Development and Immunogenicity Study of Subunit Vaccines Based on Spike Proteins of Porcine Epidemic Diarrhea Virus and Porcine Transmissible Gastroenteritis Virus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus Culture
2.2. Experimental Mice
2.3. Optimization and Synthesis of Genes for Expression and Purification of Antigens
2.4. SDS–PAGE and Western Blotting Assay
2.5. Vaccine Preparation and Animal Immunization
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Determination of Neutralizing Antibody
2.8. Enzyme-Linked Immuno-Spot (ELISPOT)
2.9. Statistical Analysis
3. Results
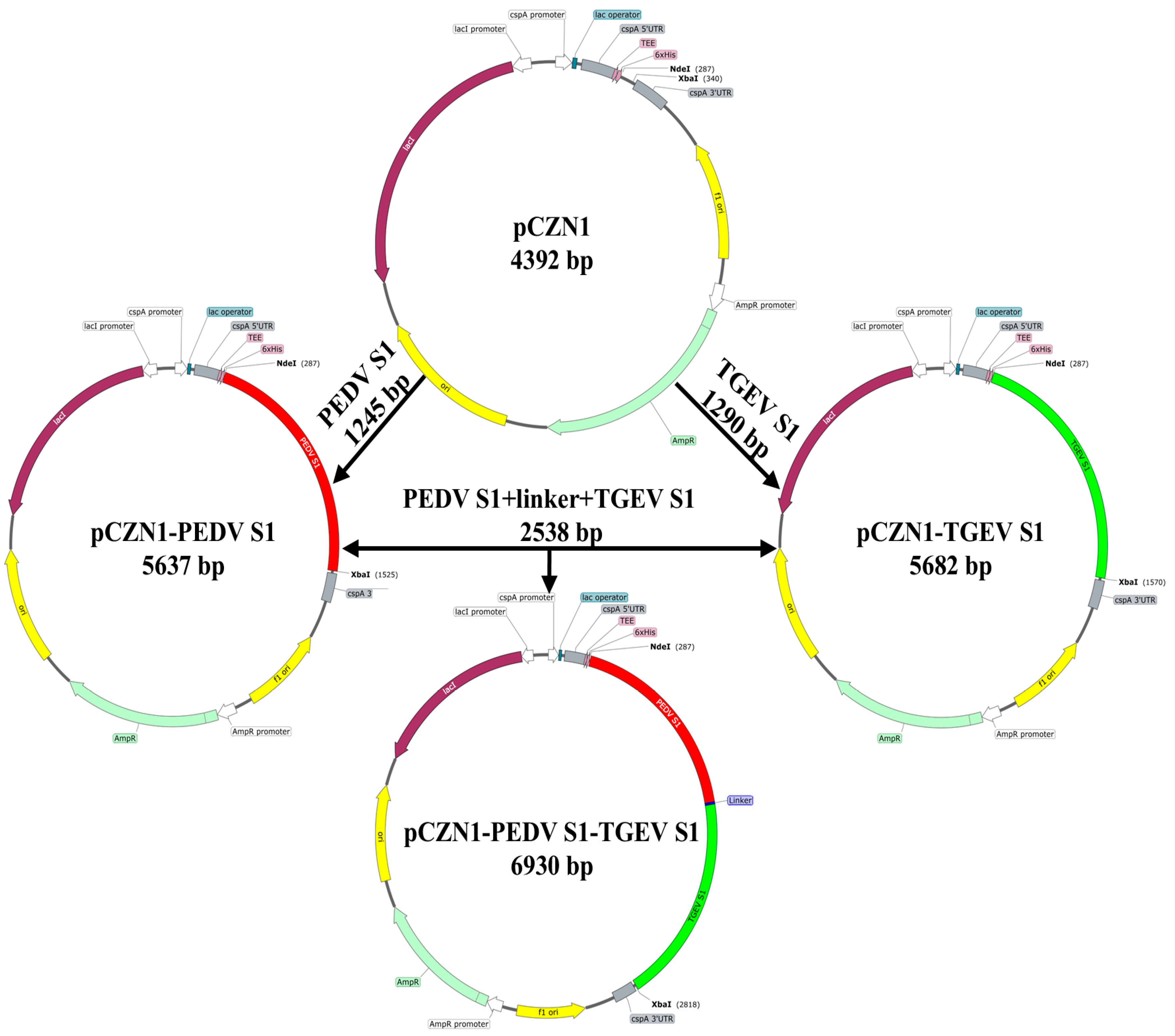
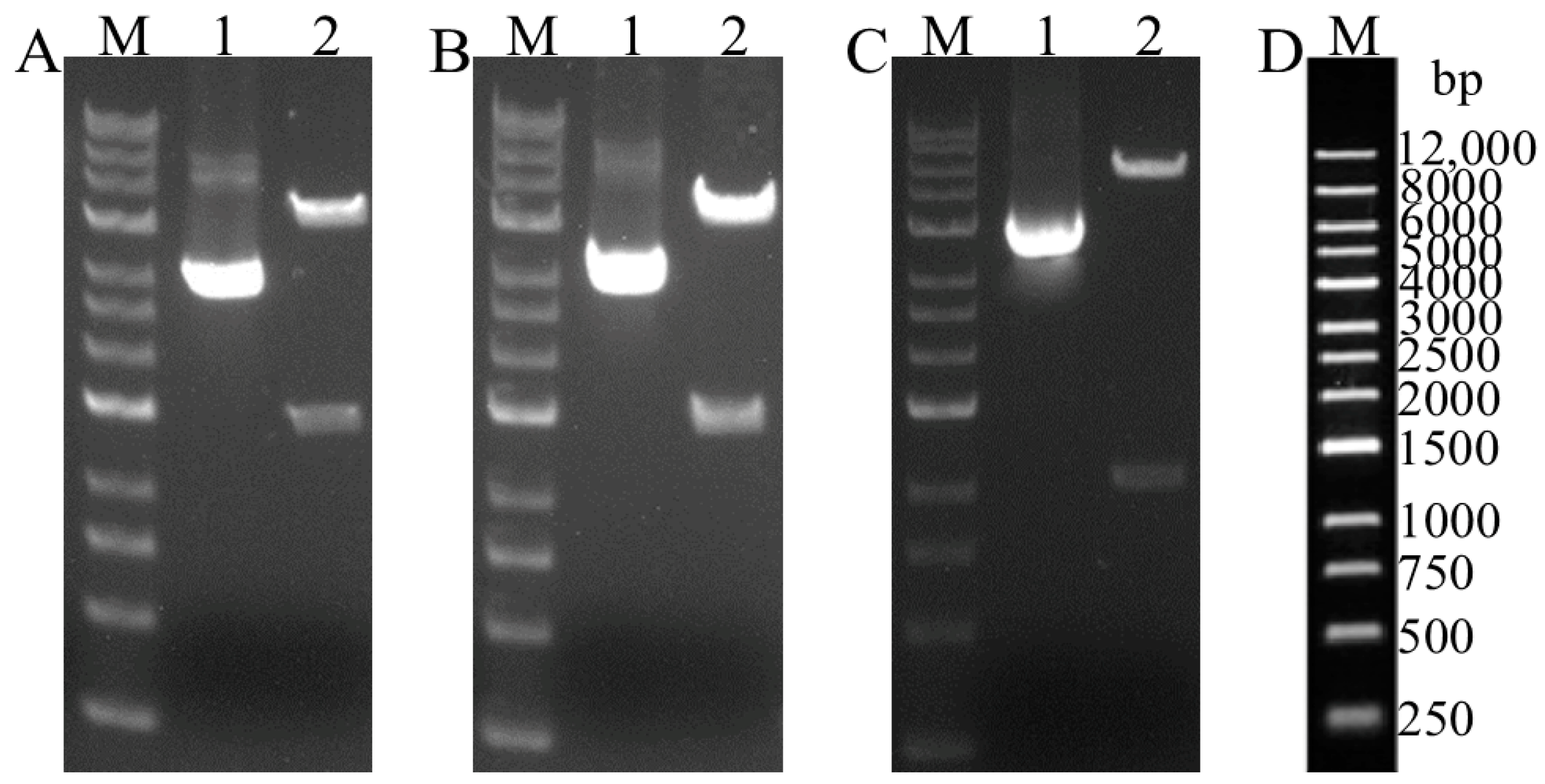
3.1. Construction of Recombinant Expression Vectors
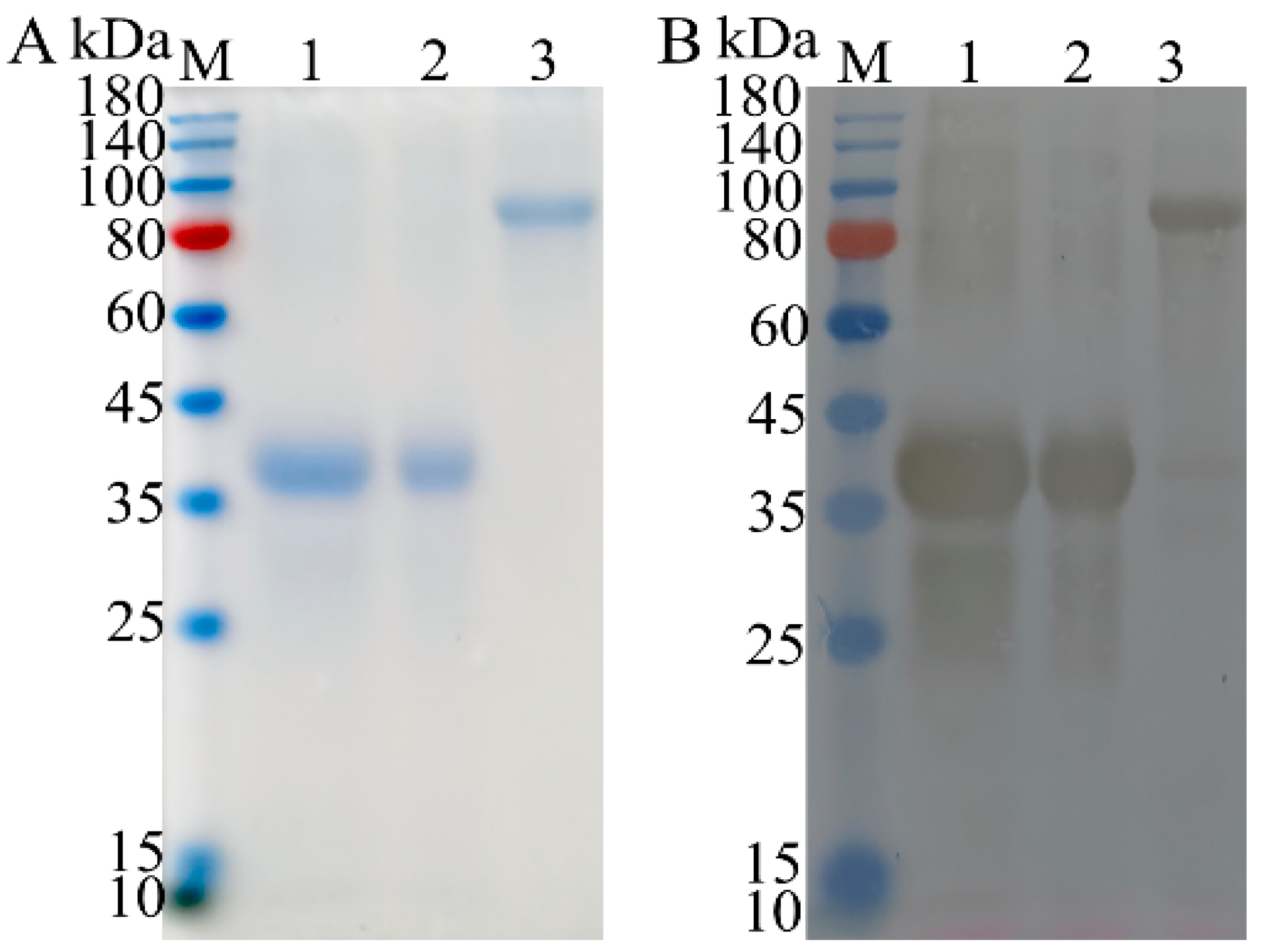
3.2. Expression and Purification of Recombinant Proteins (Supplemantary Material Figure S1)
3.3. Specific Antibody Levels Detection
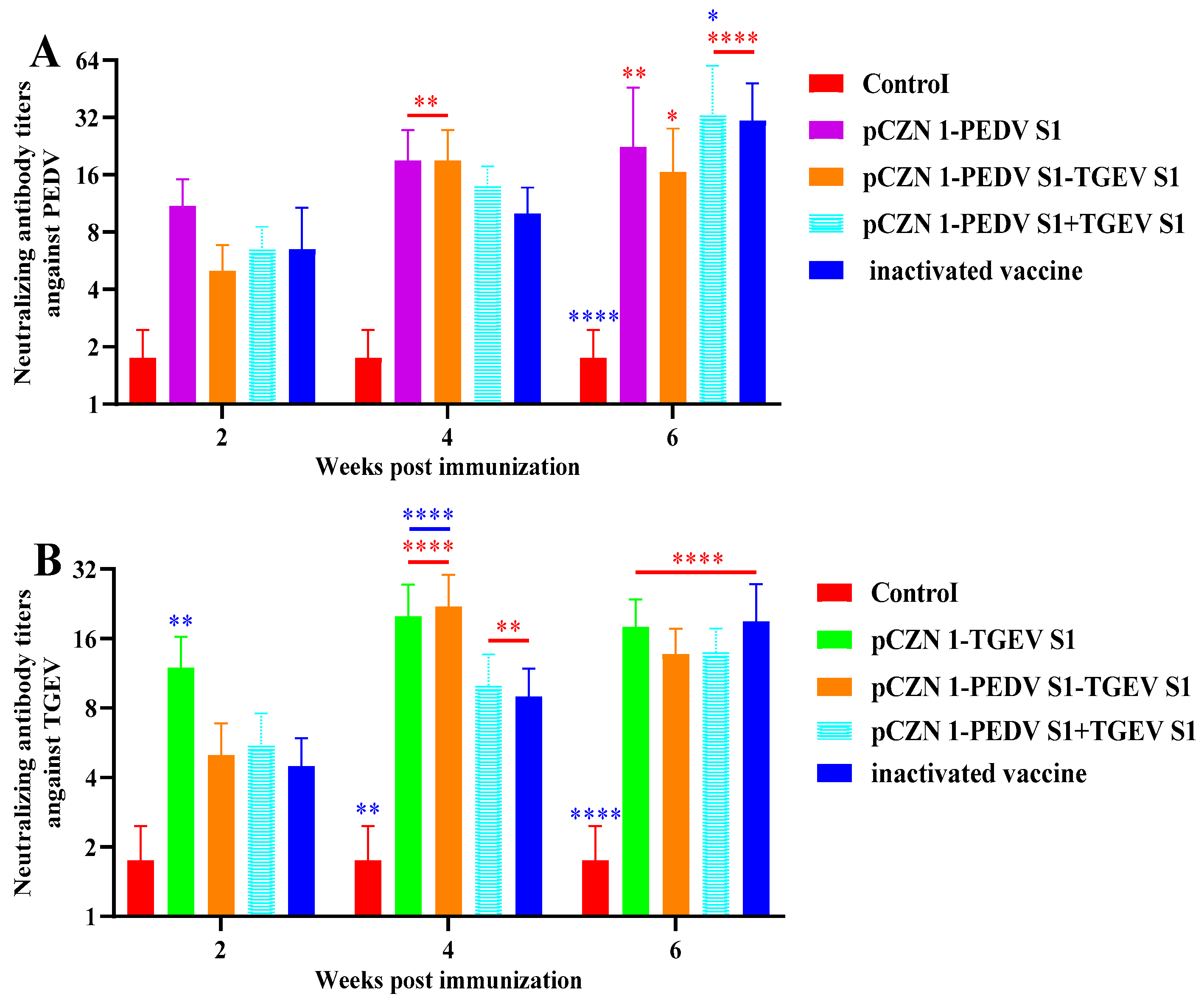
3.4. Neutralizing Antibodies of PEDV and TGEV
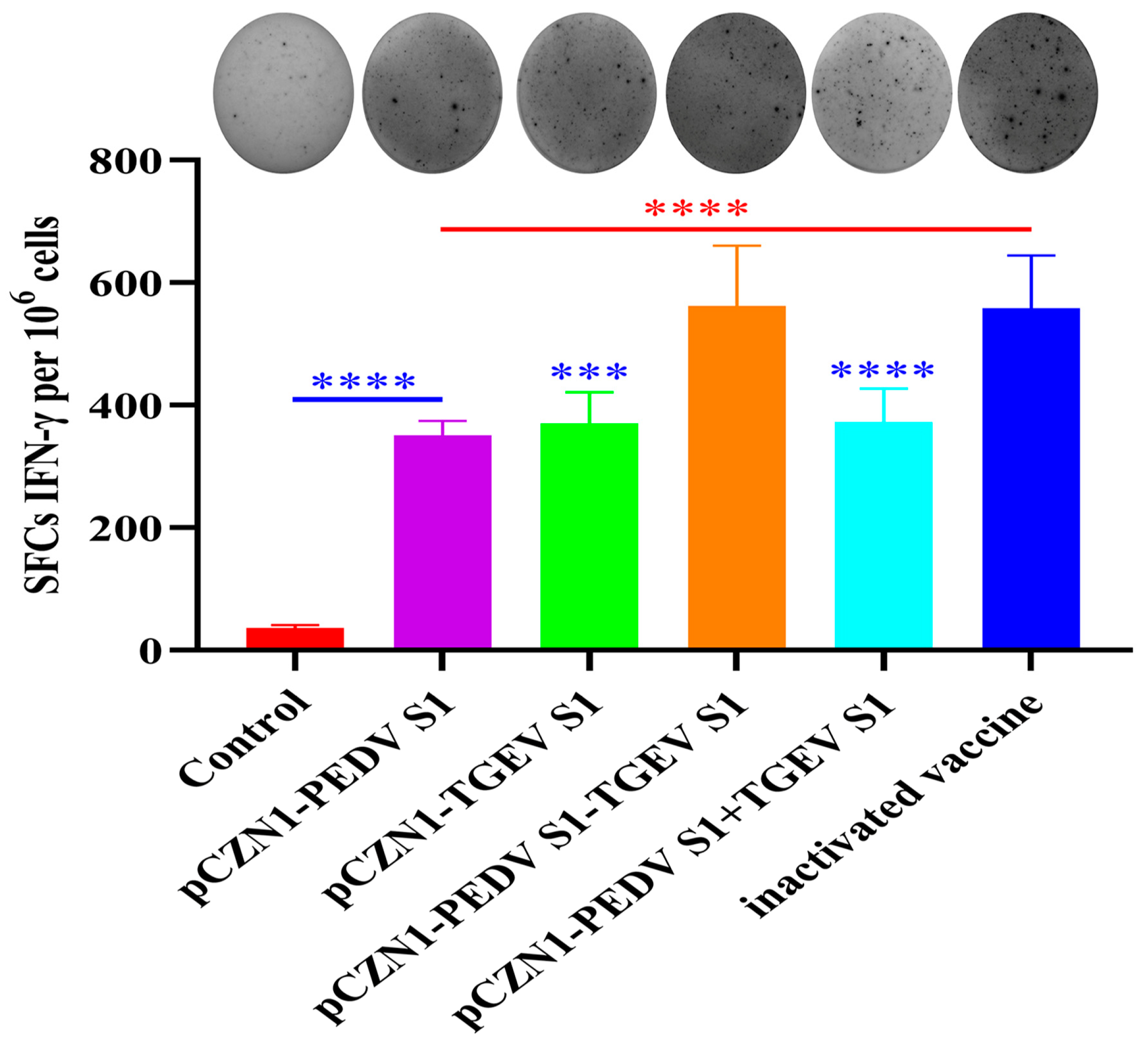
3.5. The Effect of Recombinant Subunit Vaccines on Cytokine Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mcintosh, K. Coronaviruses: A Comparative Review. In Current Topics in Microbiology and Immunology/Ergebnisse der Mikrobiologie und Immunitätsforschung; Springer: Berlin/Heidelberg, Germany, 1974; pp. 85–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Liao, X.; Huang, X.; Cao, S.; Wen, X.; Wen, Y.; Wu, R.; Liu, W. Construction of a bivalent DNA vaccine co-expressing S genes of transmissible gastroenteritis virus and porcine epidemic diarrhea virus delivered by attenuated Salmonella typhimurium. Virus Genes 2016, 52, 354–364. [Google Scholar] [CrossRef]
