J. Anim. Sci. Biotechnol. 2018, 9, 82. [Google Scholar] [CrossRef]
- Harris, C.L.; Wang, B.; Deavila, J.M.; Busboom, J.R.; Maquivar, M.; Parish, S.M.; McCann, B.; Nelson, M.L.; Du, M. Vitamin A administration at birth promotes calf growth and intramuscular fat development in Angus beef cattle. J. Anim. Sci. Biotechnol. 2018, 9, 55. [Google Scholar] [CrossRef]
- Awawdeh, M.S.; Eljarah, A.H.; Ababneh, M.M. Multiple injections of vitamin E and selenium improved the reproductive performance of estrus-synchronized Awassi ewes. Trop. Anim. Health Prod. 2019, 51, 1421–1426. [Google Scholar] [CrossRef]
- Silver, R.J. Liquid A Drops. Technical Report for Veterinarian Use Only. VBS Direct Ltd. 1 Mill View Close, Bulkeley, Cheshire, SY14 8DB. 2024. Available online: https://vbsdirect.co.uk/files/Vitamin_A_Tech_Report2.pdf (accessed on 10 February 2024).
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A update: Forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Kono, M.; Goletz, P.W.; Crouch, R.K. 11-cis- and all-trans-retinols can activate rod opsin: Rational design of the visual cycle. Biochemistry 2008, 47, 7567–7571. [Google Scholar] [CrossRef]
- Gaetano, C.; Catalano, A.; Illi, B.; Felici, A.; Minucci, S.; Palumbo, R.; Facchiano, F.; Mangoni, A.; Mancarella, S.; Mühlhauser, J.; et al. Retinoids induce fibroblast growth factor-2 production in endothelial cells via retinoic acid receptor alpha activation and stimulate angiogenesis in vitro and in vivo. Circ. Res. 2001, 88, E38–E47. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Gong, M.; He, Y.; Li, Q.; He, T.; Bi, Y. All-trans retinoic acid inhibits proliferation, migration, invasion and induces differentiation of hepa1-6 cells through reversing EMT in vitro. Int. J. Oncol. 2016, 48, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Brandebourg, T.D.; Hu, C.Y. Regulation of differentiating pig preadipocytes by retinoic acid. J. Anim. Sci. 2005, 83, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Nordling, S.; Vasavada, H.H.; Butcher, E.C.; Hirschi, K.K. Retinoic Acid Promotes Endothelial Cell Cycle Early G1 State to Enable Human Hemogenic Endothelial Cell Specification. Cell Rep. 2020, 33, 108465. [Google Scholar] [CrossRef] [PubMed]
- Cortes, P.L.; Tiwary, A.K.; Puschner, B.; Crespo, R.M.; Chin, R.P.; Bland, M.; Shivaprasad, H.L. Vitamin A deficiency in turkey poults. J. Vet. Diagn. Investig. 2006, 18, 489–494. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Teixeira, F.M.E.; Sato, M.N. Impact of retinoic acid on immune cells and inflammatory diseases. Mediat. Inflamm. 2018, 2018, 3067126. [Google Scholar] [CrossRef]
- Bono, M.R.; Tejon, G.; Flores-Santibañez, F.; Fernandez, D.; Rosemblatt, M.; Sauma, D. Retinoic Acid as a Modulator of T Cell Immunity. Nutrients 2016, 8, 349. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta 2012, 1821, 70–77. [Google Scholar] [CrossRef]
- Senoo, H.; Imai, K.; Mezaki, Y.; Miura, M.; Morii, M.; Fujiwara, M.; Blomhoff, R. Accumulation of vitamin A in the hepatic stellate cell of arctic top predators. Anat. Rec. 2012, 295, 1660–1668. [Google Scholar] [CrossRef]
- Dogru Pekiner, B. Vitamin E as an antioxidant. J. Fac. Pharm. Ank. 2003, 32, 243–267. [Google Scholar] [CrossRef]
- Ehizuelen Ebhohimen, I.; Stephen Okanlawon, T.; Ododo Osagie, A.; Norma Izevbigie, O. Vitamin E in Human Health and Oxidative Stress Related Diseases; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Pallauf, J.; Gohil, K.; Weber, S.U.; Packer, L.; Rimbach, G. Effect of selenium and vitamin E deficiency on differential gene expression in rat liver. Biochem. Biophys. Res. Commun. 2001, 285, 470–475. [Google Scholar] [CrossRef]
