Effect of Exogenous Melatonin on Performance and Mastitis in Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
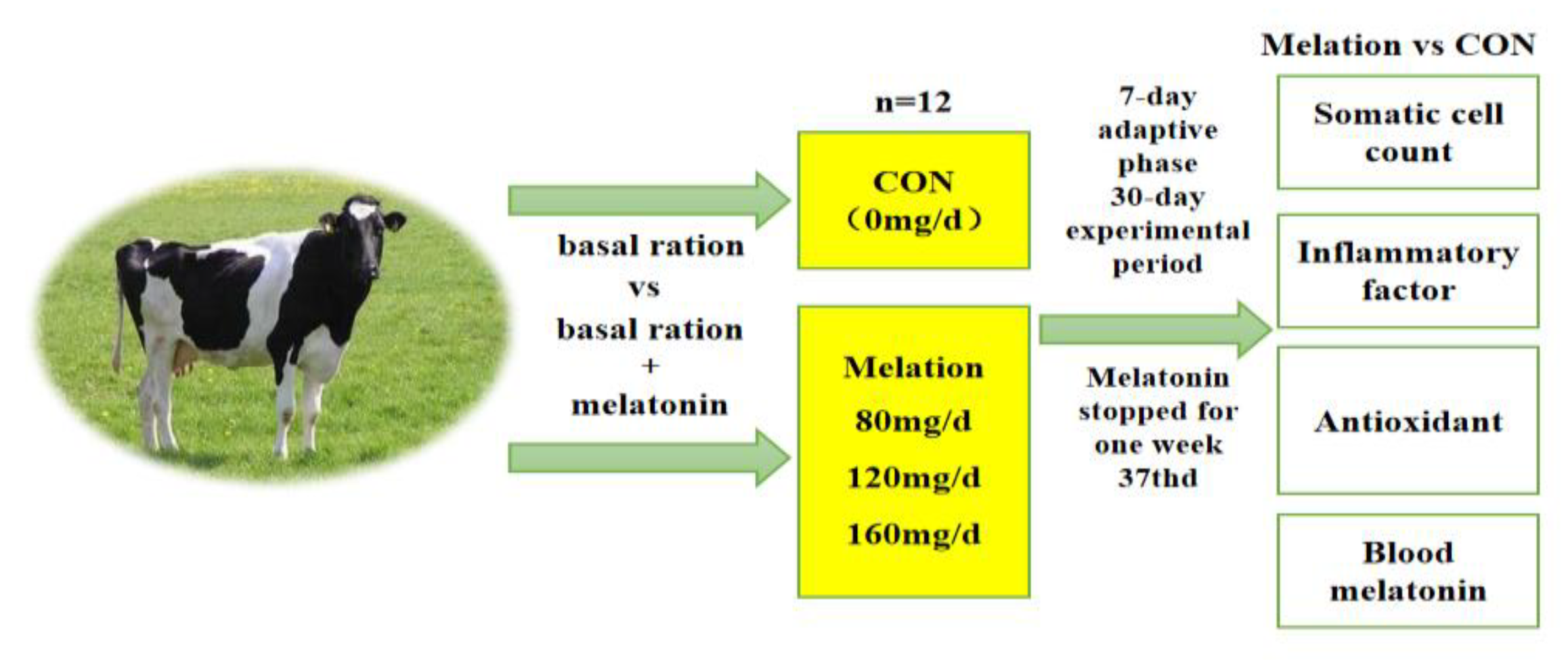
2.2. Experimental Design, Diet, and Management
2.3. Sample Collection
2.4. Sample Testing and Analysis
2.5. Statistical Analyses
3. Results
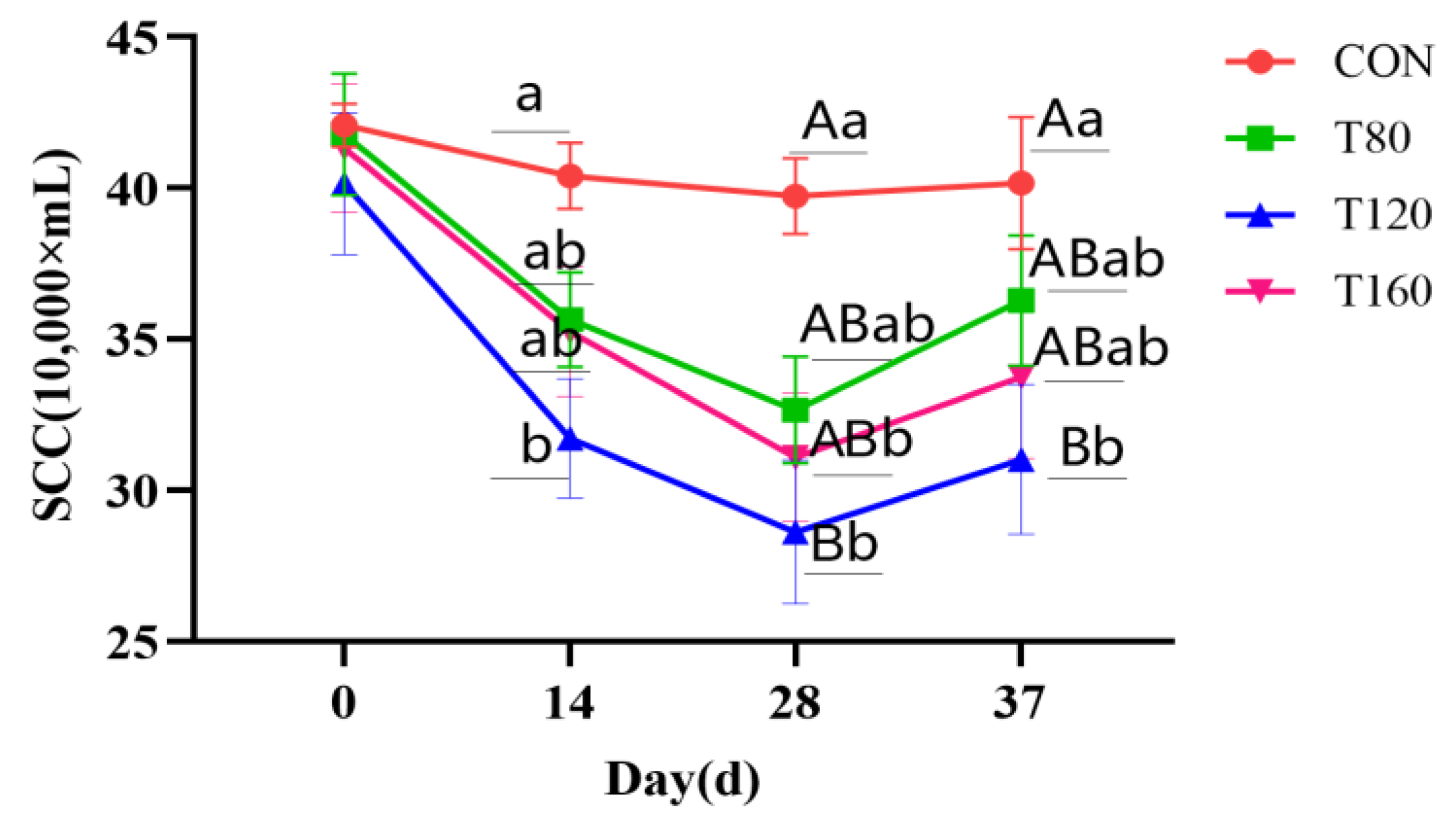
3.1. Effect of Feeding Melatonin and Cessation of Feeding for 1 Week on Somatic Cell Counts in Dairy Cows
3.2. Effects of Feeding Melatonin and Effects 1 Week after Melatonin Cessation on the Performance of Dairy Cows
3.3. Effects of Feeding Melatonin and Effects 1 Week after Feeding Cessation on Serum Antioxidant Indices in Dairy Cows
3.4. Effects of Feeding Melatonin and Effects 1 Week after Feeding Cessation on Serum Inflammatory Indices in Dairy Cows
3.5. Effects of Feeding Melatonin Supplementation and Effects 1 Week after Feeding Cessation on Serum Level Melatonin in Dairy Cows
4. Discussion
4.1. Effect of Exogenous Melatonin on Somatic Cell Number in Dairy Cows
4.2. Effect of Exogenous Melatonin on Milk Yield and Milk Composition of Dairy Cows
4.3. Effect of Exogenous Melatonin on Antioxidant Indexes in Dairy Cows
4.4. Effect of Exogenous Melatonin on Inflammatory Response in Dairy Cows
4.5. Effect of Exogenous Melatonin on Serum Melatonin in Dairy Cows
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonestroo, J.; Voort, M.V.D.; Fall, N.; Emanuelson, U.; Klaas, I.C.; Hogeveen, H. Estimating the nonlinear association of online somatic cell count, lactate dehydrogenase, and electrical conductivity with milk yield. J. Dairy Sci. 2022, 105, 3518–3529. [Google Scholar] [CrossRef] [PubMed]
- Omaleki, L.; Browning, G.F.; Allen, J.L.; Markham, P.F.; Barber, S.R. Molecular epidemiology of an outbreak of clinical mastitis in sheep caused by Mannheimia haemolytica. Vet. Microbiol. 2016, 191, 82–87. [Google Scholar] [CrossRef]
- Swinkels, J.M.; Hilkens, A.; Zoche-Golob, V.; Kroemker, V.; Buddiger, M.; Jansen, J.; Lam, T.J.G.M. Social influences on the duration of antibiotic treatment of clinical mastitis in dairy cows. J. Dairy Sci. 2015, 98, 2369–2380. [Google Scholar] [CrossRef]
