Replacement of Monensin with a Proprietary Tannin-Blend Additive in Calf-Fed Holstein Steer Diets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Use
2.2. Animals, Diet, and Experimental Design
2.3. Feedlot Performance Measures
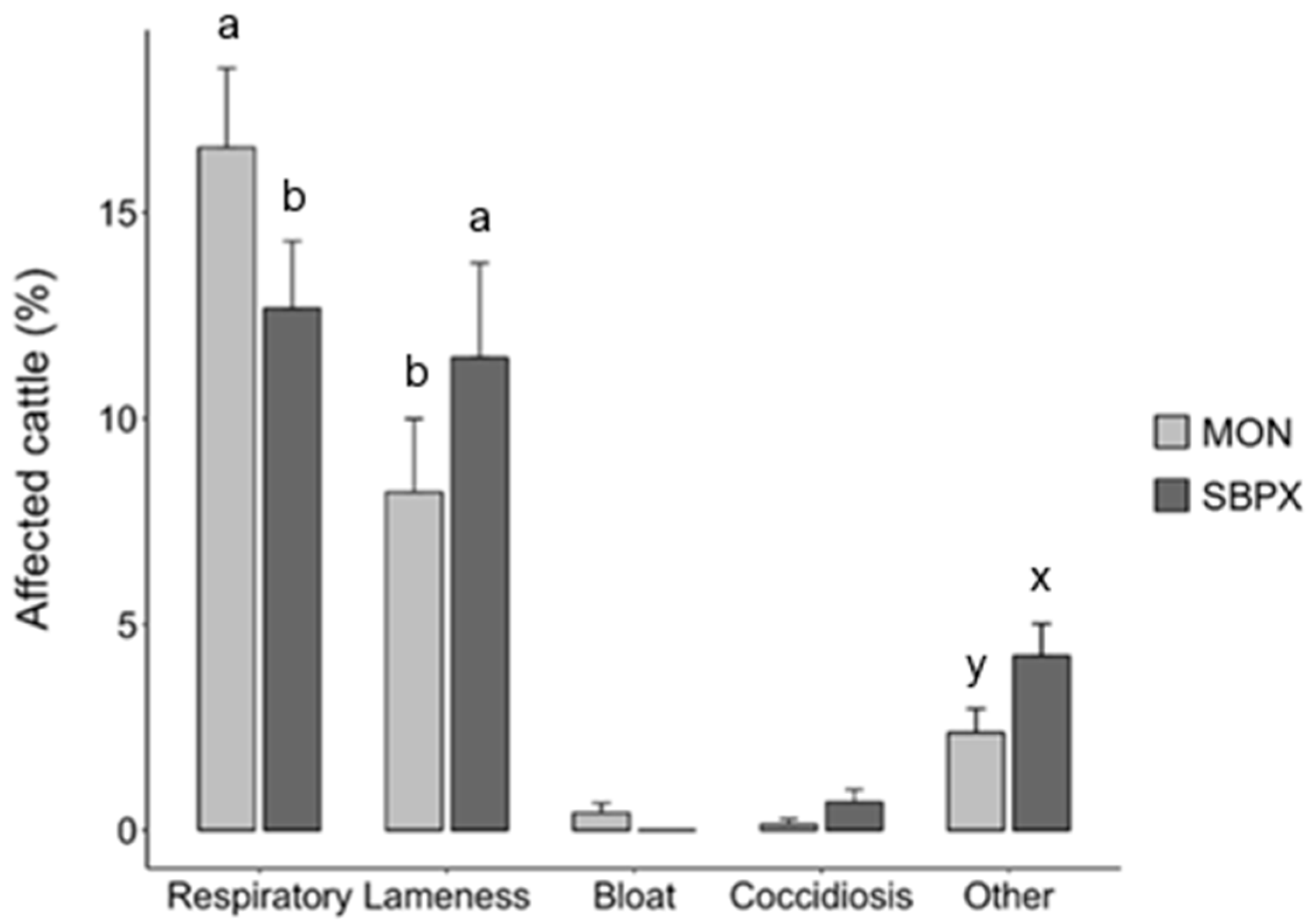
2.4. Measures of Health Response
2.5. Carcass Performance Measures
2.6. Phytochemical Quantification
2.7. Statistical Analysis
3. Results
3.1. Performance Responses
3.2. Health Responses
3.3. Carcass Responses
3.4. Phytochemical Status
4. Discussion
4.1. Performance Responses
4.2. Health-Related Outcomes
4.2.1. Phytochemical Status
4.2.2. Health Responses
4.3. Carcass Responses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Duffield, T.F.; Merrill, J.K.; Bagg, R.N. Meta-analysis of the effects of monensin in beef cattle on feed efficiency, body weight gain, and dry matter intake. J. Anim. Sci. 2012, 90, 4583–4592. [Google Scholar] [CrossRef]
- Duff, G.C.; McMurphy, C.P. Feeding Holstein steers from start to finish. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 281–297. [Google Scholar] [CrossRef]
- Goodrich, R.D.; Garrett, J.E.; Gast, D.R.; Kirick, M.A.; Larson, D.A.; Meiske, J.C. Influence of monensin on the performance of cattle. J. Anim. Sci. 1984, 58, 1484–1498. [Google Scholar] [CrossRef]
- Schelling, G.T. Monensin mode of action in the rumen. J. Anim. Sci. 1984, 58, 1518–1527. [Google Scholar] [CrossRef]
- Raun, A.P.; Cooley, C.O.; Potter, E.L.; Rathmacher, R.P.; Richardson, L.F. Effect of monensin on feed efficiency of feedlot cattle. J. Anim. Sci. 1976, 43, 670–677. [Google Scholar] [CrossRef]
- Richardson, L.F.; Raun, A.P.; Potter, E.L.; Cooley, C.O.; Rathmacher, R.P. Effect of monensin on rumen fermentation in vitro and in vivo. J. Anim. Sci. 1976, 43, 657–664. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unraveling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Mangan, J.L. Nutritional effects of tannins in animal feeds. Nutr. Res. Rev. 1988, 1, 209–231. [Google Scholar] [CrossRef]
- Swain, T. Tannins and lingnins. In Herbivores, Their Interaction with Secondary Plant Metabolites, 1st ed.; Rosenthal, G.A., Janzen, D.H., Eds.; Academic Press: New York, NY, USA, 1979; Volume 1, pp. 657–682. [Google Scholar]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Rivera-Méndez, C.; Plascencia, A.; Torrentera, N.; Zinn, R.A. Effect of level and source of supplemental tannin on growth performance of steers during the late finishing phase. J. Appl. Anim. Res. 2017, 45, 199–203. [Google Scholar] [CrossRef]
- Barajas, R.B.; Cervantes, J.; Espino, M.A.; Camacho, A.; Verdugo, M.; Flores, L.R.; Loneli, J.J.; Romo, J.A. Effect of tannin extracts supplementation on feedlot performance and plasma urea nitrogen of yearling bulls fed dry-ground corn-based diets containing corn-DDG and cane molasses. J. Anim. Sci. 2012, 90 (Suppl. S3), 600. [Google Scholar]