- Rimbach, G.; Minihane, A.M.; Majewicz, J.; Fischer, A.; Pallauf, J.; Virgli, F.; Weinberg, P.D. Regulation of cell signalling by vitamin E. Proc. Nutr. Soc. 2002, 61, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A.; Gysin, R.; Kempná, P.; Munteanu, A.; Negis, Y.; Villacorta, L.; Visarius, T.; Zingg, J.M. Vitamin E mediates cell signaling and regulation of gene expression. Ann. N. Y Acad. Sci. 2004, 1031, 86–95. [Google Scholar] [CrossRef]
- Zingg, J.M. Vitamin E: A Role in Signal Transduction. Annu. Rev. Nutr. 2015, 35, 135–173. [Google Scholar] [CrossRef]
- Ciarcià, G.; Bianchi, S.; Tomasello, B.; Acquaviva, R.; Malfa, G.A.; Naletova, I.; La Mantia, A.; Di Giacomo, C. Vitamin E and Non-Communicable Diseases: A Review. Biomedicines 2022, 10, 2473. [Google Scholar] [CrossRef]
- Hynd, P.I. (Ed.) Digestion in the mono-gastric animal. In Animal Nutrition: From Theory to Practice; CSIRO Publishing: Clayton South, Australia, 2019; pp. 42–63. [Google Scholar]
- Tesoriere, L.; Bongiorno, A.; Pintaudi, A.M.; D’Anna, R.; D’Arpa, D.; Livrea, M.A. Synergistic interactions between vitamin A and vitamin E against lipid peroxidation in phosphatidylcholine liposomes. Arch. Biochem. Biophys. 1996, 326, 57–63. [Google Scholar] [CrossRef]
- Tesoriere, L.; Ciaccio, M.; Bongiorno, A.; Riccio, A.; Pintaudi, A.M.; Livrea, M.A. antioxidant activity of all-trans-retinol in homogeneous solution and in phosphatidylcholine liposomes. Arch. Biochem. Biophys. 1993, 307, 217–223. [Google Scholar] [CrossRef]
- Guilland, J.-C. Les interactions entre les vitamines A, D, E et K: Synergie et/ou competition. OCL 2011, 18, 59–67. [Google Scholar] [CrossRef]
- Moore, T. The effect of vitamin E deficiency on vitamin A reserves of the rat. Biochem. J. 1940, 34, 1321. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Pang, S.-J.; Zhang, K.-W.; Li, P.-Y.; Li, P.-G.; Yang, C. Dietary vitamin A modifies the gut microbiota and intestinal tissue transcriptome, impacting intestinal permeability and the release of inflammatory factors, thereby influencing Aβ pathology. Front. Nutr. 2024, 11, 1367086. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Gonzalez, A.R.; Burrola-Barraza, M.E.; Dominguez-Viveros, J.; Chavez-Martinez, A. Rumen microorganisms and fermentation. Arch. Med. Vet. 2014, 46, 349–361. [Google Scholar] [CrossRef]
- Mitchell, G.E., Jr.; Little, C.O.; Hayes, B.W. Pre-intestinal destruction of vitamin A by ruminants fed nitrate. J. Anim. Sci. 1967, 26, 827–829. [Google Scholar] [CrossRef]
- Salinas-Chavira, J.; Arrizon, A.A.; Barreras, A.; Chen, C.Z.; Plascencia, A.; Zinn, R.A. Evaluation of supplemental vitamin A and E on 56-day growth performance, dietary net energy, and plasma retinol and tocopherol concentrations in Holstein steer calves. Prof. Anim. Sci. 2014, 30, 510–514. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. A cross-species perspective: β-carotene supplementation effects in poultry, swine, and cattle—A review. J. Appl. Anim. Res. 2024, 52, 2321957. [Google Scholar] [CrossRef]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Nutrient Requirements of Beef Cattle; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Nutrient Requirements of Dairy Cattle; National Academic Science: Washington, DC, USA, 2001. [Google Scholar]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Nutrient Requirements of Beef Cattle; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- GfE (German Society of Nutrition Physiology). Recommended Energy and Nutrient Supply for Dairy Cows and Growing Cattle, 8th ed.; GfE: Frankfurt am Main, Germany, 2001. [Google Scholar]
- INRA (National Institute of Agricultural Research). INRA Feeding System for Ruminants; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018. [Google Scholar]