- Saif, L.J. Comparative Pathogenesis of Enteric Viral Infections of Swine. In Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1999; Volume 473, pp. 47–59. [Google Scholar] [CrossRef]
- Chattha, K.; Roth, J.; Saif, L.J. Strategies for design and application of enteric viral vaccines. Annu. Rev. Anim. Biosci. 2015, 3, 375–395. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, L.; Ma, J.; Zhou, Q.F.; Song, Y.; Sun, B.; Chen, R.; Xie, Q.; Bee, Y. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field strains in south China. Virus Genes 2012, 45, 181–185. [Google Scholar] [CrossRef]
- Huang, X.; Chen, J.; Yao, G.; Guo, Q.; Wang, J.; Liu, G. A TaqMan-probe-based multiplex real-time RT-qPCR for simultaneous detection of porcine enteric coronaviruses. Appl. Microbiol. Biotechnol. 2019, 103, 4943–4952. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, W.; Wang, D.; Wang, W.; Chang, X.; Li, Y.; Li, J.; Fan, B.; Zhou, J.; Guo, R.; et al. Development of a colloidal gold immunochromatographic strip for the simultaneous detection of porcine epidemic diarrhea virus and transmissible gastroenteritis virus. Front. Microbiol. 2024, 19, 1418959. [Google Scholar] [CrossRef]
- Boniotti, M.; Papetti, A.; Lavazza, A.; Alborali, G.; Sozzi, E.; Chiapponi, C.; Faccini, S.; Bonilauri, P.; Cordioli, P.; Marthaler, D. Porcine epidemic diarrhea virus and discovery of a recombinant swine enteric coronavirus, Italy. Emerg. Infect. Dis. 2016, 22, 83–87. [Google Scholar] [CrossRef]