- Nascimento, K.S.; Bomfim, L.E.L.M.; Couto, V.R.M.; Silva, M.B.; Lopes, A.L.A.; Fernandes, M.H.M.R.; Manella, M.Q.; Junior, M.V.C.F.; Fernandes, J.J.R. Growth performance and carcass characteristics of bulls fed tannins associated with or not with monensin. Transl. Anim. Sci. 2024, 8, txae136. [Google Scholar] [CrossRef]
- Manella, M.Q.; Coan, R.; Mesquita, W.; Paula, E.; Arnandes, R.B. PSXI-20 Proteic dry supplements enriched with a blend of tannin-based extract enhance the performance of grass-fed cattle during the rainy season in tropical regions. J. Anim. Sci. 2024, 102, 762–763. [Google Scholar] [CrossRef]
- Carvalho, P.H.V.; Latack, B.C.; Ferraz, M.V.C.; Nolasco, L.J.R.P.; Meireles, W.R.; Oliveira, H.O.M.; Zinn, R.A. Influence of low-level tannin supplementation on comparative growth performance of Holstein and Angus × Holstein cross calf-fed concentrate-based finishing diets for 328 d. J. Anim. Sci. 2024, 102, skae087. [Google Scholar] [CrossRef]
- Krueger, W.K.; Gutierrez-Bañuelos, H.; Carstens, G.E.; Min, B.R.; Pinchak, W.E.; Gomez, R.R.; Anderson, R.C.; Krueger, N.A.; Forbes, T.D.A. Effects of dietary tannin source on performance, feed efficiency, ruminal fermentation, and carcass and non-carcass traits in steers fed a high-grain diet. Anim. Feed. Sci. Technol. 2010, 159, 1–9. [Google Scholar] [CrossRef]
- Tabke, M.C.; Sarturi, J.O.; Galyean, M.L.; Trojan, S.J.; Brooks, J.C.; Johnson, B.J.; Martin, J.; Baggerman, J.; Thompson, A.J. Effects of tannic acid on growth performance, carcass characteristics, digestibility, nitrogen volatilization, and meat lipid oxidation of steers fed steam-flaked corn-based finishing diets. J. Anim. Sci. 2017, 95, 5124–5136. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- FASS. Federation of Animal Science Societies, and Consortium for Developing a Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. In Guide for the Care and Use of Agricultural Animals in Research and Teaching, 3rd ed.; FASS—Federation of Animal Science Societies: Champaign, IL, USA, 2010. [Google Scholar]
- Krusinski, L.; Maciel, I.C.F.; van Vliet, S.; Ahsin, M.; Lu, G.; Rowntree, J.E.; Fenton, J.I. Measuring the phytochemical richness of meat: Effects of grass/grain finishing systems and grapeseed extract supplementation on the fatty acid and phytochemical content of beef. Foods 2023, 12, 3547. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. R Package; Version 1.1.4; dplyr: A Grammar of Data Manipulation; RStudio: Boston, MA, USA, 2023. [Google Scholar]
- Wickham, H.; Henry, L. R Package; Version 1.0.2; purr: Functional Programming Tools; RStudio: Boston, MA, USA, 2023. [Google Scholar]
- Wickham, H. R Package; Version 1.0.0; forcats: Tools for Working with Categorical Variables (Factors); RStudio: Boston, MA, USA, 2023. [Google Scholar]
- Wickham, H.; Vaughan, D.; Girlich, M. R Package; Version 1.3.1; tidyr: Tidy Messy Data; RStudio: Boston, MA, USA, 2023. [Google Scholar]