- MOA (Ministry of Agriculture of the People’s Republic of China). Feeding Standard of Beef Cattle (NY/T 815-2004); Agriculture Press: Beijing, China, 2004. [Google Scholar]
- CVB Table Booklet Feeding of Ruminants. Nutrient Requirements for Cattle, Sheep and Goats and Nutritional Values of Feeding Ingredients for Ruminants; CVB-series no 66; Stichting CVB: Lelystad, the Netherlands, 2022. [Google Scholar]
- McDowell, L.R. Vitamin Supplementation. In Vitamins in Animal Nutrition: Comparative Aspects to Human Nutrition; McDowell, L.R., Ed.; Academic Press Inc.: Cambridge, MA, USA, 1989; pp. 422–431. [Google Scholar]
- Kırkpınar, F.; Açıkgöz, Z. Feeding. In Animal Husbandry and Nutrition; Yücel, B., Taşkin, T., Eds.; IntechOpen: London, UK, 2018; pp. 97–114. [Google Scholar] [CrossRef]
- Green, A.S.; Tang, G.; Lango, J.; Klasing, K.C.; Fascetti, A.J. Domestic cats convert [2H8]-β-carotene to [2H4]-retinol following a single oral dose. J. Anim. Physiol. Anim. Nutr. 2012, 96, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Shastak, Y.; Pelletier, W. Pet Wellness and Vitamin A: A Narrative Overview. Animals 2024, 14, 1000. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef]
- Brush, A.H. Metabolism of carotenoid pigments in birds. FASEB J. 1990, 4, 2969–2977. [Google Scholar] [CrossRef]
- Green, A.S.; Fascetti, A.J. Meeting the Vitamin A Requirement: The Efficacy and Importance of β-Carotene in Animal Species. Sci. World J. 2016, 2016, 7393620. [Google Scholar] [CrossRef] [PubMed]
- Cobb 500 Broiler Performance & Nutrition Supplement. 2022. Available online: https://www.cobb-vantress.com/assets/Cobb-Files/product-guides/5502e86566/2022-Cobb500-Broiler-Performance-Nutrition-Supplement.pdf (accessed on 26 July 2023).
- ROSS Broiler (Aviagen): Nutrition Specifications. 2022. Available online: https://aviagen.com/eu/brands/ross/products/ross-308 (accessed on 28 July 2023).
- Hy-Line W-36 Commercial Layers. 2020. Available online: https://www.hyline.com/filesimages/Hy-Line-Products/Hy-Line-Product-PDFs/W-36/36%20COM%20ENG.pdf (accessed on 25 July 2023).
- Lohmann LSL-Lite Management Guide. 2014. Available online: https://lohmann-breeders.com/media/strains/cage/management/LOHMANN-LSL-Lite-Cage-1.pdf (accessed on 25 August 2016).
- Hybrid Turkeys Vitamin and Trace Mineral Supplementation. 2016. Available online: https://www.hybridturkeys.com/en/resources/commercial-management/feed-and-water/vitamin-and-trace-mineral-supplementation/ (accessed on 28 July 2023).
- Nicholas, B.U.T. Heavy Lines Feeding Guidelines. 2015. Available online: https://www.aviagenturkeys.com/uploads/2015/11/20/NU06%20Feeding%20Guidelines%20for%20Nicholas%20&%20BUT%20Heavy%20Lines%20EN.pdf (accessed on 28 July 2023).
- ROSS Parent Stock. 2016. Nutrition Specifications. Available online: http://www.garantitavukculuk.com/doc/Ross308-PS-NS-EN[82].pdf (accessed on 16 June 2024).
- Cobb. 2020. Breeder Management. Accessed Supplement. Available online: https://cobbgenetics.com/assets/Cobb-Files/Cobb500-Fast-Feather-Breeder-Management-Supplement.pdf (accessed on 16 June 2024).
- PIC. 2016. PIC Nutrient Specification Manual. Available online: https://www.pic.com/wp-content/uploads/sites/3/2018/10/Nutrient-Specifications-Manual_2016_English_Metric.pdf (accessed on 16 June 2024).
- BHZP. 2016. Bundes Hybrid Zucht Programm, Dahlenburg-Ellringen, Germany. Available online: https://www.bhzp.de/en/ (accessed on 16 June 2024).
- Topigs Norsvin. 2016. Rearing Gilts and Sows. Manual Topigs 20. Available online: https://topigsnorsvin.com/tn-content/uploads/2020/02/Feeding-Manual-Norsvin-Duroc.pdf (accessed on 16 June 2024).
- Hypor (A Hendrix Genetics Company). 2017. Wean to Finish Feeding Guide Hypor Maxter. Available online: https://www.hypor.com/en/resources/management-guides/ (accessed on 14 May 2019).
