Review of the Current Status on Ruminant Abortigenic Pathogen Surveillance in Africa and Asia
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
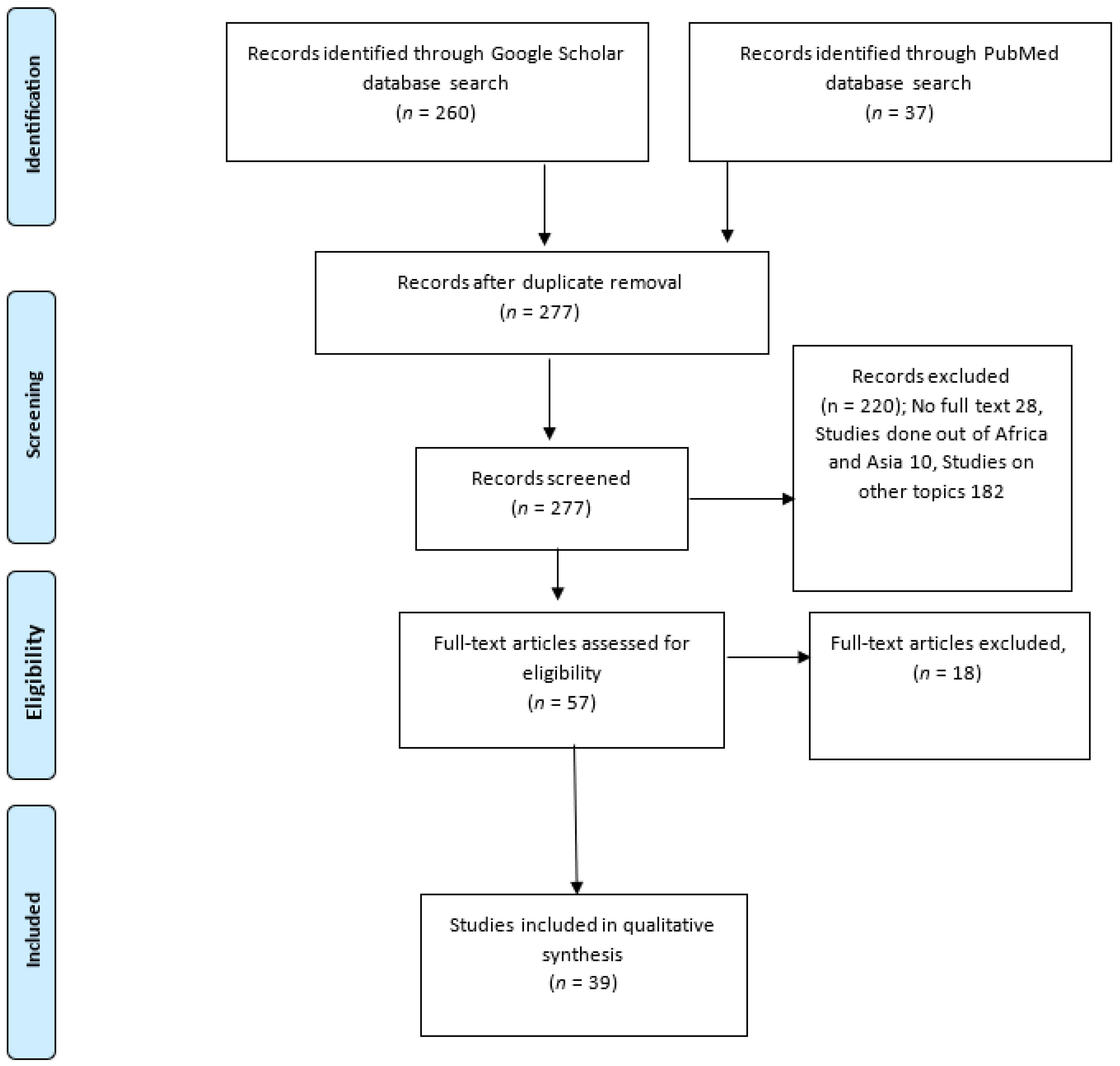
2.1. Study Design and Systematic Review Protocol
2.2. Search Strategy
2.3. Exclusion Criteria
- (i)
- If the numerator (i.e., number positive) and denominator (i.e., number tested) information were not reported at the species and sample type levels;
- (ii)
- If they were in a language other than English. When required, a third reviewer (TK) served as a tiebreaker, independently reviewing articles to resolve disagreements between the two primary reviewers.
2.4. Article Selection and Data Extraction
2.5. Analysis
3. Results
Median Sero-Prevalence of Abortigenic Pathogens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tibary, A. Abortion in Cattle–Reproductive System. Available online: https://www.msdvetmanual.com/reproductive-system/abortion-in-large-animals/abortion-in-cattle (accessed on 12 October 2021).
