The Diagnostic Value of qPCR Quantification of Paenibacillus larvae in Hive Debris and Adult Bees for Predicting the Onset of American Foulbrood
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Validation of qPCR for the Quantification of P. larvae in Adult Bee Samples
2.3. qPCR Quantification of P. larvae in Adult Bee and Hive Debris Samples
2.4. Statistical Analysis
3. Results
3.1. Validation of qPCR for the Quantification of P. larvae in Adult Bee Samples
3.2. P. larvae Counts in Colonies with Varying Degrees of Disease Severity
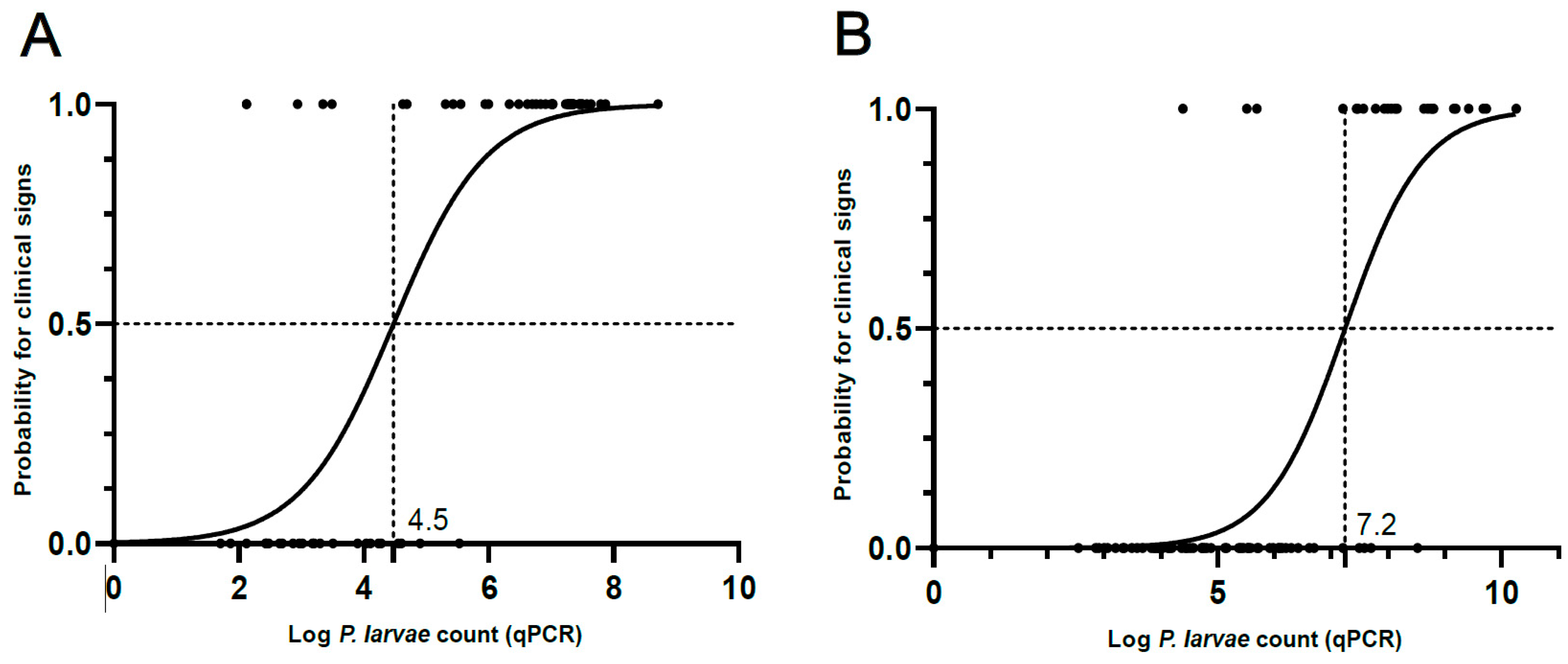
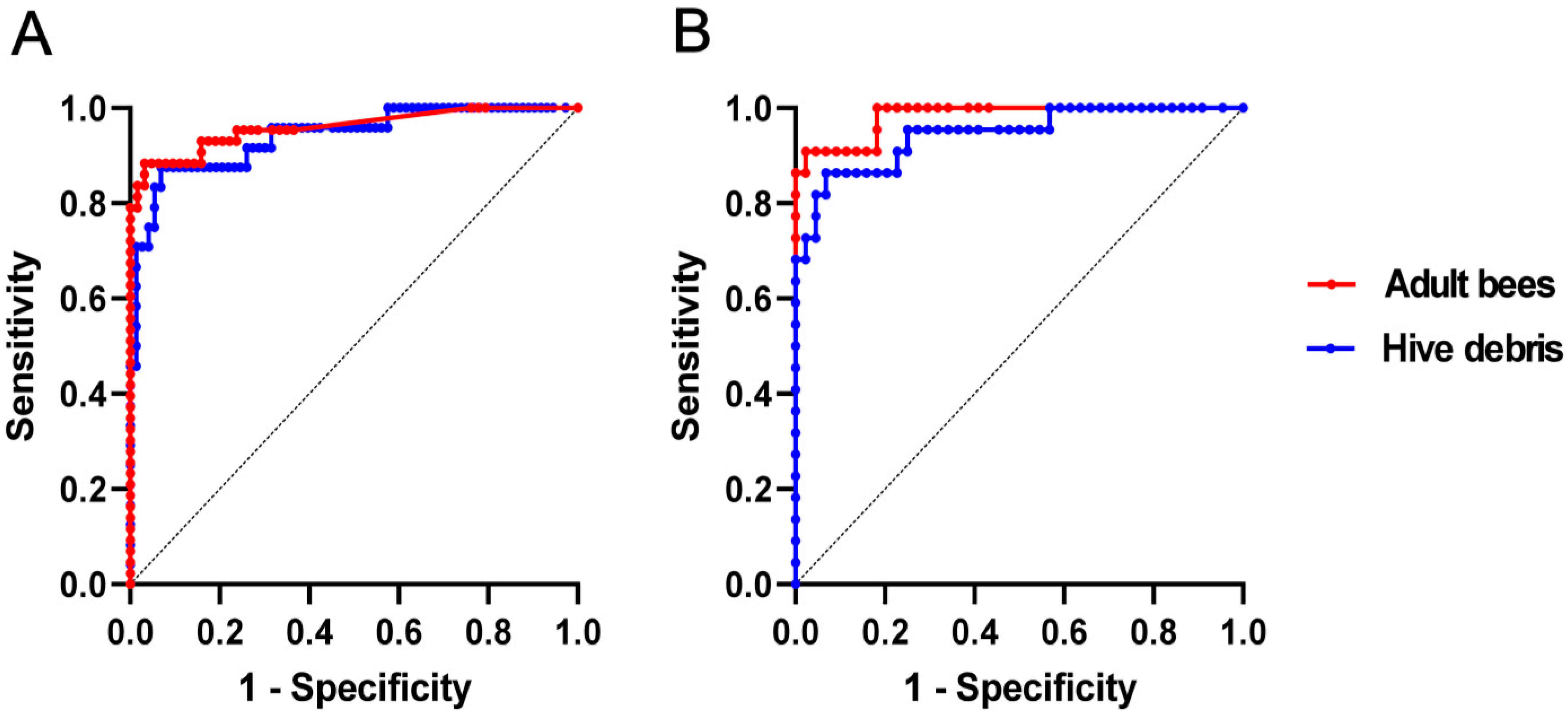
3.3. Binary Logistic Regression and ROC Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Organisation for Animal Health (WOAH). Chapter 3.2.2. American Foulbrood of honey bees (infection of honey bees with Paenibacillus larvae). In WOAH Terrestrial Manual 2023; 16p, Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.02.02_AMERICAN_FOULBROOD.pdf (accessed on 10 January 2024).