- Dziuba, M.; Caixeta, L.S.; Boyum, B.; Godden, S.; Royster, E.; Rowe, S. Negatively controlled trial investigating the effects of dry cow therapy on clinical mastitis and culling in multiparous cows. J. Dairy Sci. 2023, 106, 5687–5695. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.J.; Konturek, P.C.; Brzozowska, I.; Pawlik, M.; Pawlik, W.W. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT). J. Physiol. Pharmacol. 2007, 58, 381–405. [Google Scholar] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: Implications for an ancient antioxidant system. Cell. Mol. Biol. Res. 1995, 41, 391–395. [Google Scholar]
- Sprenger, J.; Hardeland, R.; Fuhrberg, B.; Han, S.Z. Melatonin and Other 5-Methoxylated Indoles in Yeast: Presence in High Concentrations and Dependence on Tryptophan Availability. Cytologia 1999, 64, 209–213. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Gitto, E.; Pellegrino, S.; Gitto, P.; Barberi, I.; Reiter, R.J. Oxidative stress of the newborn in the pre- and postnatal period and the clinical utility of melatonin. J. Pineal Res. 2009, 46, 128–139. [Google Scholar] [CrossRef]
- Yang, M.; Shi, J.; Tian, J.; Tao, J.; Chai, M.; Wang, J.; Xu, Z.; Song, Y.; Zhu, K.; Ji, P. Exogenous melatonin reduces somatic cell count of milk in Holstein cows. Sci. Rep. 2017, 7, 43280. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.; Sanchez, J.; Andres, S. Effect of melatonin implants on somatic cell counts in dairy goats. Indian J. Anim. Sci. 2014, 84, 1075–1076. [Google Scholar] [CrossRef]
- Brzezinski, A. Melatonin in Humans. N. Engl. J. Med. 1997, 16, 186–195. [Google Scholar] [CrossRef]
- Huang, S.H.; Liao, C.L.; Chen, S.J.; Shi, L.G.; Lin, L.; Chen, Y.W.; Cheng, C.P.; Sytwu, H.K.; Shang, S.T.; Lin, G.J. Melatonin possesses an anti-influenza potential through its immune modulatory effect-ScienceDirect. J. Funct. Foods 2019, 58, 189–198. [Google Scholar] [CrossRef]
- Qiu, Y.; Yin, H.; Wang, S.; Zang, C.; Yu, Y.; Li, Y.; Liu, T.; Nan, B.; Yang, K.; Tan, S.; et al. Establishment of Mouse Mastitits Model and Preventive Effect of Melatonin on Mastitis in Mice. Chin. J. Anim. Nutr. 2023, 35, 7379–7388. [Google Scholar]
- Ministry of Agriculture of the PRC. Feeding Standard of Dairy Cattle; Ministry of Agriculture of the PRC: Beijing, China, 2004. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Baro, J.A.; Perez, M.A.; Grillo, G.J. Method Comparison for Diagnose of Subclinical Mastitis and Milk Quality Determination in Raw Milk. In Proceedings of the Instrumentation and Measurement Technology Conference, Ottawa, ON, Canada, 17–19 May 2005. IMTC 2005. [Google Scholar]