- Guo, J.; Lai, Y.; Yang, Z.; Song, W.; Zhou, J.; Li, Z.; Su, W.; Xiao, S.; Fang, L. Coinfection and nonrandom recombination drive the evolution of swine enteric coronaviruses. Emerg. Microbes Infect. 2024, 13, 2332653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tang, C.; Yue, H.; Ren, Y.; Song, Z. Viral metagenomics analysis demonstrates the diversity of viral flora in piglet diarrhoeic faeces in China. J. Gen. Virol. 2014, 95, 1603–1611. [Google Scholar] [CrossRef]
- Wood, E.N. An apparently new syndrome of porcine epidemic diarrhoea. Vet. Rec. 1977, 100, 243–244. [Google Scholar] [CrossRef]
- Pensaert, M.; Bouck, P.D. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef]
- Debouck, P.; Pensaert, M. Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. Am. J. Vet. Res. 1980, 41, 219. [Google Scholar] [CrossRef]
- Sun, D.; Wang, X.; Wei, S.; Chen, J.; Feng, L. Epidemiology and vaccine of porcine epidemic diarrhea virus in China: A mini-review. J. Vet. Med. Sci. 2016, 78, 355–363. [Google Scholar] [CrossRef]
- Paarlberg, P. Updated Estimated Economic Welfare Impacts of Porcine Epidemic Diarrhea Virus (PEDV); Department of Agricultural Economics, Purdue University: Lafayette, IN, USA, 2014. [Google Scholar] [CrossRef]
- Weng, L.; Weersink, A.; Poljak, Z.; Lange, K.D.; Massow, M.V. An economic evaluation of intervention strategies for Porcine Epidemic Diarrhea (PED). Prev. Vet. Med. 2016, 134, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dickerman, A.; Pieyro, P.; Li, L.; Meng, X. Origin, Evolution, and Genotyping of Emergent Porcine Epidemic Diarrhea Virus Strains in the United States. Mbio 2013, 4. [Google Scholar] [CrossRef]
- Kocherhans, R.; Bridgen, A.; Ackermann, M.; Tobler, K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes 2001, 23, 137–144. [Google Scholar] [CrossRef]