- Genersch, E. American foulbrood in honeybees and its causative agent, Paenibacillus larvae. J. Invertebr. Pathol. 2010, 103, S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Commission Delegated Regulation (EU) 2018/1629 of 25 July 2018 Amending the List of Diseases Set out in Annex II to Regulation (EU) 2016/429 of the European Parliament and of the Council on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (‘Animal Health Law’). Available online: https://eur-lex.europa.eu/eli/reg_del/2018/1629/oj (accessed on 5 June 2024).
- Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (‘Animal Health Law’). Available online: https://eur-lex.europa.eu/eli/reg/2016/429/oj (accessed on 12 June 2024).
- Legal Information System of the Republic of Slovenia (LISRS). Rulebook on Measures to Identify, Suppress, Notify and Prevent Severe Bee Brood Rot (Official Gazette of the Republic of Slovenia, No. 10/20 and 61/20); Legal Information System of the Republic of Slovenia: Ljubljana, Slovenia, 2020; p. 5. Available online: https://pisrs.si/pregledPredpisa?id=PRAV13788 (accessed on 12 February 2024).
- Bassi, S.; Carpana, E.; Bergomi, P.; Galletti, G. Detection and quantification of Paenibacillus larvae spores in samples of bees, honey and hive debris as a tool for American foulbrood risk assessment. Bull. Insectology 2018, 71, 235–241. Available online: http://www.bulletinofinsectology.org/pdfarticles/vol71-2018-235-241bassi.pdf (accessed on 3 February 2024).
- Stephan, J.G.; de Miranda, J.R.; Forsgren, E. American foulbrood in a honeybee colony: Spore-symptom relationship and feedbacks. BMC Ecol. 2020, 20, e15. [Google Scholar] [CrossRef]
- Kušar, D.; Papić, B.; Zajc, U.; Zdovc, I.; Golob, M.; Žvokelj, L.; Knific, T.; Avberšek, J.; Ocepek, M.; Pislak Ocepek, M. Novel TaqMan PCR assay for the quantification of Paenibacillus larvae spores in bee-related samples. Insects 2021, 12, 1034. [Google Scholar] [CrossRef]
- Bassi, S.; Galletti, G.; Carpana, E.; Palminteri, S.; Bosi, F.; Loglio, G.; Carra, E. Powdered sugar examination as a tool for the assessment of Paenibacillus larvae infection levels in honey bee colonies. Front. Vet. Sci. 2022, 9, e853707. [Google Scholar] [CrossRef]
- Nordström, S.; Forsgren, E.; Fries, I. Comparative diagnosis of American foulbrood using samples of adult honey bees and honey. J. Apic. Sci. 2002, 46, 5–12. [Google Scholar]
- Lindström, A.; Fries, I. Sampling of adult bees for detection of American foulbrood (Paenibacillus larvae subsp. larvae) spores in honey bee (Apis mellifera) colonies. J. Apic. Res. 2005, 44, 82–86. [Google Scholar] [CrossRef]
- Gillard, M.; Charriere, J.D.; Belloy, L. Distribution of Paenibacillus larvae spores inside honey bee colonies and its relevance for diagnosis. J. Invertebr. Pathol. 2008, 99, 92–95. [Google Scholar] [CrossRef]
- Gende, L.; Satta, A.; Ligios, V.; Ruiu, L.; Buffa, F.; Fernandez, N.; Churio, S.; Eguaras, M.; Fiori, M.; Floris, I. Searching for an American foulbrood early detection threshold by the determination of Paenibacillus larvae spore load in worker honey bees. Bull. Insectology 2011, 64, 229–233. [Google Scholar]
- Forsgren, E.; Laugen, A.T. Prognostic value of using bee and hive debris samples for the detection of American foulbrood disease in honey bee colonies. Apidologie 2014, 45, 10–20. [Google Scholar] [CrossRef]