- Fox, J.; Weisberg, S.; Price, B.; Adler, D.; Bates, D.; Buad-Bovy, G.; Bolker, B.; Ellison, S.; Firth, D.; Friendly, M.; et al. R Package; Version 2023; car: Companion to Applied Regression; RStudio: Boston, MA, USA, 2023. [Google Scholar]
- Kassambra, A. R Package; rstatix: Pipe-Friendly Framework for Basic Statistical Tests; RStudio: Boston, MA, USA, 2021. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Christensen, R. R Package; Version 2023; ordinal—Regression Models for Ordinal Data; RStudio: Boston, MA, USA, 2023. [Google Scholar]
- Herve, M. R Package; Version 2023; Package ‘RVAideMemoire’: Testing and plotting procedures for biostatistics; RStudio: Boston, MA, USA, 2023. [Google Scholar]
- Lenth, R.V.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H. R Package; Version 2023; emmeans: Estimated Marginal Means, aka Least-Squares Means; RStudio: Boston, MA, USA, 2023. [Google Scholar]
- Wickham, H. R Package; ggplot2: Elegant graphics for data analysis; RStudio: Boston, MA, USA, 2016. [Google Scholar]
- Fitri, A.; Yanza, Y.R.; Jayanegara, A.; Ridwan, R.; Astuti, W.D.; Sarwono, K.A.; Fidriyanto, R.; Rohmatussolihat, R.; Widyastuti, Y.; Obitsu, T. Divergence effects between dietary Acacia and Quebracho tannin extracts in nutrient utilization, performance, and methane emission of ruminants: A meta-analysis. Anim. Sci. J. 2022, 93, e13765. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, M. Tannins: Their adverse role in ruminant nutrition. J. Agric. Food Chem. 1984, 32, 447–453. [Google Scholar] [CrossRef]
- Koenig, K.M.; Beauchemin, K.A. Effect of feeding condensed tannins in high protein finishing diets containing corn distiller grains on ruminal fermentation, nutrient digestibility, and route of nitrogen excretion in beef cattle. J. Anim. Sci. 2018, 96, 4398–4413. [Google Scholar] [CrossRef]
- Ferracini, J.G.; Lelis, A.L.J.; Polli, D.; Gasparim, M.B.; Feba, L.T.; Prado, I.N.D.; Millen, D.D. Feedlot performance of Nellore bulls fed high-concentrate diets containing the association of tannins and saponins with sodium monensin. R. Bras. Zootec. 2024, 53, e20230104. [Google Scholar] [CrossRef]
- Owens, F.N.; Secrist, D.S.; Hill, W.J.; Gill, D.R. Acidosis in cattle: A review. J. Anim. Sci. 1998, 76, 275–286. [Google Scholar] [CrossRef]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martinez, G.D.; Miranda-Romero, L.A.; Hernandez-Garcia, P.A. Effects of dietary tannins supplementation on growth performance, rumen fermentation, and enteric methane emissions in beef cattle: A meta-analysis. Sustainability 2021, 13, 7410. [Google Scholar] [CrossRef]
- Vilhelmova-Ilieva, N.; Galabov, A.S.; Mileva, M. Tannins as Antiviral Agents. In Tannins—Structural Properties, Biological Properties and Current Knowledge; Aires, A., Ed.; IntechOpen: London, UK, 2020; Volume 1. [Google Scholar]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Kroon, P.A.; Clifford, M.N.; Crozier, A.; Day, A.J.; Donovan, J.L.; Manach, C.; Williamson, G. How should we assess the effects of exposure to dietary polyphenols in vitro? Am. J. Clin. Nutr. 2004, 80, 15–21. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon, and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Motilya, M.J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef]