- Geary, U.; Lopez-Villalobos, N.; Begley, N.; McCoy, F.; O’Brien, B.; O’Grady, L.; Shalloo, L. Estimating the effect of mastitis on the profitability of Irish dairy farms. J. Dairy Sci. 2012, 95, 3662–3673. [Google Scholar] [CrossRef] [PubMed]
- Rhoda, D.A.; Pantoja, J.C.F. Using mastitis records and somatic cell count data. (Special Issue: Mastitis in dairy cows.). Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 347–361. [Google Scholar] [CrossRef]
- Yao, S.; Wu, H.; Ma, H.; Fu, Y.; Liu, G. Effects of rumen bypass melatonin feeding (RBMF) on milk quality and mastitis of Holstein cows. PeerJ 2020, 8, e9147. [Google Scholar] [CrossRef]
- Jumnake, A.R.; Patodkar, V.R.; Gavali, N.E.; Sardar, V.M.; Mehere, P.V. Advances in Melatonin Research—A Review. Chron. Aquat. Sci. 2024, 1, 152–174. [Google Scholar] [CrossRef]
- Is, P.; Chung, M.; Raman, G. Breastfeeding and Maternal and Infant Health Outcomes In Develope. Evid. Report. 2007, 153, 1–186. [Google Scholar]
- Lonnerdal, B. Infant formula and infant nutrition: Bioactive proteins of human milk and implications for composition of infant formulas. Am. J. Clin. Nutr. 2014, 99, 712S–717S. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ai, Y.; Zhang, S. Signalling pathways and nutritional regulation of milk protein synthesis. Livest. Vet. Med. 2014, 46, 133–136. [Google Scholar]
- Hu, X.; Wu, L.; Liu, L.; Li, C.; Lv, Z.; Duan, G.; Yue, H.; Chen, J.; Liu, C.; Zhao, J. Determination of lactose in low-lactose and lactose-free buttermilk by ion chromatography. J. Dairy Sci. Technol. 2019, 42, 21–24. [Google Scholar]
- Avilés, R.; Delgadillo, J.A.; Flores, J.A.; Duarte, G.; Vielma, J.; Flores, M.J.; Petrovski, K.; Zarazaga, L.A.; Hernández, H. Melatonin administration during the dry period stimulates subsequent milk yield and weight gain of offspring in subtropical does kidding in summer. J. Dairy Sci. 2019, 102, 11536–11543. [Google Scholar] [CrossRef]
- Auldist, M.J.; Turner, S.A.; Mcmahon, C.D.; Prosser, C.G. Effects of melatonin on the yield and composition of milk from grazing dairy cows in New Zealand. J. Dairy Res. 2007, 74, 52. [Google Scholar] [CrossRef]
- Molik, E.; Bonczar, G.; Żebrowska, A.; Misztal, T.; Zięba, D. Effect of day length and exogenous melatonin on chemical composition of sheep milk. Arch. Fur Tierz. 2011, 54, 177–187. [Google Scholar] [CrossRef]