- Zabrodski, M.W.; Epp, T.; Wilson, G.; Moshynskyy, I.; Sharafi, M.; Reitsma, L.; Castano Ospina, M.; DeBruyne, J.E.; Wentzell, A.; Wood, S.C.; et al. Establishment of apiary-level risk of American foulbrood through the detection of Paenibacillus larvae spores in pooled, extracted honey in Saskatchewan. Sci. Rep. 2022, 12, e8848. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, J.; Reinecke, A.; Sibum, N.; Fünfhaus, A.; Aumeier, P.; Otten, C.; Genersch, E. A comparison of different matrices for the laboratory diagnosis of the epizootic American foulbrood of honey bees. Vet. Sci. 2023, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, D.C.; Alippi, A.M.; Antúnez, K.; Aronstein, K.A.; Budge, G.; De Koker, D.; De Smet, L.; Dingman, D.W.; Evans, J.D.; Foster, L.J.; et al. Standard methods for American foulbrood research. J. Apic. Res. 2013, 52, 28. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization of the United Nations (FAO). Istituto Zooprofilattico Sperimentale del Lazio e della Toscana M. Aleandri (IZSLT), Apimondia, Chinese Academy of Agricultural Sciences, 2021. Good Beekeeping Practices for Sustainable Apiculture. FAO Animal Production and Health Guidelines No. 25. Rome. [CrossRef]
- Pernal, S.F.; Albright, R.L.; Melathopoulos, A.P. Evaluation of the shaking technique for the economic management of American foulbrood disease of honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2008, 101, 1095–1104. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Goksuluk, D.; Korkmaz, S.; Zararsiz, G.; Karaagaoglu, A.E. easyROC: An interactive web-tool for ROC curve analysis using R language environment. R J. 2016, 8/2, 2016. [Google Scholar] [CrossRef]
- Martínez, J.; Simon, V.; Gonzalez, B.; Conget, P. A real-time PCR-based strategy for the detection of Paenibacillus larvae vegetative cells and spores to improve the diagnosis and the screening of American foulbrood. Lett. Appl. Microbiol. 2010, 50, 603–610. [Google Scholar] [CrossRef]
- Rossi, F.; Amadoro, C.; Ruberto, A.; Ricchiuti, L. Evaluation of quantitative PCR (qPCR) Paenibacillus larvae targeted assays and definition of optimal conditions for its detection/quantification in honey and hive debris. Insects 2018, 9, 165. [Google Scholar] [CrossRef]
- Crudele, S.; Ricchiuti, L.; Ruberto, A.; Rossi, F. Quantitative PCR (qPCR) vs. culture-dependent detection to assess honey contamination by Paenibacillus larvae. J. Apic. Res. 2019, 59, 218–222. [Google Scholar] [CrossRef]
- Wilhelm, E.; Korschineck, I.; Sigmund, M.; Paulsen, P.; Hilbert, F.; Rossmanith, W. Monitoring of Paenibacillus larvae in Lower Austria through DNA-based detection without de-sporulation: 2018 to 2022. Vet. Sci. 2023, 10, 213. [Google Scholar] [CrossRef]
- Genersch, E.; Forsgren, E.; Pentikäinen, J.; Ashiralieva, A.; Rauch, S.; Kilwinski, J.; Fries, I. Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies classification. Int. J. Syst. Evol. Microbiol. 2006, 56, e501511. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Ashiralieva, A.; Hedtke, K.; Genersch, E. Negative correlation between individual-insect-level virulence and colony-level virulence of Paenibacillus larvae, the etiological agent of American foulbrood of honeybees. Appl. Environ. Microbiol. 2009, 75, 3344–3347. [Google Scholar] [CrossRef] [PubMed]