- Xu, Z.; Meenu, M.; Chen, P.; Xu, B. Comparative study on phytochemical profiles and antioxidant capacities of chestnuts produced in different geographic area in China. Antioxidants 2020, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Marín-Martinez, R.; Veloz-García, R.; Veloz-Rodríguez, R.; Guzmán-Maldonado, S.H.; Loarca-Pina, G.; Cardador-Martinez, A.; Guevara-Olvera, L.; Miranda-López, R.; Torres-Pacheco, I.; Pérez, C.P.; et al. Antimutagenic and antioxidant activities of quebracho phenolics (Schinopsis balansae) recovered from tannery wastewaters. Bioresour. Technol. 2009, 100, 434–439. [Google Scholar] [CrossRef]
- Echegaray, N.; Gomez, B.; Barba, F.J.; Franco, D.; Estévez, M.; Caraballo, J.; Marszalek, K.; Lorenzo, J.M. Chestnuts and by-products as source of natural antioxidants in meat and meat products: A review. Trends Food Sci. Technol. 2018, 82, 110–121. [Google Scholar] [CrossRef]
- Liu, X.L.; Hao, Y.W.; Jin, L.; Xu, Z.J.; McAllister, T.A.; Wang, Y. Anti-Escherichia coli O157:H7 properties of purple prairie clover and sainfoin condensed tannins. Molecules 2013, 18, 2183–2199. [Google Scholar] [CrossRef]
- Min, B.R.; Pinchak, W.E.; Merkel, R.; Walker, S.; Tomita, G.; Anderson, R.C. Comparative antimicrobial activity of tannin extracts from perennial plants on mastitis pathogens. Sci. Res. Essays 2008, 3, 66–73. [Google Scholar]
- Chung, K.T.; Lu, Z.; Chou, M.W. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem. Toxicol. 1998, 36, 1053–1060. [Google Scholar] [CrossRef]
- Rajamanickam, K.; Yang, J.; Sakharkar, M.K. Phytochemicals as alternatives to antibiotics against major pathogens involved in bovine respiratory disease (BRD) and bovine mastitis (BM). Bioinformation 2019, 15, 32–35. [Google Scholar] [CrossRef]
- McGill, J.L.; Sacco, R.E. The immunology of bovine respiratory disease: Recent advancements. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 333–348. [Google Scholar] [CrossRef]
- Di Pasquale, G.L.; Ostedgaard, L.; Vermeer, D.; Swaim, W.D.; Karp, P.; Chiorini, J.A. Bovine AAV transcytosis inhibition by tannic acid results in functional expression of CTFR in vitro and altered biodistribution in vivo. Gene Ther. 2012, 19, 576–581. [Google Scholar] [CrossRef]
- Sgoifo Rossi, C.A.; Grossi, S.; Compiani, R.; Baldi, G. Effect of a blend of essential oils, bioflavonoids, and tannins on production performance, health, immune functionality, and antioxidant status in fattening beef cattle. Large Anim. Rev. 2023, 29, 163–170. [Google Scholar]