- AWT (Arbeitsgemeinschaft für Wirkstoffe in der Tierernährung e.V.). Vitamins in Animal Nutrition: Vitamin E; AgriMedia: Eisenberg, Germany, 2002; p. 50. ISBN 3-86037-167-3. [Google Scholar]
- Asmundson, V.S.; Kratzer, F.H. Observation on vitamin A deficiency in turkey breeding stock. Poult. Sci. 1952, 31, 71–73. [Google Scholar] [CrossRef]
- Lima, H.J.D.; Souza, L.A.Z. Vitamin A in the diet of laying hens: Enrichment of table eggs to prevent nutritional deficiencies in humans. Worlds Poult. Sci. J. 2018, 74, 619–626. [Google Scholar] [CrossRef]
- Feng, Y.L.; Xie, M.; Tang, J.; Huang, W.; Zhang, Q.; Hou, S.S. Effects of vitamin A on growth performance and tissue retinol of starter White Pekin ducks. Poult. Sci. 2019, 98, 2189–2192. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.P.; Greaves, J.P.; Scott, M.G. Nutritional blindness in the cat. Exp. Eye Res. 1964, 3, 357–364. [Google Scholar] [CrossRef]
- Murkey, S.P.; Agarwal, A.; Pandit, P.; Kumar, S.; Jaiswal, A. Unveiling the Spectrum of Ophthalmic Manifestations in Nutritional Deficiencies: A Comprehensive Review. Cureus 2023, 15, e50311. [Google Scholar] [CrossRef]
- Palludan, B. The teratogenic effect of vitamin A deficiency in pigs. Acta Vet. Scand. 1961, 2, 32–59. [Google Scholar] [CrossRef]
- Orengo, J.; Hernández, F.; Martínez-Miró, S.; Sánchez, C.J.; Peres Rubio, C.; Madrid, J. Effects of Commercial Antioxidants in Feed on Growth Performance and Oxidative Stress Status of Weaned Piglets. Animals 2021, 11, 266. [Google Scholar] [CrossRef]
- Blaxter, K.L. The significance of selenium and vitamin E in nutrition. Muscular dystrophy in farm animals: Its cause and prevention. Proc. Nutr. Soc. 1962, 21, 211–216. [Google Scholar] [CrossRef]
- Hurley, W.L.; Doane, R.M. Recent developments in the roles of vitamins and minerals in reproduction. J. Dairy Sci. 1989, 72, 784–804. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Kitagawa, M.; Imai, H.; Yamada, M. The roles of vitamin A for cytoplasmic maturation of bovine oocytes. J. Reprod. Dev. 2005, 51, 23–35. [Google Scholar] [CrossRef]
- Threadgold, T.; Greenwood, E.C.; Van Wettere, W. Identifying Suitable Supplements to Improve Piglet Survival during Farrowing and Lactation. Animals 2021, 11, 2912. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M.; Burr, G.O. The anti-sterility fat soluble vitamin E. Proc. Natl. Acad. Sci. USA 1925, 11, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jiang, Z.; Jiang, S.; Li, L.; Lin, X.; Gou, Z.; Fan, Q. Dietary Vitamin A Supplementation Improved Reproductive Performance by Regulating Ovarian Expression of Hormone Receptors, Caspase-3 and Fas in Broiler Breeders. Poult. Sci. 2015, 95, 30–40. [Google Scholar] [CrossRef]
- Verstegen, J.; Dhaliwal, G.; Verstegen-Onclin, K. Canine and feline pregnancy loss due to viral and non-infectious causes: A review. Theriogenology 2008, 70, 304–319. [Google Scholar] [CrossRef]
- Barber, T.; Esteban-Pretel, G.; Marín, M.P.; Timoneda, J. Vitamin a deficiency and alterations in the extracellular matrix. Nutrients 2014, 6, 4984–5017. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Vitamin E as an in vitro and in vivo antioxidant. Ann. N. Y. Acad. Sci. 1989, 570, 7–22. [Google Scholar] [CrossRef]
- Shastak, Y.; Gordillo, A.; Pelletier, W. The relationship between vitamin A status and oxidative stress in animal production. J. Appl. Anim. Res. 2023, 51, 546–553. [Google Scholar] [CrossRef]
- Vetinfo. Liquid Vitamins for Dogs. 2024. Available online: https://www.vetinfo.com/liquid-vitamins-for-dogs.html (accessed on 23 June 2024).