- Spivak, M.; Reuter, G.S. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 2001, 32, 555–565. [Google Scholar] [CrossRef]
- Evans, J.D.; Armstrong, T.N. Antagonistic interactions between honey bee bacterial symbionts and implications for disease. BMC Ecol. 2006, 6, e4. [Google Scholar] [CrossRef]
- Seeley, T.D.; Tarpy, D.R. Queen promiscuity lowers disease within honeybee colonies. Proc. Biol. Sci. 2007, 274, 67–72. [Google Scholar] [CrossRef]
- Daisley, B.A.; Pitek, A.P.; Chmiel, J.A.; Al, K.F.; Chernyshova, A.M.; Faragalla, K.M.; Burton, J.P.; Thompson, G.J.; Reid, G. Novel probiotic approach to counter Paenibacillus larvae infection in honey bees. ISME J. 2020, 14, 476–491. [Google Scholar] [CrossRef]
- Erban, T.; Ledvinka, O.; Kamler, M.; Nesvorna, M.; Hortova, B.; Tyl, J.; Titera, D.; Markovic, M.; Hubert, J. Honeybee (Apis mellifera)-associated bacterial community affected by American foulbrood: Detection of Paenibacillus larvae via microbiome analysis. Sci. Rep. 2017, 7, e5084. [Google Scholar] [CrossRef]
- Žugelj, A.; Papić, B.; Zdovc, I.; Zajc, U.; Golob, M.; Avberšek, J.; Kušar, D. ERIC and WGS typing of Paenibacillus larvae in Slovenia: Investigation of ERIC I outbreaks. Insects 2021, 12, 362. [Google Scholar] [CrossRef]



| AFB-Unaffected Colonies | AFB-Affected Colonies | Sum | |||
|---|---|---|---|---|---|
| Disease Severity | 0 | 1 | 2 | 3 | |
| Adult Bees (total) | 63 | 15 | 13 | 15 | 106 |
| Hive Debris (total) | 73 | 8 | 12 * | 4 * | 97 |
| Paired Sample Subset (Adult Bees and Hive Debris) | 44 | 8 | 11 * | 3 * | 2 × 66 |
| Measure | Adult Bees (n = 106) | Hive Debris (n = 97) |
|---|---|---|
| Youden’s J Index (Optimal Criterion) | 0.9 | 0.8 |
| Optimal Cut-Off (log P. larvae Count) | 4.6 | 7.2 |
| Sensitivity | 0.884 | 0.875 |
| Specificity | 0.968 | 0.932 |
| Positive Predictive Value | 0.950 | 0.808 |
| Negative predictive Value | 0.924 | 0.958 |
| Positive Likelihood Ratio | 27.837 | 12.775 |
| Negative Likelihood Ratio | 0.120 | 0.134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papić, B.; Žvokelj, L.; Pislak Ocepek, M.; Hočevar, B.; Kozar, M.; Rus, R.; Zajc, U.; Kušar, D. The Diagnostic Value of qPCR Quantification of Paenibacillus larvae in Hive Debris and Adult Bees for Predicting the Onset of American Foulbrood. Vet. Sci. 2024, 11, 442. https://doi.org/10.3390/vetsci11090442
Papić B, Žvokelj L, Pislak Ocepek M, Hočevar B, Kozar M, Rus R, Zajc U, Kušar D. The Diagnostic Value of qPCR Quantification of Paenibacillus larvae in Hive Debris and Adult Bees for Predicting the Onset of American Foulbrood. Veterinary Sciences. 2024; 11(9):442. https://doi.org/10.3390/vetsci11090442
Chicago/Turabian StylePapić, Bojan, Lucija Žvokelj, Metka Pislak Ocepek, Barbara Hočevar, Monika Kozar, Rene Rus, Urška Zajc, and Darja Kušar. 2024. "The Diagnostic Value of qPCR Quantification of Paenibacillus larvae in Hive Debris and Adult Bees for Predicting the Onset of American Foulbrood" Veterinary Sciences 11, no. 9: 442. https://doi.org/10.3390/vetsci11090442
APA StylePapić, B., Žvokelj, L., Pislak Ocepek, M., Hočevar, B., Kozar, M., Rus, R., Zajc, U., & Kušar, D. (2024). The Diagnostic Value of qPCR Quantification of Paenibacillus larvae in Hive Debris and Adult Bees for Predicting the Onset of American Foulbrood. Veterinary Sciences, 11(9), 442. https://doi.org/10.3390/vetsci11090442