- Heuer, C.; Schukken, Y.H.; Jonker, L.J.; Wilkinson, J.I.; Noorhuizen, J.P. Effect of monensin on blood ketone bodies, incidence, and recurrence of disease and fertility in dairy cows. J. Dairy Sci. 2001, 84, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Duffield, T.F.; Bagg, R.; DesCoteaux, L.; Bouchard, E.; Brodeur, M.; DuTremblay, D.; Keefe, G.; LeBlanc, S.; Dick, P. Prepartum monensin for the reduction of energy associated disease in postpartum dairy cows. J. Dairy Sci. 2002, 85, 397–405. [Google Scholar] [CrossRef]
- Duffield, T.F.; Leslie, K.E.; Sandals, D.; Lissemore, K.; McBride, B.W.; Lumsden, J.H.; Dick, P.; Bagg, R. Effect of monensin-controlled release capsule on cow health and reproductive performance. J. Dairy Sci. 1999, 82, 2377–2384. [Google Scholar] [CrossRef]
- Beckett, S.; Lean, I.; Dyson, R.; Tranter, W.; Wade, L. Effects of monensin on the reproduction, health, and milk production of dairy cows. J. Dairy Sci. 1998, 81, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Majak, W.; McAllister, T.A. Frothy bloat in ruminants: Cause, occurrence, and mitigation strategies. Anim. Feed Sci. Technol. 2012, 172, 103–114. [Google Scholar] [CrossRef]
- Li, Y.; Tanner, G.; Larkin, P. The DMACA-HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- Waghorn, G.C.; McNabb, W.C. Consequences of plant phenolic compounds for productivity and health of ruminants. Proc. Nurt. Soc. 2003, 62, 383–392. [Google Scholar] [CrossRef] [PubMed]
- McMahon, L.R.; McAllister, T.A.; Berg, B.P.; Majak, W.; Acharya, S.N.; Popp, J.D.; Coulman, B.E.; Wang, Y.; Cheng, K.J. A review of the effects of forage condensed tannins on ruminal fermentation and bloat in grazing cattle. Can. J. Plant Sci. 2000, 80, 469–485. [Google Scholar] [CrossRef]
- Gadberry, S.; Lalman, D.; White, F.; Linneen, S.; Beck, P. Meta-analysis of the effects of monensin on growth and bloat of cattle on pasture. Transl. Anim. Sci. 2022, 6, txac031. [Google Scholar] [CrossRef]
- Nagaraja, T.G. Ionophores and antibiotics in ruminants. In Biotechnology in Animal Feeds and Feeding, 1st ed.; Wallace, R.J., Chesson, A., Eds.; VCH Publishers Inc.: New York, NY, USA, 1995; pp. 173–204. [Google Scholar]
- Bergen, W.G.; Bates, D.B. Ionophores: Their effect on production efficiency and mode of action. J. Anim. Sci. 1984, 58, 1465–1483. [Google Scholar] [CrossRef]
- Min, B.R.; Hart, S.P.; Miller, D.; Tomita, G.M.; Loetz, E.; Sahlu, T. The effects of grazing forage containing condensed tannins on gastro-intestinal parasite infection and milk composition in Angora does. Vet. Parasitol. 2005, 130, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Butter, N.L.; Dawson, J.M.; Wakelin, D.; Buttery, P.J. Effect of dietary condensed tannins on gastrointestinal nematodes. J. Agric. Sci. 2001, 137, 461–469. [Google Scholar] [CrossRef]
- Parisi, F.; Mancini, S.; Mazzei, M.; Forzan, M.; Turchi, B.; Perrucci, S.; Poli, A.; Paci, G. Effect of dietary supplementation of a mix of chestnut and quebracho tannins on intestinal morphology, bacterial load, Eimeria spp. oocyst excretion, and immune response after vaccination in rabbits. Am. J. Anim. Vet. Sci. 2018, 13, 94–103. [Google Scholar] [CrossRef]