- Finno, C.J. Veterinary Pet Supplements and Nutraceuticals. Nutr. Today 2020, 55, 97–101. [Google Scholar] [CrossRef]
- EMA (European Medicines Agency). Injectable Veterinary Medicinal Products Containing Vitamin A for Use in Food Producing Species. EMEA/V/A/141. 2021. Available online: https://www.ema.europa.eu/en/medicines/veterinary/referrals/injectable-veterinary-medicinal-products-containing-vitamin-use-food-producing-species (accessed on 27 June 2024).
- EFSA (European Food Safety Authority). Scientific opinion on the safety and efficacy of Vitamin A (Retinyl acetate, retinyl palmitate and retinyl propionate) as a feed additive for all animal species and categories. EFSA J. 2013, 11, 3037. [Google Scholar] [CrossRef]
- Galli, G.M.; Andretta, I.; Martinez, N.; Wernick, B.; Shastak, Y.; Gordillo, A.; Gobi, J. Stability of vitamin A at critical points in pet-feed manufacturing and during premix storage. Front. Vet. Sci. 2024, 11, 1309754. [Google Scholar] [CrossRef]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian. Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, J.S.; Kim, S.; Lee, J.H.; Shin, Y.C.; Choi, W.I. Super-Antioxidant Vitamin A Derivatives with Improved Stability and Efficacy Using Skin-Permeable Chitosan Nanocapsules. Antioxidants 2023, 12, 1913. [Google Scholar] [CrossRef]
- Pignitter, M.; Somoza, V. Sustainable Nutrition in a Changing World; The Stability of Vitamins A and E in Edible Oils; Springer International Publishing: Cham, Switzerland, 2017; pp. 295–305. [Google Scholar]
- Tadros, T.F. Surfactants, Industrial Applications. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 423–438. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- WHO; UNICEF; IVACG Task Force. Vitamin A Supplements: A Guide to Their Use in the Treatment and Prevention of Vitamin A Deficiency and Xerophthalmia, 2nd ed.; World Health Organization: Geneva, Switzerland, 1997; Available online: https://iris.who.int/bitstream/handle/10665/41947/9241545062.pdf?sequence=1&isAllowed=y (accessed on 27 May 2024).
- WHO. Guideline: Vitamin A Supplementation in Infants and Children 6–59 Months of Age; World Health Organization: Geneva, Switzerland, 2011; Available online: https://iris.who.int/bitstream/handle/10665/44664/9789241501767_eng.pdf (accessed on 27 May 2024).
- Yang, Y.; McClements, D.J. Vitamin E bioaccessibility: Influence of carrier oil type on digestion and release of emulsified α-tocopherol acetate. Food Chem. 2013, 141, 473–481. [Google Scholar] [CrossRef] [PubMed]
- BASF (Badische Anilin- und Sodafabrik). Vitamins. In BASF Technical Handbook Animal Nutrition; BASF SE, Animal Nutrition: Ludwigshafen, Germany, 2014; pp. 48–70. [Google Scholar]
- Broze, G.; Pucci, C.; Navarro, A. Formulation of Transparent and Nutritive Microemulsions. Patent Application MX2014000135A. 2012. Available online: https://patents.google.com/patent/MX2014000135A/en (accessed on 5 July 2024).
- Ravichandran, V.; Lee, M.; Nguyen Cao, T.G.; Shim, M.S. Polysorbate-Based Drug Formulations for Brain-Targeted Drug Delivery and Anticancer Therapy. Appl. Sci. 2021, 11, 9336. [Google Scholar] [CrossRef]
- Yoshida, K.; Sekine, T.; Matsuzaki, F.; Yanaki, T.; Yamaguchi, M. Stability of vitamin A in oil-in-water-in-oil-type multiple emulsions. J. Amer Oil Chem. Soc. 1999, 76, 1–6. [Google Scholar] [CrossRef]
- Song, Y.; Day, C.M.; Afinjuomo, F.; Tan, J.E.; Page, S.W.; Garg, S. Advanced Strategies of Drug Delivery via Oral, Topical, and Parenteral Administration Routes: Where Do Equine Medications Stand? Pharmaceutics 2023, 15, 186. [Google Scholar] [CrossRef]
- Polania Gutierrez, J.J.; Munakomi, S. Intramuscular Injection; StatPearls Publishing. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556121/ (accessed on 30 June 2024).