- Givens, M.D. A Clinical, Evidence-Based Approach to Infectious Causes of Infertility in Beef Cattle. Theriogenology 2006, 66, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Semango, G.; Hamilton, C.M.; Kreppel, K.; Katzer, F.; Kibona, T.; Lankester, F.; Allan, K.J.; Thomas, K.M.; Claxton, J.R.; Innes, E.A.; et al. The Sero-Epidemiology of Neospora Caninum in Cattle in Northern Tanzania. Front. Vet. Sci. 2019, 6, 327. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, N.A.; Thomas, L.F.; Cook, E.A.J.; de Glanville, W.A.; Atkinson, P.M.; Wamae, C.N.; Fèvre, E.M. The Sero-Epidemiology of Coxiella Burnetii in Humans and Cattle, Western Kenya: Evidence from a Cross-Sectional Study. PLoS Neglected Trop. Dis. 2016, 10, e0005032. [Google Scholar] [CrossRef] [PubMed]
- Madzingira, O.; Fasina, F.O.; Kandiwa, E.; Musilika-Shilongo, A.; Chitate, F.; van Heerden, H. A Retrospective Sero-Epidemiological Survey of Bovine Brucellosis on Commercial and Communal Farming Systems in Namibia from 2004 to 2018. Trop Anim Health Prod 2020, 52, 3099–3107. [Google Scholar] [CrossRef]
- Oyas, H.; Holmstrom, L.; Kemunto, N.P.; Muturi, M.; Mwatondo, A.; Osoro, E.; Bitek, A.; Bett, B.; Githinji, J.W.; Thumbi, S.M.; et al. Enhanced Surveillance for Rift Valley Fever in Livestock during El Niño Rains and Threat of RVF Outbreak, Kenya, 2015-2016. PLoS Neglected Trop. Dis. 2018, 12, e0006353. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.-Y.; Jeoung, H.-Y.; Yeh, J.-Y.; Cho, Y.-S.; Choi, J.-S.; Lee, J.-Y.; Cho, I.-S.; Yoo, H.-S. Serological Surveillance Studies Confirm the Rift Valley Fever Virus Free Status in South Korea. Trop. Anim. Health Prod. 2015, 47, 1427–1430. [Google Scholar] [CrossRef]
- Esubalew, S.; Tarekegn, Z.S.Z.; Jemberu, W.T.; Nigatu, S.D.; Kussa, M.; Tsegaye, A.A.; Asteraye, G.B.; Bogale, B.; Kebede, M.C. Seroepidemiology of Toxoplasma Gondii in Small Ruminants in Northwest Ethiopia. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100456. [Google Scholar] [CrossRef]
- Njiro, S.M.; Kidanemariam, A.G.; Tsotetsi, A.M.; Katsande, T.C.; Mnisi, M.; Lubisi, B.A.; Potts, A.D.; Baloyi, F.; Moyo, G.; Mpofu, J.; et al. A Study of Some Infectious Causes of Reproductive Disorders in Cattle Owned by Resource-Poor Farmers in Gauteng Province, South Africa. J. S. Afr. Vet. Assoc. 2011, 82, 213–218. [Google Scholar] [CrossRef]
- Moore, D.; Reichel, M.; Spath, E.; Campero, C. Neospora Caninum Causes Severe Economic Losses in Cattle in the Humid Pampa Region of Argentina. Trop. Anim. Health Prod. 2013, 45, 1237–1241. [Google Scholar] [CrossRef]
- Nicolino, R.R.; Capanema, R.O.; de Oliveira, C.S.F.; Pastrana, M.E.O.; Lopes, L.B.; Haddad, J.P.A. Estimating the Abortion Risk Difference in Neospora Caninum Seropositive Dairy Cattle in Brazil. Ciência Rural 2015, 45, 1629–1633. [Google Scholar] [CrossRef]
- Semango, G.; Yoder, J.; Kibona, T.; Claxton, J.R.; Buza, J.; Mmbaga, B.T.; Johnson, S.S.; Cleaveland, S.; Lankester, F. Economic Burden of Livestock Abortions in Northern Tanzania. J. Agric. Appl. Econ. 2024, 56, 195–215. [Google Scholar] [CrossRef]
- Berezowski, J. Veterinary Surveillance. EOLSS 2002, 6, 153. [Google Scholar]
- OIE Animal Health Surveillance. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_surveillance_general.pdf (accessed on 18 May 2024).
- Tonouhewa, A.B.N.; Akpo, Y.; Sherasiya, A.; Sessou, P.; Adinci, J.M.; Aplogan, G.L.; Youssao, I.; Assogba, M.N.; Farougou, S. A Serological Survey of Toxoplasma Gondii Infection in Sheep and Goat from Benin, West-Africa. J. Parasit. Dis. 2019, 43, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, U.W.; Bagnall, R.; Perrett, K.; Bosch, B.; Horner, R.; Gummow, B. A Serological Prevalence Survey of Brucella Abortus in Cattle of Rural Communities in the Province of KwaZulu-Natal, South Africa. J. S. Afr. Vet. Assoc. 2008, 79, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Gomo, C.; de Garine-Wichatitsky, M.; Caron, A.; Pfukenyi, D.M. Survey of Brucellosis at the Wildlife-Livestock Interface on the Zimbabwean Side of the Great Limpopo Transfrontier Conservation Area. Trop. Anim. Health Prod. 2012, 44, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Alhaji, N.B.; Babalobi, O.O.; Wungak, Y.; Ularamu, H.G. Participatory Survey of Rift Valley Fever in Nomadic Pastoral Communities of North-Central Nigeria: The Associated Risk Pathways and Factors. PLoS Neglected Trop. Dis. 2018, 12, e0006858. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Häsler, B.; Komba, E.; Sindato, C.; Rweyemamu, M.; Mlangwa, J. Towards an Integrated Animal Health Surveillance System in Tanzania: Making Better Use of Existing and Potential Data Sources for Early Warning Surveillance. BMC Vet. Res. 2021, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Chethan Kumar, H.B.; Hiremath, J.; Yogisharadhya, R.; Balamurugan, V.; Jacob, S.S.; Manjunatha Reddy, G.B.; Suresh, K.P.; Shome, R.; Nagalingam, M.; Sridevi, R.; et al. Animal Disease Surveillance: Its Importance & Present Status in India. Indian J. Med. Res. 2021, 153, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Namayanja, J.; Dione, M.; Kungu, J.M. Stakeholders’ Perceptions on Performance of the Livestock Disease Surveillance System in Uganda: A Case of Pallisa and Kumi Districts. Pastoralism 2019, 9, 12. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- UN Statistics Division. Available online: https://unstats.un.org/home/ (accessed on 20 August 2021).