- Wang, Q.; Vlasova, A.; Kenney, S.; Saif, L.J. Emerging and re-emerging coronaviruses in pigs. Curr. Opin. Virol. 2019, 34, 39–49. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Liu, J.; Qu, Q.; Gai, W.; Zhang, H.; Xiao, X.; Wei, R. Research advances in antigenic epitopes of structural proteins of PEDV. Chin. Vet. Sci. 2024, 54, 1651–1657. [Google Scholar] [CrossRef]
- Doyle, L.; Hutchings, L. A transmissible gastroenteritis in pigs. J. Am. Vet. Med. Assoc. 1946, 108, 257–259. [Google Scholar] [CrossRef][Green Version]
- Zhao, S.; Gao, Q.; Qin, T.; Yin, Y.; Lin, J.; Yu, Q.; Yang, Q. Effects of virulent and attenuated transmissible gastroenteritis virus on the ability of porcine dendritic cells to sample and present antigen. Vet. Microbiol. 2014, 171, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, H. Porcine enteric coronaviruses: An updated overview of the pathogenesis, prevalence, and diagnosis. Vet. Res. Commun. 2021, 45, 75–86. [Google Scholar] [CrossRef]
- Chen, F.; Knutson, T.; Rossow, S.; Saif, L.; Marthaler, D. Decline of transmissible gastroenteritis virus and its complex evolutionary relationship with porcine respiratory coronavirus in the United States. Sci. Rep. 2019, 9, 3953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, W.; Yuan, L.; Yang, Q. Transferrin receptor 1 is a supplementary receptor that assists transmissible gastroenteritis virus entry into porcine intestinal epithelium. Cell Commun. Signal 2018, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhao, Z.; Li, G.; Suo, S.; Shi, N.; Yin, J.; Zarlenga, D.; Ren, X. Bacterial expression of antigenic sites A and D in the spike protein of transmissible gastroenteritis virus and evaluation of their inhibitory effects on viral infection. Virus Genes 2011, 43, 335–341. [Google Scholar] [CrossRef]