- Rahnfeld, L.; Luciani, P. Injectable lipid-based depot formulations: Where do we stand? Pharmaceutics 2020, 12, 567. [Google Scholar] [CrossRef]
- Brief, S.; Chew, B.P. Effects of vitamin A and beta-carotene on reproductive performance in gilts. J. Anim. Sci. 1985, 60, 998–1004. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs-The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Coffey, M.T.; Britt, J.H. Enhancement of sow reproductive performance by beta-carotene or vitamin A. J. Anim. Sci. 1993, 71, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Silveira, P.R.; Fernandes, L.C.O.; Filho, J.C.M.; Junior, W.B. Efeito da vitamina A no desempenho reprodutivo de porcas [Effect of vitamin A on the reproductive performance of sows]. Rev. Bras. Zootec. 1997, 27, 743–748. [Google Scholar]
- Whaley, S.L.; Hedgpeth, V.S.; Farin, C.E.; Martus, N.S.; Jayes, F.C.L.; Britt, J.H. Influence of vitamin A injection before mating on oocyte development, follicular hormones, and ovulation in gilts fed high-energy diets. J. Anim. Sci. 2000, 78, 1598–1607. [Google Scholar] [CrossRef]
- Tokach, M.D.; Goodband, R.D.; Nelssen, J.L. Influence of a single injection of beta-carotene and or vitamin A at weaning on subsequent reproductive performance of sows. In Kansas State University Swine Day; Kansas State University: Mannhattan, KS, USA, 1994; p. 11. [Google Scholar]
- Pusateri, A.E.; Diekman, M.A.; Singleton, W.L. Failure of vitamin A to increase litter size in sows receiving injections at various stages of gestation. J. Anim. Sci. 1999, 77, 1532–1535. [Google Scholar] [CrossRef]
- Ryan, T.; Liu, J.; Chu, A.; Wang, L.; Blais, A.; Skerjanc, I.S. Retinoic acid enhances skeletal myogenesis in human embryonic stem cells by expanding the premyogenic progenitor population. Stem Cell Rev. Rep. 2012, 8, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Chen, X.; Zhao, J.; Li, Q.; Li, X.; Wang, Y.; Wang, B.; Zhao, J. Vitamin A injection at birth improves muscle growth in lambs. Anim Nutr. 2023, 14, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhao, J.; Zhang, W.; Li, X.; Ji, B.; Zhao, J. Vitamin a potentiates sheep myoblasts myogenic differentiation through BHLHE40-modulated ID3 expression. BMC Genom. 2024, 25, 244. [Google Scholar] [CrossRef]
- Smołucha, G.; Steg, A.; Oczkowicz, M. The Role of Vitamins in Mitigating the Effects of Various Stress Factors in Pigs Breeding. Animals 2024, 14, 1218. [Google Scholar] [CrossRef]
- Akinmoladun, O.F.; Muchenje, V.; Fon, F.N.; Mpendulo, C.T. Small Ruminants: Farmers’ Hope in a World Threatened by Water Scarcity. Animals 2019, 9, 456. [Google Scholar] [CrossRef]
- Amazan, D.; Rey, A.I.; Fernández, E.; López-Bote, C.J. Natural vitamin E (d-α-tocopherol) supplementation in drinking water prevents oxidative stress in weaned piglets. Livest. Sci. 2012, 145, 55–62. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar] [PubMed]
- Weir, M.; Ward, E. Giving Liquid Medication to Dogs. VCA Animal Hospitals. 2024. Available online: https://vcahospitals.com/know-your-pet/giving-liquid-medication-to-dogs (accessed on 27 June 2024).