- Swai, E.S.; Hulsebosch, J.; Van der Heijden, W. Prevalence of Genital Campylobacteriosis and Trichomonosis in Crossbred Breeding Bulls Kept on Zero-Grazed Smallholder Dairy Farms in the Tanga Region of Tanzania. J. S. Afr. Vet. Assoc. 2005, 76, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Otim, C.P.; Ocaido, M.; Okuna, N.M.; Erume, J.; Ssekitto, C.; Wafula, R.Z.O.; Kakaire, D.; Walubengo, J.; Okello, A.; Mugisha, A. Disease and Vector Constraints Affecting Cattle Production in Pastoral Communities of Ssembabule District, Uganda. Livest. Res. Rural. Dev. 2004, 16, 1–5. [Google Scholar]
- Sandhu, K.S.; Ball, M.S.; Kumar, H.; Sharma, S.; Sidhu, P.K.; Sreekumar, C.; Dubey, J.P. Seroprevalence of Neospora Caninum Antibodies in Cattle and Water Buffaloes in India. J. Parasitol. 2007, 93, 1374–1377. [Google Scholar]
- Chevalier, V.; Thiongane, Y.; Etter, E.; Lancelot, R. Serological Follow up of Rift Valley Fever in a Sahelian Ecosystem 2004; CIRAD: Paris, France, 2007. [Google Scholar]
- Matope, G.; Bhebhe, E.; Muma, J.B.; Lund, A.; Skjerve, E. Risk Factors for Brucella Spp. Infection in Smallholder Household Herds. Epidemiol. Infect. 2011, 139, 157–164. [Google Scholar] [CrossRef]
- Kroc, J.M.; Ochi, E.B. Short-Communication: Sero-Survey of Anti Rift Valley Fever Virus (RVFV) Antibodies in Sheep and Goats in Kapoeta, Eastrn Equatoria State, Sudan. Sudan J. Vet. Res. 2009, 24, 65–67. [Google Scholar]
- Jonker, A.; Michel, A. Retrospective Study of Bacterial and Fungal Causes of Abortion in Domestic Ruminants in Northern Regions of South Africa (2006–2016). Aust. Vet. J. 2021, 99, 66–71. [Google Scholar] [CrossRef]
- Ferede, Y.; Mengesha, D.; Mekonen, G. Study on the Seroprevalence of Small Ruminant Brucellosis in and around Bahir Dar, North West Ethiopia. Ethiop. Vet. J. 2011, 15, 2. [Google Scholar] [CrossRef]
- Degefa, T.; Duressa, A.; Duguma, R. Brucellosis and Some Reproductive Problems of Indigenous Arsi Cattle in Selected Arsi Zone’s of Oromia Regional State, Ethiopia. Glob. Vet. 2011, 7, 45–53. [Google Scholar]
- Barkallah, M.; Gharbi, Y.; Hassena, A.B.; Slima, A.B.; Mallek, Z.; Gautier, M.; Greub, G.; Gdoura, R.; Fendri, I. Survey of Infectious Etiologies of Bovine Abortion during Mid- to Late Gestation in Dairy Herds. PLoS ONE 2014, 9, e91549. [Google Scholar] [CrossRef]
- Moiane, B.T. Rift Valley Fever in Mozambique; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2017. [Google Scholar]
- Matope, G.; Bhebhe, E.; Muma, J.B.; Oloya, J.; Madekurozwa, R.L.; Lund, A.; Skjerve, E. Seroprevalence of Brucellosis and Its Associated Risk Factors in Cattle from Smallholder Dairy Farms in Zimbabwe. Trop. Anim. Health Prod. 2011, 43, 975–982. [Google Scholar] [CrossRef]
- Jafarizadeh, A.; Pourbakhsh, S.A.; Tadayon, K.; Jamshidian, M.; Ashtari, A. Mixed Infection Zones May Be Important in the Epidemiology of Contagious Agalactia. J. Vet. Res. 2016, 60, 159–162. [Google Scholar] [CrossRef][Green Version]
- Ligi, J.; Sengupta, P.P.; Rudramurthy, G.R.; Rahman, H. A Pilot Sero-Survey for Surra in Livestock in Karnataka by ELISA Using Flagellar Antigen of Trypanosoma Evansi. Int. J. Fundam. Appl. Sci. 2015, 4, 99–103. [Google Scholar]
- Hwang, J.-M.; Kim, J.G.; Yeh, J.-Y. Serological Evidence of Bluetongue Virus Infection and Serotype Distribution in Dairy Cattle in South Korea. BMC Vet. Res. 2019, 15, 255. [Google Scholar] [CrossRef]
- Bronsvoort, B.M.; Kelly, R.F.; Freeman, E.; Callaby, R.; Bagninbom, J.M.; Ndip, L.; Handel, I.G.; Tanya, V.N.; Morgan, K.L.; Ngwa, V.N.; et al. A Cross-Sectional, Population-Based, Seroepidemiological Study of Rift Valley Fever in Cameroonian Cattle Populations. Front. Vet. Sci. 2022, 9, 897481. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.M.; Kibona, T.; Claxton, J.R.; de Glanville, W.A.; Lankester, F.; Amani, N.; Buza, J.J.; Carter, R.W.; Chapman, G.E.; Crump, J.A.; et al. Prospective Cohort Study Reveals Unexpected Aetiologies of Livestock Abortion in Northern Tanzania. Sci. Rep. 2022, 12, 11669. [Google Scholar] [CrossRef]
- Govindasamy, K.; Etter, E.M.C.; Geertsma, P.; Thompson, P.N. Progressive Area Elimination of Bovine Brucellosis, 2013–2018, in Gauteng Province, South Africa: Evaluation Using Laboratory Test Reports. Pathogens 2021, 10, 1595. [Google Scholar] [CrossRef]
- Fafetine, J.M.; Coetzee, P.; Mubemba, B.; Nhambirre, O.; Neves, L.; Coetzer, J.A.W.; Venter, E.H. Rift Valley Fever Outbreak in Livestock, Mozambique, 2014. Emerg. Infect. Dis. 2016, 22, 2165–2167. [Google Scholar] [CrossRef]
- Khajuria, B.K.; Malik, M.A.; Tiwari, A.; Sharma, N.; Wazir, V.S. Seroprevalence Studies of Brucellosis at Organized and Unorganized Cattle Farms in North India. Int. J. Agric. Environ. Biotechnol. 2014, 7, 499. [Google Scholar] [CrossRef]
- Hekal, S.H.A.; Al-Gaabary, M.H.; El-Sayed, M.M.; Sobhy, H.M.; Fayed, A.A.A. Seroprevalence of Some Infectious Transboundry Diseases in Cattle Imported from Sudan to Egypt. J. Adv. Vet. Anim. Res. 2019, 6, 92. [Google Scholar] [CrossRef]