- Suo, S.; Wang, X.; Zarlenga, D.; Bu, R.; Ren, Y.; Ren, X. Phage display for identifying peptides that bind the spike protein of transmissible gastroenteritis virus and possess diagnostic potential. Virus Genes 2015, 51, 51–56. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, Y.; Lin, C.; Tan, M.; Wan, P.; Xie, B.; Xiong, L.; Ji, H. Epidemiological monitoring and genetic variation analysis of pathogens associated with porcine viral diarrhea in southern China from 2021 to 2023. Front. Microbiol. 2024, 15, 1303915. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Yan, Q.; Zhang, X.A.; Zeng, W.; Xie, K.; Yuan, Z.; Liu, X.; Liu, X.; Zhang, L.; Wu, K.; et al. Virus-like particle vaccines with epitopes from porcine epidemic virus and transmissible gastroenteritis virus incorporated into self-assembling ADDomer platform provide clinical immune responses in piglets. Front. Immunol. 2023, 14, 1251001. [Google Scholar] [CrossRef]
- Kong, F.; Jia, H.; Xiao, Q.; Fang, L.; Wang, Q. Prevention and control of swine enteric coronaviruses in China: A review of vaccine development and application. Vaccines 2023, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Iglesias, A.; Sanchez, C.M.; Penzes, Z.; Sola, I.; Enjuanes, L.; Zuñiga, S. Recombinant chimeric transmissible gastroenteritis Virus (TGEV)—Porcine epidemic diarrhea virus (PEDV) virus provides protection against virulent PEDV. Viruses 2019, 11, 682. [Google Scholar] [CrossRef]