- Zhang, L.; Hou, Y.; Ma, Z.; Xie, J.; Fan, J.; Jiao, Y.; Wang, F.; Han, Z.; Liu, S.; Ma, D. Effect of oral vitamin A supplementation on host immune response to infectious bronchitis virus infection in specific pathogen-free chicken. Poult. Sci. 2023, 102, 102701. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, S.E.; Winyard, P.G.; Guo, R.; Kidd, B.; Merry, P.; Langrish-Smith, A.; Hansen, C.; Ramm, S.; Blake, D.R. Putative analgesic activity of repeated oral doses of vitamin E in the treatment of rheumatoid arthritis. Results of a prospective placebo controlled double blind trial. Ann. Rheum. Dis. 1997, 56, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.; Saxena, R.; Gupta, V. Efficacy of vitamin E in knee osteoarthritis management of North Indian geriatric population. Ther. Adv. Musculoskelet. Dis. 2012, 4, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; de Oliveira El Warrak, A.; Troncy, E.; Beaudry, F.; Chorfi, Y. Anti-inflammatory response of dietary vitamin E and its effects on pain and joint structures during early stages of surgically induced osteoarthritis in dogs. Can. J. Vet. Res. 2013, 77, 191–198. [Google Scholar]
- EFSA (European Food Safety Authority). Scientific opinion of the panel on additives and products or substances used in animal feed (FEEDAP) on a request from the European commission on the consequences for the consumer of the use of Vitamin A in animal nutrition. EFSA J. 2008, 873, 1–81. [Google Scholar] [CrossRef]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Durjava, M.F.; Kouba, M.; Lopez-Alonso, M.; Lopez Puente, S.L.; et al. Scientific Opinion on the assessment of a feed additive consisting of all-rac-alpha-tocopheryl acetate (vitamin E) for all animal species for the renewal of its authorisation (NHU Europe GmbH). EFSA J. 2021, 19, 6533. [Google Scholar] [CrossRef]
- Kelly, D.N.; Sleator, R.D.; Murphy, C.P.; Conroy, S.B.; Judge, M.M.; Berry, D.P. Large variability in feeding behavior among crossbred growing cattle. J. Anim. Sci. 2020, 98, skaa216. [Google Scholar] [CrossRef]
- Ferguson, T.I.; Emery, S.; Price-Davies, R.; Cosslett, A.G. A review of stability issues associated with vitamins in parenteral nutrition. e-SPEN J. 2014, 9, e49–e53. [Google Scholar] [CrossRef]
- Somagond, Y.M.; Alhussien, M.N.; Dang, A.K. Repeated injection of multivitamins and multiminerals during the transition period enhances immune response by suppressing inflammation and oxidative stress in cows and their calves. Front. Immunol. 2023, 14, 1059956. [Google Scholar] [CrossRef] [PubMed]
- Wooten, H.; Kim, H.; Rakhshandeh, A.R.; Rakhshandeh, A. Glucocorticoid Receptor Agonists to Improve the Productivity and Health of Early-Weaned Pigs: What is the Best Method of Delivery? Animals 2020, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.A.; Suzuki, K. Genetics of Residual Feed Intake in Cattle and Pigs: A Review. Asian-Australas. J. Anim. Sci. 2009, 22, 747–755. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Pišlar, M.; Kristl, A.; Roškar, R. Comprehensive Stability Study of Vitamin D3 in Aqueous Solutions and Liquid Commercial Products. Pharmaceutics 2021, 13, 617. [Google Scholar] [CrossRef]
- Gibbs, M.; Winnig, M.; Riva, I.; Dunlop, N.; Waller, D.; Klebansky, B.; Logan, D.W.; Briddon, S.J.; Holliday, N.D.; McGrane, S.J. Bitter taste sensitivity in domestic dogs (Canis familiaris) and its relevance to bitter deterrents of ingestion. PLoS ONE 2022, 17, e0277607. [Google Scholar] [CrossRef]
- Ford, R.B.; Mazzaferro, E.M. Diagnostic and Therapeutic Procedures. In Kirk and Bistner’s Handbook of Veterinary Procedures and Emergency Treatment; Elsevier: Amsterdam, The Netherlands, 2006; pp. 449–572. [Google Scholar] [CrossRef]
- Adenot, C.C.; Abdelhakim, H.E. Palatability assessment of oral dosage forms for companion animals: A systematic review. J. Drug Deliv. Sci. Technol. 2022, 77, 103841. [Google Scholar] [CrossRef]