- Camacho, A.; Cervantes, B.J.; Espino, M.A.; Verdugo, M.; Flores, L.R.; Romo, J.A.; Barajas, R. W295 Influence of addition to tannins-extract in low concentration of dietary dry matter on carcass characteristics of bull-calves. J. Anim. Sci. 2011, 89 (Suppl. S1), 615. [Google Scholar]
- Barajas, R.; Cervantes, B.J.; Espino, M.A.; Camacho, A.; Verdugo, M.; Flores, L.R.; Romo, J.A. Interaction of tannin extract and zilpaterol hydrochloride supplementation on feedlot performance of bulls. J. Anim. Sci. 2013, 91 (Suppl. S2), 8. [Google Scholar]
- Manella, M.Q.; Campanini, A.; Boin, C.; Paula, E.; Arnandes, R.B.; Lopes, V. Use of a commercial blend of tannins as feed additive for feed lot cattle on large pen trial improves carcass weight and carcass feed conversion. J. Anim. Sci. 2024, 102 (Suppl. S3), 778–779. [Google Scholar] [CrossRef]
| Finishing Diet | |
|---|---|
| Formulated DM inclusion, % | |
| Steam-flaked corn | 59.43 |
| Roughage blend | 12.96 |
| Dried distillers grain | 8.14 |
| Bakery waste | 11.40 |
| Fat blend | 4.26 |
| Protein supplement | 1.43 |
| Mineral supplement 1 | 2.37 |
| Chemical analysis | |
| Dry matter, % | 82.90 |
| Crude protein, % | 13.31 |
| Acid detergent fiber (ADF), % | 7.92 |
| Neutral detergent fiber (NDF), % | 17.29 |
| Physically effective NDF, % | 8.28 |
| Crude fat, % | 8.65 |
| NEM, Mcal/kg | 2.34 |
| NEG, Mcal/kg | 1.47 |
- 1 Supplement was formulated to include 25.842% calcium, 15.000% salt, and 1.078% magnesium.
| Treatment | ||||
|---|---|---|---|---|
| MON | SBPX | SEM 1 | p-Value 2 | |
| Pens, n | 10 | 10 | ||
| Days on trial | 341 | 341 | 2.7 | 0.99 |
| Total days on feed | 357 | 358 | 2.0 | 0.19 |
| Initial SBW, kg | 140 y | 143 x | 3.6 | 0.06 |
| Final SBW, kg | 569 | 570 | 2.7 | 0.82 |
| DMI, kg/day | 7.34 b | 7.62 a | 0.060 | <0.01 |
| ADG, kg | 1.26 | 1.25 | 0.006 | 0.71 |
| Gain:Feed | 0.17 a | 0.16 b | 0.001 | <0.01 |
| Daily cost 3, USD | 3.97 b | 4.11 a | 0.049 | <0.01 |
| Daily per head DM deliveries CV, % | 20.95 a | 19.75 b | 0.858 | <0.01 |
| Natural program fallouts 4, % | 8.12 | 6.09 | 1.371 | 0.13 |
| Shipped for natural program 5, % | 89.57 | 91.13 | 1.619 | 0.31 |
- 1 The largest standard error of the mean.
- 2 Tested as the ANOVA significance of the main effect.
- 3 Daily cost references are cost-specific to feed and other daily production inputs but do not include costs associated with processing or health treatment.
- 4 Cattle that required antibiotic health treatment that nullified their eligibility to be harvested in the intended natural program.
- 5 Cattle that remained eligible to be harvested in the intended natural program and that were likewise eligible for associated premiums.
- a, b Means without a common superscript are different by pairwise comparison (p ≤ 0.05).