- Shin, J.A.; Chung, J.S.; Cho, S.H.; Kim, H.J.; Yoo, Y.D. Romo1 expression contributes to oxidative stress-induced death of lung epithelial cells. Biochem. Biophys. Res. Commun. 2013, 439, 315–320. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, J.B.; Liu, W.; Yao, X.R.; Guo, H.; Jin, Z.L.; Zhang, M.J.; Yuan, B.; Jiang, H.; Kim, N.H. Leonurine improves invitro porcine embryo development competence by reducing reactive oxygen species production and protecting mitochondrial function. Theriogenology 2020, 156, 116–123. [Google Scholar] [CrossRef]
- Choudhary, P.K.; Ishwar, A.K.; Kumar, R.; Niyogi, D.; Kumar, M. Effect of exogenous melatonin and different photoperiods on oxidative status and antioxidant enzyme activity in Chhotanagpuri ewe. Vet. World 2018, 11, 130–134. [Google Scholar] [CrossRef]
- Dong, N.; Xue, C.; Zhang, L.; Zhang, T.; Wang, C.; Bi, C.; Shan, A. Oleanolic acid enhances tight junctions and ameliorates inflammation in Salmonella typhimurium-induced diarrhea in mice via the TLR4/NF-kappa B and MAPK pathway. Food Funct. 2020, 11, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.; Blaser, H.; Mak, T.W. Regulation of tumour necrosis factor signalling: Live or let die. Nat. Rev. Immunol. 2015, 15, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Tca, A. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.S.; Kaufmann, D.E. Protective and detrimental roles of IL-10 in HIV pathogenesis. Eur. Cytokine Netw. 2010, 21, 208–214. [Google Scholar]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62, e12394. [Google Scholar] [CrossRef]
- Contreras-Correa, Z.E.; Messman, R.D.; Swanson, R.M.; Lemley, C.O. Melatonin in Health and Disease: A Perspective for Livestock Production. Biomolecules 2023, 13, 490. [Google Scholar] [CrossRef]
- Malpaux, B.; Viguie, C.; Skinner, D.C. Seasonal breeding in sheep: Mechanism of action of melatonin. Anim. Reprod. Sci. 1996, 42, 109–117. [Google Scholar] [CrossRef]
- Cevik, M.; Kocyigit, A.; Yilmazer, C. Effects of melatonin implantation on the fertility potentials of Kivircik and Charollais ewes and rams during the non-breeding season. Pol. J. Vet. Sci. 2017, 20, 501–506. [Google Scholar] [CrossRef]