- Dumlu, B. Importance of Nano-Sized Feed Additives in Animal Nutrition. J. Agric. Prod. 2024, 5, 55–72. [Google Scholar] [CrossRef]
- Hassan, A.A.; Mansour, M.K.; El Hamaky, A.M.; Sayed El Ahl, R.M.; Oraby, N.H. Nanomaterials and nanocomposite applications in veterinary medicine. Multifunct. Hybrid Nanomater. Sustain. Agri-Food Ecosyst. 2020, 2020, 583–638. [Google Scholar] [CrossRef]
- Praca, F.G.; Viegas, J.S.R.; Peh, H.Y.; Garbin, T.N.; Medina, W.S.G.; Bentley, M.V.L.B. Microemulsion co-delivering vitamin A and vitamin E as a new platform for topical treatment of acute skin inflammation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110639. [Google Scholar] [CrossRef]


| Committee | Adequate Intake of Vitamin A, IU/kg BW | |||
|---|---|---|---|---|
| Growing Heifers | Dry Cows | Lactating Cows, (<35 kg milk/d) | Lactating Cows, (>35 kg milk/d) | |
| NASEM [58] | 110 | 110 | 110 | n/a |
| NASEM [17] | 110 | 110 | 110 | 110 + (1000 × (milk-35)) |
| Adequate Intake of Vitamin E, IU/kg BW | ||||
| NASEM [58] | 0.8 | 1.6 | 0.8 | n/a |
| NASEM [17] | 0.8 | 1.6 | 0.8 (3) * | 0.8 (3) * |
| Committee | Adequate Intake of Vitamin A, IU/kg DM Intake | ||
|---|---|---|---|
| Growing and Finishing | Gestating Cows | Lactating Cows | |
| NASEM [57] | 2200 | 2800 | 3900 |
| NASEM [59] | 2200 | 2800 | 3900 |
| Adequate Intake of Vitamin E, IU/kg DM Intake | |||
| NASEM [57] | 15–60 | - | - |
| NASEM [59] | 15–60 | 15–20 | 40–60 |
| Animal Species | Vitamin A Recommendation, IU/kg Feed | Vitamin E Recommendation, IU/kg Feed | Source |
|---|---|---|---|
| Broilers | 10,000–13,000 | 55–80 | [71,72] |
| Laying hens | 8000–13,000 | 20–30 | [73,74] |
| Turkeys | 5000–12,500 | 20–100 | [75,76] |
| Broiler breeders | 10,000–13,000 | 100 | [77,78] |
| Piglets | 8000–16,000 | 84–150 | [79,80,81,82] |
| Finishing gilts and barrows | 4000–10,000 | 33–80 | [79,80,81,82] |
| Lactating gilts and sows | 9920–14,000 | 60–100 | [79,80,81,82] |
| Dogs | 8000–12,000 | 80–120 | [83] |
| Cats | 15,000–25,000 | 100–150 | [83] |
| Disorder | Animal Model | Compromised Organ/Tissue |
|---|---|---|
| Immune deficiency | Chick, pig | Mononuclear phagocyte system |
| Myopathic disorders | Rabbit, duck, lamb, calf, turkey, chicken | Heart, skeletal muscles, gizzard |
| Reproductive dysfunction | ||
| embryonic apoptosis | Hen, turkey, cow | Embryonic circulatory system |
| infertility (male) | Rooster, rabbit | Testes |
| Kidney, pancreas, liver, brain, blood | ||
| necrobiosis | Pig | Liver |
| erythrocyte hemolysis | Chick, calf | Red blood cells |
| hypoproteinemia | Chick, turkey | Ricin |
| cerebral softening | Chick, duckling | Encephalon |
| hemorrhagic diathesis | Chick, turkey | Vascular system |
| nephrosis | Mink, rat | Renal tubular |
| yellow fat disease | Pig | Adipose tissue |
| Parameter | 0 IU (n = 9) | 150,000 IU (n = 7) | 300,000 IU (n = 9) | SE |
|---|---|---|---|---|
| Birth to weaning | ||||
| Birth weight, kg | 35.1 | 35.6 | 35.6 | 0.58 |
| Average daily gain, kg/d | 0.88 b | 0.98 a | 1.00 a | 0.02 |
| Backgrounding | ||||
| Weaning weight at d 210, kg | 219.2 | 248.7 | 246.0 | 5.98 |
| Gain/feed ratio, kg | 0.149 | 0.153 | 0.156 | 0.031 |
| Finishing | ||||
| Weight at 308 d, kg | 312.1 b | 333.0 ab | 339.7 a | 8.65 |
| Feed/gain ratio, kg | 4.35 | 4.76 | 4.79 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shastak, Y.; Pelletier, W. Review of Liquid Vitamin A and E Formulations in Veterinary and Livestock Production: Applications and Perspectives. Vet. Sci. 2024, 11, 421. https://doi.org/10.3390/vetsci11090421
Shastak Y, Pelletier W. Review of Liquid Vitamin A and E Formulations in Veterinary and Livestock Production: Applications and Perspectives. Veterinary Sciences. 2024; 11(9):421. https://doi.org/10.3390/vetsci11090421
Chicago/Turabian StyleShastak, Yauheni, and Wolf Pelletier. 2024. "Review of Liquid Vitamin A and E Formulations in Veterinary and Livestock Production: Applications and Perspectives" Veterinary Sciences 11, no. 9: 421. https://doi.org/10.3390/vetsci11090421