- x, y Means without a common superscript are different by pairwise comparison (p ≤ 0.10).
| Treatment | ||||
|---|---|---|---|---|
| MON | SBPX | SEM 1 | p-Value 2 | |
| Pens, n | 10 | 10 | ||
| Head per pen | 72 | 73 | ||
| Total Morbidity, % | 25.49 | 26.03 | 2.987 | 0.82 |
| First Treatment Protocol, % | 22.77 | 23.79 | 2.508 | 0.65 |
| First Pull, % | 15.49 | 17.62 | 1.760 | 0.27 |
| Second Pull, % | 4.94 | 4.96 | 1.043 | 0.98 |
| Third Pull, % | 1.97 x | 0.96 y | 0.643 | 0.10 |
| Second Treatment Protocol, % | 1.79 | 1.63 | 0.581 | 0.80 |
| Third Treatment Protocol, % | 0.79 | 0.55 | 0.393 | 0.54 |
| Treatment cost, USD/hd | 10.08 | 8.50 | 1.660 | 0.35 |
| Bullers, % | 5.91 | 5.75 | 1.141 | 0.90 |
| Railers, % | 0.70 | 0.69 | 0.313 | 0.97 |
| Total Mortality, % | 1.54 | 2.11 | 0.612 | 0.41 |
- 1 The largest standard error of the mean.
- 2 Tested as the ANOVA significance of the main effect.
- x, y Means without a common superscript are different by pairwise comparison (p ≤ 0.10).
| Treatment | ||||
|---|---|---|---|---|
| MON | SBPX | SEM 1 | p-Value 2 | |
| Hot carcass weight, kg | 362.3 | 363.9 | 2.08 | 0.53 |
| 12th rib fat, cm. | 0.95 | 0.95 | 0.025 | 0.90 |
| Ribeye area, sq. cm. | 74.94 | 74.39 | 0.643 | 0.44 |
| Quality grade distribution 3 | 0.22 | |||
| Prime, % | 13.25 | 10.94 | 1.346 | 0.20 |
| Choice, % | 84.23 | 86.32 | 1.448 | 0.29 |
| Select, % | 1.42 | 2.02 | 0.701 | 0.37 |
| Standard, % | 0.79 | 0.30 | 0.351 | 0.25 |
| Yield grade distribution 3 | 0.79 | |||
| USDA YG 1, % | 2.20 | 2.42 | 0.599 | 0.79 |
| USDA YG 2, % | 8.07 | 6.44 | 1.797 | 0.23 |
| USDA YG 3, % | 88.56 | 90.24 | 1.930 | 0.31 |
| USDA YG 4, % | 0.25 | 0.24 | 0.252 | 0.97 |
| USDA YG 5, % | 0.32 | 0.00 | 0.222 | 0.99 |
| Total liver abscess, % | 10.08 | 12.27 | 1.277 | 0.21 |
- 1 The largest standard error of the mean.
- 2 Tested as the ANOVA significance of the main effect.
- 3 Tested using ordinal logistic regression.
| Treatment | |||||
|---|---|---|---|---|---|
| Polyphenol 1 | MON | SBPX | SEM 2 | p-Value 3 | |
| Alkaloids | Hordenine | 75.75 | 25.48 | 32.42 | 0.05 |
| Flavonoids | Epicatechin gallate | 0.001 | 0.04 | 0.03 | 0.25 |
| Epigallocatechin gallate | <LOQ | 0.04 | 0.01 | <0.01 | |
| Apigenin | 0.71 | 1.53 | 1.30 | 0.04 | |
| Chrysoeriol | 0.02 | 0.34 | 0.25 | 0.02 | |
| Diosmetin | 0.05 | 0.38 | 0.26 | 0.06 | |
| Apiin | 0.63 | 1.78 | 0.88 | 0.52 | |
| Isovitexin | 0.07 | 0.42 | 0.28 | 0.16 | |
| Linarin | 0.20 | 1.25 | 0.84 | 0.10 | |
| Orientin | <LOQ | 0.32 | 0.23 | <0.01 | |
| Schaftoside | 0.84 | 4.15 | 2.90 | 0.16 | |
| Sophoricoside | 0.13 | 0.72 | 0.06 | <0.01 | |
| Hyperoside | 1.99 | 2.2 | 0.07 | 0.10 | |
| Narcissoside | 0.12 | 0.82 | 0.56 | 0.43 | |
| Rutin | 0.06 | 1.48 | 0.95 | 0.09 | |
| Isorhamnetin | 21.74 | 2.81 | 13.22 | 0.17 | |
| Kaempferide | <LOQ | 0.21 | 0.17 | <0.01 | |