- Lindahl-Rajala, E.; Hoffman, T.; Fretin, D.; Godfroid, J.; Sattorov, N.; Boqvist, S.; Lundkvist, Å.; Magnusson, U. Detection and Characterization of Brucella Spp. in Bovine Milk in Small-Scale Urban and Peri-Urban Farming in Tajikistan. PLoS Neglected Trop. Dis. 2017, 11, e0005367. [Google Scholar] [CrossRef]
- Barkallah, M.; Jribi, H.; Ben Slima, A.; Gharbi, Y.; Mallek, Z.; Gautier, M.; Fendri, I.; Gdoura, R. Molecular Prevalence of Chlamydia and Chlamydia-like Bacteria in Tunisian Domestic Ruminant Farms and Their Influencing Risk Factors. Transbound. Emerg. Dis. 2018, 65, e329–e338. [Google Scholar] [CrossRef]
- Abdeltif, B.; Tennah, S.; Derdour, S.Y.; Temim, A.; Boufendi, H.; Ghalmi, F. The First Study on Seroprevalence and Risk Factors of Neospora Caninum Infection in Pregnant Local Cows from Northeast Algeria. Vet. World 2022, 15, 442–448. [Google Scholar] [CrossRef]
- De Glanville, W.A.; Allan, K.J.; Nyarobi, J.M.; Thomas, K.M.; Lankester, F.; Kibona, T.J.; Claxton, J.R.; Brennan, B.; Carter, R.W.; Crump, J.A.; et al. An Outbreak of Rift Valley Fever among Peri-Urban Dairy Cattle in Northern Tanzania. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 1082–1090. [Google Scholar] [CrossRef]
- Troupin, C.; Ellis, I.; Doukouré, B.; Camara, A.; Keita, M.; Kagbadouno, M.; Bart, J.-M.; Diallo, R.; Lacôte, S.; Marianneau, P.; et al. Seroprevalence of Brucellosis, Q Fever and Rift Valley Fever in Domestic Ruminants in Guinea in 2017–2019. BMC Vet. Res. 2022, 18, 64. [Google Scholar] [CrossRef]
- Djellata, N. Seroprevalence of Infectious Bovine Rhinotracheitis in Aborted Cows in Algeria. Vet. Stanica 2024, 55, 311. [Google Scholar] [CrossRef]
- Al-Mubarak, A.I.A.; Hussen, J.; Kandeel, M.; Al-Kubati, A.A.G.; Falemban, B.; Skeikh, A.; Hemida, M.G. Risk-Associated Factors Associated with the Bovine Viral Diarrhea Virus in Dromedary Camels, Sheep, and Goats in Abattoir Surveillance and Semi-Closed Herd System. Vet. World 2022, 15, 1924–1931. [Google Scholar] [CrossRef]
- Messele, Y.E.; Girmay, G.; Emeru, B.A.; Bora, S.K.; Gudeta, W.F.; Dersso, B.S.; Tegegne, D.T.; Hurrisa, B.U.; Yalew, S.T.; Werid, G.M. Seroprevalence of Major Infectious Causes of Dairy Cattle Reproductive Problems in Central Ethiopia; Research Square: Durham, NC, USA, 2021. [Google Scholar]
- Yitagesu, E.; Jackson, W.; Kebede, N.; Smith, W.; Fentie, T. Prevalence of Bovine Abortion, Calf Mortality, and Bovine Viral Diarrhea Virus (BVDV) Persistently Infected Calves among Pastoral, Peri-Urban, and Mixed-Crop Livestock Farms in Central and Northwest Ethiopia. BMC Vet. Res. 2021, 17, 87. [Google Scholar] [CrossRef]
- Naveena, T.; Sarangi, L.N.; Rana, S.K.; Prasad, A.; Prabha, T.S.; Jhansi, D.; Ponnanna, N.M.; Sharma, G.K. Seroprevalence to Common Infectious Abortifacient and Infertility Causing Agents in the Dairy Herds of India. Iran J. Vet. Res. 2022, 23, 189–195. [Google Scholar]
- Mohammed, F.U.; Ibrahim, S.; Musa, G.A.; Kaltungo, B.Y.; Danbirni, S.; Kwaga, J.K. Brucella Infection in Migratory Cattle Herds in Jigawa State Nigeria: A Cross Sectional Study. Sokoto J. Vet. Sci. 2020, 18, 191–194. [Google Scholar] [CrossRef]
- El-Mohamady, R.; Gerges, A.M.; Abd-Elhafeiz, Y.G.M. Investigation of The Association Between Bovine Viral Diarrhea Virus and Neospora Caninum as a Cause of Abortion in Cattle. J. Appl. Vet. Sci. 2021, 7, 11–17. [Google Scholar] [CrossRef]
- Akoko, J.M.; Mwatondo, A.; Muturi, M.; Wambua, L.; Abkallo, H.M.; Nyamota, R.; Bosire, C.; Oloo, S.; Limbaso, K.S.; Gakuya, F.; et al. Mapping Brucellosis Risk in Kenya and Its Implications for Control Strategies in Sub-Saharan Africa. Sci. Rep. 2023, 13, 20192. [Google Scholar] [CrossRef] [PubMed]
- Deb Nath, N.; Ahmed, S.S.U.; Malakar, V.; Hussain, T.; Chandra Deb, L.; Paul, S. Sero-Prevalence and Risk Factors Associated with Brucellosis in Dairy Cattle of Sylhet District, Bangladesh: A Cross-Sectional Study. Vet. Med. Sci. 2023, 9, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Marumo, B.; Hlokwe, T.M.; Kayoka-Kabongo, P.N. Seroprevalence of Brucellosis in Communal and Smallholder Cattle Farming in North West Province, South Africa. Onderstepoort J. Vet. Res. 2023, 90, 2114. [Google Scholar] [CrossRef]
- Jaismon, P.A.; Sushmitha, A.P.; Verma, M.R.; Singh, Y.P.; Borthakur, U.; Kumar, S.; Sharun, K.; Dhama, K. Prevalence of Bovine Brucellosis in India: A Meta-Analysis. Vet. Q. 2023, 43, 1–9. [Google Scholar] [CrossRef]
- Mohammadian, B.; Noaman, V.; Emami, S.J. Molecular Survey on Prevalence and Risk Factors of Anaplasma Spp. Infection in Cattle and Sheep in West of Iran. Trop. Anim. Health Prod. 2021, 53, 266. [Google Scholar] [CrossRef]
- Chambaro, H.M.; Sasaki, M.; Simulundu, E.; Silwamba, I.; Sinkala, Y.; Gonzalez, G.; Squarre, D.; Fandamu, P.; Lubaba, C.H.; Munyeme, M.; et al. Co-Circulation of Multiple Serotypes of Bluetongue Virus in Zambia. Viruses 2020, 12, 963. [Google Scholar] [CrossRef]
- Daniels, P.W.; Sendow, I.; Pritchard, L.I.; Eaton, B.T. Regional Overview of Bluetongue Viruses in South-East Asia: Viruses, Vectors and Surveillance. Vet. Ital. 2004, 40, 94–100. [Google Scholar]
- FAO Animal Genetic Resources. Strategies for Improved Use and Conservation. Available online: https://www.fao.org/3/ah806e/AH806E12.htm (accessed on 17 April 2024).