- Flinn, T.; Gunn, J.R.; Kind, K.L.; Swinbourne, A.M.; Weaver, A.C.; Kelly, J.M.; Walker, S.K.; Gatford, K.L.; van Wettere, W.H.E.J.; Kleemann, D.O. Short communication: Maternal melatonin implants improve twin Merino lamb survival. J. Anim. Sci. 2020, 98, skaa344. [Google Scholar] [CrossRef]
- Lovenberg, W.; Jequier, E.; Sjoerdsma, A. Tryptophan Hydroxylation: Measurement in Pineal Gland, Brainstem, and Carcinoid Tumor. Science 1967, 155, 217–219. [Google Scholar] [CrossRef]
- Deguchi, T.; Axelrod, J. Sensitive assay for serotonin N-acetyltransferase activity in rat pineal. Anal. Biochem. 1972, 50, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Shi, J. Study of Melation on Reducing Somatic Cells and Improving Reprucuctive Performance in Dairy Cows. Ph.D. Thesis, China Agricultural University, Beijing, China, 2015. [Google Scholar]
- Delagrange, P.; Jockers, R. Melatonin and energy homeostasis: Peripheral versus central regulation. Minerva Endocrinol. 2003, 28, 313–320. [Google Scholar] [PubMed]
| Ingredients | Content | Nutrient Components 2 | Content |
|---|---|---|---|
| Alfalfa | 15.20 | DM | 96.42 |
| Corn silage | 24.17 | OM | 92.64 |
| Tablet corn | 6.47 | CP | 15.57 |
| Corn | 24.23 | EE | 3.96 |
| Concentrate 1818 1 | 17.95 | NDF | 38.39 |
| Fatty powder | 0.75 | ADF | 16.45 |
| Cottonseed meal | 4.04 | Ca | 0.85 |
| Cottonseed | 1.41 | P | 0.46 |
| Beet granules | 4.78 | NEL/(MJ/kg) | 6.60 |
| Methionine | 0.06 | ||
| Sodium bicarbonate | 0.56 | ||
| NaCl | 0.38 | ||
| Total | 100.00 |
| Trial Period | Items | CON | T80 | T120 | T160 | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Time | Treatment × Time | |||||||
| 0–30 day Melatonin feeding period | Milk yield (kg/day) | 36.33 | 37.05 | 37.91 | 37.32 | 0.445 | 0.093 | 0.794 | 0.999 |
| Milk fat (%) | 3.62 b | 3.79 ab | 3.87 a | 3.79 ab | 0.062 | 0.034 | <0.001 | 0.793 | |
| Milk fat yield (kg/day) | 1.31 Bb | 1.40 ABa | 1.47 Aa | 1.41ABa | 0.027 | 0.001 | <0.001 | 0.814 | |
| Milk lactose (%) | 4.69 | 4.62 | 4.64 | 4.67 | 0.033 | 0.405 | 0.729 | 0.995 | |
| Milk lactose yield (kg/day) | 1.71 | 1.71 | 1.76 | 1.74 | 0.024 | 0.378 | 0.95 | 0.999 | |
| Milk protein (%) | 3.16 Bb | 3.18 Bb | 3.31 Aa | 3.20 ABb | 0.032 | 0.006 | 0.040 | 0.772 | |
| Milk protein yield (kg/day) | 1.15 Bb | 1.18 Bb | 1.26 Aa | 1.19 ABb | 0.019 | 0.001 | 0.004 | 0.969 | |
| Non-fat milk solids | 8.63 | 8.51 | 8.56 | 8.58 | 0.062 | 0.578 | 0.191 | 0.994 | |