| Phenolic acids | Gallic acid | 0.01 | 0.09 | 0.04 | 0.57 |
| 2,6-Dihydroxybenzoic acid | <LOQ | 0.06 | 0.03 | <0.01 | |
| 3,4-Dihydroxybenzoic acid | 0.05 | 0.04 | 0.02 | 0.04 | |
| 4-Hydroxybenzoic acid | 2.33 | 2.91 | 0.47 | 0.65 | |
| Syringic acid | 0.03 | 0.04 | 0.02 | 0.52 | |
| Vanillic acid | 0.14 | 0.28 | 0.11 | 0.42 | |
| Caffeic acid | 0.08 | 0.49 | 0.00 | <0.01 | |
| Chlorogenic acid | 0.01 | 0.17 | 0.09 | <0.01 | |
| p-Coumaric acid | 0.14 | 0.62 | 0.19 | 0.01 | |
| trans Ferulic acid | 0.003 | 0.15 | 0.09 | 0.11 | |
| Salicylic acid | 0.14 | 0.29 | 0.05 | 0.03 | |
| 4-Ethylphenol | 5.37 | 10.26 | 4.08 | 0.02 | |
| Gut-derived phenolic metabolites | Catechol sulfate | 0.83 | 0.62 | 0.14 | 0.39 |
| p-Cresol sulfate | 51.37 | 42.02 | 9.04 | 0.63 | |
| Hippuric acid | 29.73 | 22.50 | 6.66 | 0.52 | |
| Dimethyl Sulfone | 3.72 | 2.40 | 0.77 | 0.33 | |
| Others | Ectoine | 1.94 | 0.09 | 1.60 | 0.06 |
| Ergothioneine | 3.08 | 5.87 | 1.45 | 0.28 | |
| Hercynine | 29.63 | 15.35 | 12.17 | 0.65 | |
| Hypaphorine | 1.62 | 4.78 | 1.23 | 0.04 | |
| Isocitric lactone | 6.10 | 6.88 | 1.44 | 0.80 | |
| Imperatorin | 0.11 | 0.80 | 0.65 | 0.06 | |
| Morin | 1.83 | 0.07 | 1.49 | 0.63 | |
| Trigonelline | 3.56 | 2.31 | 1.31 | 0.44 | |
| Tyrosol | 0.08 | 0.15 | 0.03 | 0.04 | |
- 1 Information about individual metabolites can be sourced from the Human Metabolome Database (HMDB).
- 2 The largest standard error of the mean.
- 3 Tested as the ANOVA significance of the main effect. <LOQ: below the lowest level of quantification.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowman-Schnug, S.M.; Fuerniss, L.K.; Cameron, J.D.; Beckett, J.L.; Ahsin, M.; van Vliet, S.; Hufstedler, G.D.; Johnson, B.J. Replacement of Monensin with a Proprietary Tannin-Blend Additive in Calf-Fed Holstein Steer Diets. Vet. Sci. 2025, 12, 166. https://doi.org/10.3390/vetsci12020166
Bowman-Schnug SM, Fuerniss LK, Cameron JD, Beckett JL, Ahsin M, van Vliet S, Hufstedler GD, Johnson BJ. Replacement of Monensin with a Proprietary Tannin-Blend Additive in Calf-Fed Holstein Steer Diets. Veterinary Sciences. 2025; 12(2):166. https://doi.org/10.3390/vetsci12020166
Chicago/Turabian StyleBowman-Schnug, Sydney M., Luke K. Fuerniss, Joe D. Cameron, Jonathon L. Beckett, Muhammad Ahsin, Stephan van Vliet, Guy D. Hufstedler, and Bradley J. Johnson. 2025. "Replacement of Monensin with a Proprietary Tannin-Blend Additive in Calf-Fed Holstein Steer Diets" Veterinary Sciences 12, no. 2: 166. https://doi.org/10.3390/vetsci12020166
APA StyleBowman-Schnug, S. M., Fuerniss, L. K., Cameron, J. D., Beckett, J. L., Ahsin, M., van Vliet, S., Hufstedler, G. D., & Johnson, B. J. (2025). Replacement of Monensin with a Proprietary Tannin-Blend Additive in Calf-Fed Holstein Steer Diets. Veterinary Sciences, 12(2), 166. https://doi.org/10.3390/vetsci12020166