- Reed, L.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Gao, Y.; Pei, C.; Sun, X.; Zhang, C.; Li, L.; Kong, X. Novel subunit vaccine based on grass carp reovirus VP35 protein provides protective immunity against grass carp hemorrhagic disease. Fish Shellfish Immunol. 2018, 75, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, F.; Wang, Y.; Guo, H.; Liu, C.; Zhao, H.; Yu, M.; Wen, Y.J. Subunit vaccine based on glycoprotein B protects pattern animal guinea pigs from tissue damage caused by infectious bovine rhinotracheitis virus. Virus Res. 2022, 320, 198899. [Google Scholar] [CrossRef]
- Xiong, C.; He, W.; Xiao, J.; Hao, G.; Pu, J.; Chen, H.; Xu, L.; Zhu, Y.; Yang, G. Assessment of the immunoprotective efficacy of recombinant 14-3-3 protein and dense granule protein 10 (GRA10) as candidate antigens for rabbit vaccines against eimeria intestinalis. Int. J. Mol. Sci. 2023, 24, 14418. [Google Scholar] [CrossRef]
- Hu, J.; Li, G.; Wang, X.; Cai, L.; Rong, M.; Li, H.; Xie, M.; Zhang, Z.; Rong, J. Development of a subunit vaccine based on fiber2 and hexon against fowl adenovirus serotype 4. Virus Res. 2021, 305, 198552. [Google Scholar] [CrossRef]
- Miao, X.; Zhang, L.; Zhou, P.; Zhang, Z.; Yu, R.; Liu, X.; Lv, J.; Wang, Y.; Guo, H.; Pan, L.; et al. Recombinant human adenovirus type 5 based vaccine candidates against GIIa- and GIIb-genotype porcine epidemic diarrhea virus induce robust humoral and cellular response in mice. Virology 2023, 584, 9–23. [Google Scholar] [CrossRef]