| Ash content | 0.70 | 0.69 | 0.69 | 0.69 | 0.005 | 0.342 | 0.108 | 0.382 | |
| Trial Period | Items | CON | T80 | T120 | T160 | SEM | p-Value | ||
| Treatment | Linear | Quadratic | |||||||
| 31–37 day At 1 week after melatonin cessation | Milk yield (kg/day) | 37.19 | 37.24 | 37.90 | 37.56 | 0.336 | 0.877 | 0.571 | 0.781 |
| Milk fat (%) | 3.73 | 3.79 | 3.88 | 3.71 | 0.073 | 0.866 | 0.951 | 0.459 | |
| Milk fat yield (kg/day) | 1.38 | 1.41 | 1.47 | 1.39 | 0.027 | 0.675 | 0.697 | 0.326 | |
| Milk lactose (%) | 4.73 | 4.71 | 4.67 | 4.66 | 0.036 | 0.902 | 0.458 | 0.996 | |
| Milk lactose yield (kg/day) | 1.76 | 1.75 | 1.77 | 1.75 | 0.021 | 0.980 | 0.975 | 0.832 | |
| Milk protein (%) | 3.15 | 3.22 | 3.33 | 3.27 | 0.041 | 0.442 | 0.189 | 0.435 | |
| Milk protein yield (kg/day) | 1.17 | 1.20 | 1.26 | 1.23 | 0.020 | 0.404 | 0.197 | 0.405 | |
| Non-fat milk solids | 8.61 | 8.44 | 8.52 | 8.57 | 0.065 | 0.826 | 0.962 | 0.427 | |
| Ash content | 0.70 | 0.69 | 0.68 | 0.70 | 0.005 | 0.436 | 0.683 | 0.120 | |
| Trial Period | Items | CON | T80 | T120 | T160 | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Time | Treatment × Time | |||||||
| 0–30 day Melatonin feeding period | SOD (U/mL) | 50.58 Bb | 53.00 ABab | 55.08 Aa | 54.18 Aa | 0.871 | 0.003 | <0.001 | 0.470 |
| GSH-Px (U/mL) | 336.63 b | 343.30 b | 361.09 a | 350.17 ab | 5.851 | 0.028 | 0.140 | 0.545 | |
| CAT (U/mL) | 32.40 | 32.68 | 34.08 | 32.81 | 0.671 | 0.306 | 0.009 | 0.488 | |
| MDA (U/mL) | 3.92 Aa | 3.57 ABb | 3.39 Bb | 3.48 Bb | 0.094 | 0.001 | 0.001 | 0.968 | |
| Trial Period | Items | CON | T80 | T120 | T160 | SEM | p-Value | ||
| Treatment | Linear | Quadratic | |||||||
| 31–37 day At 1 week after Melatonin cessation | SOD (U/mL) | 50.81 | 51.69 | 56.32 | 53.66 | 0.933 | 0.160 | 0.111 | 0.332 |
| GSH-Px (U/mL) | 330.24 | 339.20 | 363.50 | 350.52 | 7.126 | 0.398 | 0.192 | 0.447 | |
| CAT (U/mL) | 31.68 | 33.00 | 34.73 | 33.42 | 0.811 | 0.636 | 0.358 | 0.433 | |
| MDA (U/mL) | 4.05 | 3.51 | 3.48 | 3.55 | 0.098 | 0.122 | 0.077 | 0.111 | |
| Trial Period | Items | CON | T80 | T120 | T160 | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Time | Treatment × Time | |||||||
| 0–30 day Melatonin feeding period | TNF-α (pg/mL) | 40.02 Aa | 39.41 Aa | 36.60 Bb | 38.39 ABab | 0.671 | 0.003 | <0.001 | 0.072 |