- Rahman, M.M.; Islam, M.R.; Dhar, P.S. Recent Re-Emergence of Rift Valley Fever: Epidemiology, Clinical Characteristics, Transmission, Symptoms, Diagnosis, Prevention, and Treatment. Int. J. Surg. 2023, 109, 117. [Google Scholar] [CrossRef]
- FAO Manual on Livestock Disease Surveillance and Information Systems. Available online: https://www.fao.org/3/x3331e/x3331e01.htm (accessed on 22 October 2021).
- Bronner, A.; Hénaux, V.; Fortané, N.; Hendrikx, P.; Calavas, D. Why Do Farmers and Veterinarians Not Report All Bovine Abortions, as Requested by the Clinical Brucellosis Surveillance System in France? BMC Vet. Res. 2014, 10, 93. [Google Scholar] [CrossRef]
- Goutardab, F.L.; Binotab, A.; Dubozac, R.; Rasamoelina-Andriamanivode, H.; Pedronoae, M.; Hollf, D.; Peyreag, M.I.; Cappelleah, J.; Chevaliera, V.; Figuiéi, M.; et al. How to Reach the Poor? Surveillance in Low-Income Countries, Lessons from Experiences in Cambodia and Madagascar. Prev. Vet. Med. 2015, 120, 12–26. [Google Scholar] [CrossRef]
- Mtema, Z.; Changalucha, J.; Cleaveland, S.; Elias, M.; Ferguson, H.M.; Halliday, J.E.B.; Haydon, D.T.; Jaswant, G.; Kazwala, R.; Killeen, G.F.; et al. Mobile Phones as Surveillance Tools: Implementing and Evaluating a Large-Scale Intersectoral Surveillance System for Rabies in Tanzania. PLOS Med. 2016, 13, e1002002. [Google Scholar] [CrossRef]
- Karimuribo, E.D.; Mutagahywa, E.; Sindato, C.; Mboera, L.; Mwabukusi, M.; Kariuki Njenga, M.; Teesdale, S.; Olsen, J.; Rweyemamu, M. A Smartphone App (AfyaData) for Innovative One Health Disease Surveillance from Community to National Levels in Africa: Intervention in Disease Surveillance. JMIR Public Health Surveill 2017, 3, e94. [Google Scholar] [CrossRef] [PubMed]
- Karimuribo, E.; Batamuzi, E.; Massawe, L.; Silayo, R.; Mgongo, F.; Kimbita, E.; Wambura, R. Potential Use of Mobile Phones in Improving Animal Health Service Delivery in Underserved Rural Areas: Experience from Kilosa and Gairo Districts in Tanzania. BMC Vet. Res. 2016, 12, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, D.S.; Daly, K.; Nyanza, E.C.; Ngallaba, S.E.; Bull, S. Health Worker Acceptability of an mHealth Platform to Facilitate the Prevention of Mother-to-Child Transmission of HIV in Tanzania. Digit. Health 2020, 6, 2055207620905409. [Google Scholar] [CrossRef] [PubMed]
- L’Engle, K.L.; Vahdat, H.L.; Ndakidemi, E.; Lasway, C.; Zan, T. Evaluating Feasibility, Reach and Potential Impact of a Text Message Family Planning Information Service in Tanzania. Contraception 2013, 87, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.; Ishengoma, D.S.; Mmbando, B.P.; Rutta, A.S.M.; Malecela, M.N.; Mayala, B.; Lemnge, M.M.; Michael, E. Deployment and Use of Mobile Phone Technology for Real-Time Reporting of Fever Cases and Malaria Treatment Failure in Areas of Declining Malaria Transmission in Muheza District North-Eastern Tanzania. Malar. J. 2017, 16, 308. [Google Scholar] [CrossRef]
- Haberer, J.E.; Kiwanuka, J.; Nansera, D.; Wilson, I.B.; Bangsberg, D.R. Challenges in Using Mobile Phones for Collection of Antiretroviral Therapy Adherence Data in a Resource-Limited Setting. AIDS Behav. 2010, 14, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Wakadha, H.; Chandir, S.; Were, E.V.; Rubin, A.; Obor, D.; Levine, O.S.; Gibson, D.G.; Odhiambo, F.; Laserson, K.F.; Feikin, D.R. The Feasibility of Using Mobile-Phone Based SMS Reminders and Conditional Cash Transfers to Improve Timely Immunization in Rural Kenya. Vaccine 2013, 31, 987–993. [Google Scholar] [CrossRef]
- Chang, L.W.; Kagaayi, J.; Arem, H.; Nakigozi, G.; Ssempijja, V.; Serwadda, D.; Quinn, T.C.; Gray, R.H.; Bollinger, R.C.; Reynolds, S.J. Impact of a mHealth Intervention for Peer Health Workers on AIDS Care in Rural Uganda: A Mixed Methods Evaluation of a Cluster-Randomized Trial. AIDS Behav. 2011, 15, 1776–1784. [Google Scholar] [CrossRef]
- Githinji, S.; Kigen, S.; Memusi, D.; Nyandigisi, A.; Mbithi, A.M.; Wamari, A.; Muturi, A.N.; Jagoe, G.; Barrington, J.; Snow, R.W.; et al. Reducing Stock-Outs of Life Saving Malaria Commodities Using Mobile Phone Text-Messaging: SMS for Life Study in Kenya. PLoS ONE 2013, 8, e54066. [Google Scholar] [CrossRef]