- Lambe, T.; Carey, J.B.; Li, Y.; Spencer, A.; Laarhoven, A.; Mullarkey, C.; Vrdoljak, A.; Moore, A.; Gilbert, S. Immunity against heterosubtypic influenza virus induced by adenovirus and MVA expressing nucleoprotein and matrix protein-1. Sci. Rep. 2013, 3, 1443. [Google Scholar] [CrossRef] [PubMed]
- Ulaszewska, M.; Merelie, S.; Sebastian, S.; Lambe, T. Preclinical immunogenicity of an adenovirus-vectored vaccine for herpes zoster. Hum. Vaccin. Immunother. 2023, 19, 2175558. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, L.; Ma, S.; Wang, X.; Wang, Y.; Xiao, Y.; Jiang, Y.; Qiao, X.; Tang, L.; Xu, Y.; et al. Immunogenicity of eGFP-marked recombinant lactobacillus casei against transmissible gastroenteritis virus and porcine epidemic diarrhea virus. Viruses 2017, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Ren, Y.; Suo, S.; Sun, X.; Li, X.; Li, P.; Yang, W.; Li, G.; Li, L.; Schwegmann-Wessels, C.; et al. Evaluation on the efficacy and immunogenicity of recombinant DNA plasmids expressing spike genes from porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. PLoS ONE 2013, 8, e57468. [Google Scholar] [CrossRef]
- Negro, F. Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis? Swiss Med. Wkly. 2020, 150, w20249. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Foulds, K.E.; Wu, C.Y.; Seder, R.A. Th1 memory: Implications for vaccine development. Immunol. Rev. 2006, 211, 58–66. [Google Scholar] [CrossRef] [PubMed]

| Groups (n = 8) | Immunogen and Dosage | Immunization Time (d) |
|---|---|---|
| PBS (Negative control, NC) | 100 µL of Sterile PBS | 0, 14th |
| PEDV S1 + A206 adjuvant | 100 µg PEDV S1 + 100 µL A206 adjuvant | 0, 14th |
| TGEV S1 + A206 adjuvant | 100 µg TGEV S1 + 100 µL A206 adjuvant | 0, 14th |