| IL-1β (pg/mL) | 20.64 | 20.08 | 19.02 | 19.91 | 0.558 | 0.235 | <0.001 | <0.001 | |
| IL-6 (pg/mL) | 120.59 a | 118.34 ab | 115.08 b | 116.89 b | 1.175 | 0.011 | <0.001 | 0.212 | |
| IL-10 (pg/mL) | 11.06 Bb | 11.63 ABb | 12.55 Aa | 11.72 ABab | 0.298 | 0.007 | <0.001 | 0.390 | |
| Trial Period | Items | CON | T80 | T120 | T160 | SEM | p-Value | ||
| Treatment | Linear | Quadratic | |||||||
| 31–37 day At 1 week after melatonin cessation | TNF-α (pg/mL) | 38.70 | 37.95 | 36.25 | 36.86 | 0.772 | 0.699 | 0.316 | 0.671 |
| IL-1β (pg/mL) | 20.20 | 21.20 | 19.73 | 20.10 | 0.680 | 0.897 | 0.780 | 0.827 | |
| IL-6 (pg/mL) | 119.75 | 117.92 | 111.72 | 113.59 | 4.001 | 0.896 | 0.513 | 0.826 | |
| IL-10 (pg/mL) | 12.60 | 11.09 | 13.07 | 12.03 | 0.344 | 0.208 | 0.923 | 0.731 | |
| Trial Period | Items | CON | T80 | T120 | T160 | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Time | Treatment × Time | |||||||
| 0–30 day Melatonin feeding period | MT (pg/mL) | 50.42 Bb | 53.39 ABa | 55.36 Aa | 54.44 ABa | 1.041 | 0.008 | 0.019 | 0.906 |
| Trial Period | Items | CON | T80 | T120 | T160 | SEM | p-Value | ||
| Treatment | Linear | Quadratic | |||||||
| 31–37 day At 1 week after melatonin cessation | MT (pg/mL) | 50.08 | 53.21 | 54.68 | 54.47 | 1.008 | 0.358 | 0.114 | 0.412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cheng, Z.; Ma, W.; Qiu, Y.; Liu, T.; Nan, B.; Li, M.; Sun, L.; Liu, W.; Yin, H.; et al. Effect of Exogenous Melatonin on Performance and Mastitis in Dairy Cows. Vet. Sci. 2024, 11, 431. https://doi.org/10.3390/vetsci11090431
Li Y, Cheng Z, Ma W, Qiu Y, Liu T, Nan B, Li M, Sun L, Liu W, Yin H, et al. Effect of Exogenous Melatonin on Performance and Mastitis in Dairy Cows. Veterinary Sciences. 2024; 11(9):431. https://doi.org/10.3390/vetsci11090431
Chicago/Turabian StyleLi, Yunmeng, Zhiqiang Cheng, Wenting Ma, Yaqi Qiu, Tuo Liu, Bingyu Nan, Mengfei Li, Long Sun, Wentao Liu, Haina Yin, and et al. 2024. "Effect of Exogenous Melatonin on Performance and Mastitis in Dairy Cows" Veterinary Sciences 11, no. 9: 431. https://doi.org/10.3390/vetsci11090431
APA StyleLi, Y., Cheng, Z., Ma, W., Qiu, Y., Liu, T., Nan, B., Li, M., Sun, L., Liu, W., Yin, H., Wang, C., Li, X., & Zang, C. (2024). Effect of Exogenous Melatonin on Performance and Mastitis in Dairy Cows. Veterinary Sciences, 11(9), 431. https://doi.org/10.3390/vetsci11090431