- Leon, N.; Schneider, H.; Daviaud, E. Applying a Framework for Assessing the Health System Challenges to Scaling up mHealth in South Africa. BMC Med. Inf. Decis. Mak. 2012, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Horvath, T.; Azman, H.; Kennedy, G.E.; Rutherford, G.W. Mobile Phone Text Messaging for Promoting Adherence to Antiretroviral Therapy in Patients with HIV Infection. Cochrane Database Syst. Rev. 2012, 2012, CD009756. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, M.; Zurovac, D.; Ogara, E.A.A.; Chuma, J.; Kirigia, D. Assessing the Feasibility of eHealth and mHealth: A Systematic Review and Analysis of Initiatives Implemented in Kenya. BMC Res. Notes 2017, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Kiberu, V.M.; Mars, M.; Scott, R.E. Barriers and Opportunities to Implementation of Sustainable E-Health Programmes in Uganda: A Literature Review. Afr. J. Prim. Health Care Fam. Med. 2017, 9, 10. [Google Scholar] [CrossRef] [PubMed]
| Search String | Database or Further Sources | Results | Date | Comments |
|---|---|---|---|---|
| ((((ASIA[Text Word]) OR (AFRICA[Text Word]) AND (1990/1/1:2024/5/1[pdat])) AND (((GOATS[Title/Abstract]) OR (SHEEP[Title/Abstract])) OR (CATTLE[Title/Abstract]) AND (1990/1/1:2024/5/1[pdat]))) AND (ABORT*[Title/Abstract] AND (1990/1/1:2024/5/1[pdat]))) AND (surve*[Title/Abstract]) | PubMed | 37 | 1 May 2024 | PubMed search |
| abortion surveillance cattle OR sheep OR goats * * * * “Asia OR Africa” -human -people -persons -man -woman -Europe -americas -australia -pacific -“south america”1990–2024 | Google Scholar | 240 | 1 May 2024 | Google Scholar search through NM-AIST |
| s/no | Country | Region | Year | Species | Number of Species (Positive) | Pathogen(s) Detected | Study Type | Husbandry Method (Climatic Zone) | Detection Method | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tanzania | East Africa | 1996 | Cross-bred bulls; Taurine breeds [24] (Friesian, Ayrshire, and Simmental crossed with Tanzanian short-horn zebu, boran, and Sahiwal) | Campylobacter fetus 3/58, Trichomonas foetus 0/58 | Campylobacter fetus subsp. Venerealis, Trichomonas foetus | Sero-survey | Smallholder dairy farms (zero-grazing) Tropical climate | Culture and biochemical tests | [24] |
| 2 | Uganda | East Africa | 2000 | Cattle (Ankole, crosses—Fresian and Boran) | Brucella—41/143 Anaplasma 3/454 | Brucella, Anaplasma | Cross-sectional | Pastoral communities Tropical climate | RBPT, ELISA | [25] |
| 3 | India | Asia | 2002–2004 | Cattle | 35/427 (9.6%) | Neospora caninum | Cross-sectional survey | Dairy farms Tropical climate | ELISA, IFAT | [26] |
| 4 | Senegal | West Africa | 2003 | Sheep | 7/260 (2.7%) | RVFV | Serological survey | Nomadic Tropical climate | Sero neutralization test | [27] |
| 5 | Zimbabwe | Southern Africa | 2004–2005 | Cattle | 71/1291 (5.5%) | Brucella | Cross-sectional | Smallholder Subtropical climate | RBT, ELISA | [28] |
| 6 | Sudan | Central Africa | 2005 | Sheep and goats | Sheep 3/270 (1.1%) | RVFV | Sero-surveillance | Nomadic pastoralist Tropical savannah | ELISA, Hemagglutination | [29] |
| 7 | South Africa | Southern Africa | 2006–2016 | 193 cattle, 39 goats, and 57 sheep | 63/288; Brucella 21/288 (7.3%) Cattle, Trueperella pyogenes 5/288 Cattle, 1/288 sheep | Brucella, Trueperella pyogenes, E. coli, Salmonella, L. monocytogenes, C. burnetii, B. licheniformus, Rhizopus, B. abortus, Leptospira, C. pecorum, Campylobacter | Observational retrospective study | Archived samples Subtropical and temperate | Microbiology, necropsy, histopathology, PCR | [30] |
| 8 | Ethiopia | East Africa | 2008–2009 | Sheep and goats | 0/270 sheep, 2/230 goats | Brucella | Cross-sectional | Mixed farming Tropical | Rose Bengal Plate Test, Complement Fixation Test | [31] |
| 9 | Ethiopia | East Africa | 2009–2010 | Cattle | 2/370 (0.05%) | Brucella | Cross-sectional survey | Mixed farming Equatorial rainforest, Afro-alpine | Rose Bengal, Complement Fixation Test | [32] |
| 10 | Tunisia | North Africa | 2010–2012 | Cattle | 214 blood, vaginal swabs, milk. Brucella 47/150 (31.3%) RBPT, DANA PCR 46/150 (30.6%). Chlamydia 27/150 (18%), L. monocytogenes 7/150 (4.6%), Salmonella 5/150 (3.3%). Vaginal swabs; Brucella 46/150 (30.6%), Chlamydiales 27/150 (2.65%), L. monocytogenes 4/150 (2.6%) | Brucella, Chamydiales (C. abortus, C. pecorum), Listeria, Salmonella, Coxiella burnetii, Campylobacter | Cross-sectional survey | Limited pasture or tethered Mediterranean climate | PCR, Rose Bengal | [33] |
| 11 | Mozambique | Southern Africa | 2010–2016 | Cattle, goats, and sheep | Cattle 149/404 Goats 45/223 Sheep | RVFV | Sero-survey | Mixed farming Tropical to sub-tropical | ELISA, PRNT | [34] |
| 12 | Zimbabwe | Southern Africa | 2011 | Cattle (mixed breeds) | 81/1440 (5.6%) | Brucella | Cross-sectional survey | Smallholder, mixed farming (strictly separate pastures) Subtropical | ELISA | [35] |