| PEDV S1-TGEV S1 + A206 adjuvant | 100 µg PEDV S1- TGEV S1 + 100 µL A206 adjuvant | 0, 14th |
| PEDV S1 + TGEV S1 + A206 adjuvant | 50 µg PEDV S1 + 50 µg TGEV S1 + 100 µL A206 adjuvant | 0, 14th |
| Inactivated vaccines (Positive control, PC) | 200 µL | 0, 14th |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Yang, Z.; Yang, N.; Li, H.; Ma, H.; Yi, J.; Hou, H.; Han, F.; Ma, Z.; Chen, C. Development and Immunogenicity Study of Subunit Vaccines Based on Spike Proteins of Porcine Epidemic Diarrhea Virus and Porcine Transmissible Gastroenteritis Virus. Vet. Sci. 2025, 12, 106. https://doi.org/10.3390/vetsci12020106
Xu M, Yang Z, Yang N, Li H, Ma H, Yi J, Hou H, Han F, Ma Z, Chen C. Development and Immunogenicity Study of Subunit Vaccines Based on Spike Proteins of Porcine Epidemic Diarrhea Virus and Porcine Transmissible Gastroenteritis Virus. Veterinary Sciences. 2025; 12(2):106. https://doi.org/10.3390/vetsci12020106
Chicago/Turabian StyleXu, Mingguo, Zhonglian Yang, Ningning Yang, Honghuan Li, Hailong Ma, Jihai Yi, Huilin Hou, Fangfang Han, Zhongchen Ma, and Chuangfu Chen. 2025. "Development and Immunogenicity Study of Subunit Vaccines Based on Spike Proteins of Porcine Epidemic Diarrhea Virus and Porcine Transmissible Gastroenteritis Virus" Veterinary Sciences 12, no. 2: 106. https://doi.org/10.3390/vetsci12020106
APA StyleXu, M., Yang, Z., Yang, N., Li, H., Ma, H., Yi, J., Hou, H., Han, F., Ma, Z., & Chen, C. (2025). Development and Immunogenicity Study of Subunit Vaccines Based on Spike Proteins of Porcine Epidemic Diarrhea Virus and Porcine Transmissible Gastroenteritis Virus. Veterinary Sciences, 12(2), 106. https://doi.org/10.3390/vetsci12020106