| 13 | Iran | Asia | 2011–2012 | Sheep and goats | PCR: Sheep 101/274. Goats 10/25, Culture Sheep 76/274. Goats’ 9/25 | Mycoplasma spp. | Cross-sectional | Mixed farming Arid and semi-arid climate | PCR, bacterial culture | [36] |
| 14 | India | Asia | 2012–2014 | Cattle | 11/61 (18.03%) | Trypanosoma evansi | Sero-survey | Mixed farming. Tropical climate | ELISA | [37] |
| 15 | South Korea | Asia | 2012–2013 | Cattle (Holstein breed) | 37/171 and 85/466 | Blue Tongue Virus | Serological survey from National Surveillance Program | Mixed farming Temperate climate | ELISA, BTV neutralization test, RT-PCR | [38] |
| 16 | Cameroon | West Africa | 2013 | Cattle | 117/1498 | RVFV | Cross-sectional survey | Pastoralists Humid and Equatorial climate | ELISA | [39] |
| 17 | Tanzania | East Africa | 2013–2016 | Cattle, goats, and sheep | Brucella Cattle 1/71, Coxiella Goats 5/100, Sheep 1/44, Neospora Cattle 9/71, Goats 1/100, Toxoplasma Sheep 1/44, BHV-1 Cattle 2/49, BVDV Cattle 2/71, Goats 1/100, Sheep 6/44, RVFV Cattle 14/71 | Brucella, Chlamydia abortus, Coxiella burnetii, Leptospira hardjo, Neospora caninum, Toxoplasma gondii, Bluetongue Virus, Bovine Herpes Virus 1, Pestiviruses (BVDV/BDV), RVFV | Cross-sectional survey | Pastoral, agro-pastoral, and smallholder Tropical climate | ELISA, PCR | [40] |
| 18 | South Africa | Southern Africa | 2013–2018 | Cattle | 359,026 (22.1%) | Brucella | Cross-sectional survey, Provincial surveillance program | Mixed farming Subtropical and temperate | CFT, Rose Bengal Plate Test | [41] |
| 19 | Mozambique | Southern Africa | 2014 | Goats | Serology: 31/127 (24.4%) | RVFV | Outbreak investigation | Mixed farming Tropical to sub-tropical | ELISA, PCR | [42] |
| 20 | India | Asia | 2014 | Cattle | 160 RBPT 3/160 (1.8%), Standard Tube Agglutination Test (STAT) 5/160 (3.13%) | Brucella | Sero-epidemiological survey | Mixed farming Tropical climate | RBPT, STAT, Bacterial culture, Milk Ring Test | [43] |
| 21 | Nigeria | West Africa | 2015 | Cattle | 11/97 (11.3%) | RVFV | Cross-sectional survey | Nomadic pastoral Tropical monsoon climate, tropical savannah, and Sahelian hot and semi-arid | ELISA | [18] |
| 22 | Kenya | East Africa | 2016 | Cattle | 100/955, 10.5% | Coxiella burnetii | Cross-sectional survey | Mixed crop-livestock Tropical climate | ELISA | [4] |
| 23 | Egypt | North Africa | 2016–2018 | Cattle | 165/176 (93.86%) | BHV-1 | Transboundary, Import from Sudan | Nomadic Subtropical desert climate | ELISA | [44] |
| 24 | Tajikistan | Central Asia | 2016 | Cattle | 570 (58 PCR, 12 ELISA) | Brucella | Sero-prevalence | Smallholder Continental, subtropical, desert | ELISA, qPCR, DNA sequencing | [45] |
| 25 | Tunisia | North Africa | 2017 | Cattle and sheep | Cattle Waddlia 12/27, Parachlamydiaceae 8/27, Chlamydiaceae 7/27, Sheep P. acanthamoebae 9/164, C. pecorum 6/164 | Waddlia chondrophila, C. abortus, C. pecorum | Cross-sectional survey | Smallholder Mediterranean | PCR | [46] |
| 26 | Algeria | North Africa | 2017–2019 | Atlas brown cows | 650 pregnant (235(36.2%)) | Neospora caninum | Sero-prevalence | Smallholder Mediterranean | ELISA | [47] |
| 27 | Tanzania | East Africa | 2017–2019 | Cattle | 14/63 (23%) | RVFV | Prospective cohort | Pastoral, agropastoral, and smallholder Tropical climate | ELISA, RT-qPCR | [48] |
| 28 | Benin | West Africa | 2017 | Sheep and goats | Goats 83/153, Sheep 3/215 | Toxoplasma gondii | Sero-epidemiological survey | Pastoral. Steppe climate and topical humid climate | ELISA | [15] |
| 29 | Guinea | West Africa | 2017–2019 | Cattle, goats, and sheep | Brucella; Cattle 52/463, Sheep 2/486. Coxiella; Cattle 95/463, Goats 18/408, Sheep 11/486. RVF; Cattle 76/463, Goats 4/408, Sheep 5/486 | Brucella, Coxiella burnetii, RVFV | Sero-survey from archived samples | Intensive farms Samples from different prefectures Hot and humid | ELISA, Virus Neutralizing Ab | [49] |
| 30 | Algeria | North Africa | 2018–2019 | Cattle | 201/460 (43.7%) | Bovine Herpes Virus 1 | Abortion investigation | Mixed farming Mediterranean climate | ELISA | [50] |
| 31 | Saudi Arabia | Asia | 2018–2020 | Sheep and goats | Goat 3/84 (3.5%) Serum | BVDV | Sero-prevalence-Abattoir surveillance | Abattoir, semi-closed management Desert climate | ELISA | [51] |
| 32 | Ethiopia | East Africa | 2018–2019 | Cattle cross and pure breeds; Boran–Fresian cross, Boran–Jersey, Pure Jersey, and Boran | BHV-1 68/86(79.1%), BVD 33/86 (38.4%), Neospora 3/86 (3.5%), Coxiella 1/86 (1.2%) | Brucella spp., Neospora caninum, BVD, BHV-1, Coxiella burnetii | Reproductive problem investigation | Semi-intensive farming system (grazing and indoor feeding) Equatorial rainforest, Afro-alpine | ELISA | [52] |
| 33 | Ethiopia | East Africa | 2018–2019 | Cattle (Zebu, Holstein, Fresian, and crossbreed) | 0/882 (ear notch samples | BVDV | Cross-sectional survey | Peri-urban dairy farms, mixed crop–livestock farms (small holder extensive management system), pastoral herds (seasonal mobility) Equatorial rainforest, Afro-alpine | ELISA | [53] |
| 34 | India | Asia | 2019 | Cattle crossbreeds, exotic, and indigenous | BHV-1 425/1004, BVDV 604/1004, Brucella 176/1004, Coxiella 57/1004, Anaplasma 363/1004, Neospora 9/1004 | BHV-1, BVDV, Brucella, Coxiella burnetii, Neospora caninum, Anaplasma marginale | Cros-sectional | Intensive dairy farms Tropical | ELISA | [54] |
| 35 | Nigeria | West Africa | 2020 | Cattle | 61/1810 (3.37%) | Brucella | Cross-sectional | Mixed farming Tropical monsoon climate, tropical savannah, and Sahelian hot and semi-arid | SAT | [55] |
| 36 | Egypt | North Africa | 2020 | Cattle | Neospora 35/116 (30.17%), BVDV 31/116(26.72%) | Neospora caninum, BVDV | Cross-sectional | Medium-sized farms Subtropical desert climate | ELISA | [56] |
| 37 | Kenya | East Africa | 2020–2021 | Cattle | 6593(449) | Brucella | Sero-prevalence | Agro-alpine, high and medium potential, semi-arid, arid, and very arid Tropical climate | ELISA | [57] |
| 38 | Bangladesh | Asia | 2023 | Cattle (local, cross) | 66/386 (17.09%) | Brucella | Cross-sectional | Transboundary area, mixed farming Humid, warm climate | RBPT, Plate agglutination test, serum agglutination | [58] |
| 39 | South Africa | Southern Africa | 2023 | Cattle | 2% 770 | Brucella | Cross-sectional survey, abattoir survey | Communal, commercial, and non-commercial farms Subtropical and temperate | RNT, CFT, Milk Ring Test | [59] |
| Abortigenic Pathogen | Species | Cases (n) | Total Tested (N) | Median Sero-Prevalence | |||
|---|---|---|---|---|---|---|---|
| Africa | Asia | Africa | Asia | Africa | Asia | ||
| Anaplasma | Cattle | 3 | 363 | 454 | 1004 | 0.7 | 36.2 |
| BHV-1 | Cattle | 436 | 245 | 771 | 1004 | 56.5 | 24.4 |
| Bluetongue virus | Cattle | 0 | 122 | 0 | 637 | 0 | 19.2 |
| Brucella spp. | Goats | 2 | 0 | 230 | 0 | 0.87 | 0 |
| Sheep | 2 | 0 | 754 | 0 | 0.27 | 0 | |
| Cattle | 80,165 | 305 | 372,127 | 2120 | 21.5 | 14.4 | |
| BVDV | Goats | 1 | 3 | 100 | 84 | 1 | 3.6 |
| Sheep | 6 | 0 | 44 | 0 | 13.6 | 0 | |
| Cattle | 66 | 604 | 1155 | 1004 | 5.7 | 60.2 | |
| Campylobacter | Cattle | 3 | 0 | 58 | 0 | 5.2 | 0 |
| Chlamydia abortus | Cattle | 34 | 0 | 177 | 0 | 19.2 | 0 |
| Chlamydia pecorum | Sheep | 6 | 0 | 164 | 0 | 3.7 | 0 |
| Coxiella burnetii | Goats | 23 | 0 | 508 | 0 | 4.5 | 0 |
| Sheep | 12 | 0 | 530 | 0 | 2.3 | 0 | |
| Cattle | 196 | 54 | 1504 | 1004 | 13 | 5.4 | |
| Listeria | Cattle | 7 | 0 | 150 | 0 | 4.7 | 0 |
| Mycoplasma | Goats | 0 | 10 | 0 | 25 | 0 | 40 |
| Sheep | 0 | 101 | 0 | 274 | 0 | 36.9 | |
| Neospora caninum | Goats | 1 | 0 | 100 | 0 | 1 | 0 |
| Cattle | 282 | 44 | 923 | 1431 | 30.6 | 3.1 | |
| RVFV | Goats | 80 | 0 | 758 | 0 | 10.6 | 0 |
| Sheep | 15 | 0 | 1016 | 0 | 1.5 | 0 | |
| Cattle | 381 | 0 | 2596 | 0 | 14.7 | 0 | |
| Salmonella | Cattle | 5 | 0 | 150 | 0 | 3.3 | 0 |
| Toxoplasma gondii | Goats | 83 | 0 | 153 | 0 | 54.2 | 0 |
| Sheep | 4 | 0 | 259 | 0 | 1.5 | 0 | |
| Trichomonas foetus | Cattle | 0 | 0 | 58 | 0 | 0 | 0 |
| Trypanosoma evansi | Cattle | 0 | 11 | 0 | 61 | 0 | 18 |
| Waddlia chondrophila | Cattle | 12 | 0 | 27 | 0 | 44.4 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semango, G.P.; Buza, J. Review of the Current Status on Ruminant Abortigenic Pathogen Surveillance in Africa and Asia. Vet. Sci. 2024, 11, 425. https://doi.org/10.3390/vetsci11090425
Semango GP, Buza J. Review of the Current Status on Ruminant Abortigenic Pathogen Surveillance in Africa and Asia. Veterinary Sciences. 2024; 11(9):425. https://doi.org/10.3390/vetsci11090425
Chicago/Turabian StyleSemango, George Peter, and Joram Buza. 2024. "Review of the Current Status on Ruminant Abortigenic Pathogen Surveillance in Africa and Asia" Veterinary Sciences 11, no. 9: 425. https://doi.org/10.3390/vetsci11090425
APA StyleSemango, G. P., & Buza, J. (2024). Review of the Current Status on Ruminant Abortigenic Pathogen Surveillance in Africa and Asia. Veterinary Sciences, 11(9), 425. https://doi.org/10.3390/vetsci11090425







