Effects of Supplementing Rumen-Protected Glutathione on Lactation Performance, Nutrients, Oxidative Stress, Inflammation, and Health in Dairy Cows During the Transition Period
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Feeding Management
2.4. Sample Collection and Processing
2.5. Data Collection
2.6. Statistical Analysis
3. Results
3.1. DMI, Milk Yield, and Milk Composition
3.2. Effects of RPGSH Supplementation on Serum Metabolites in Dairy Cows
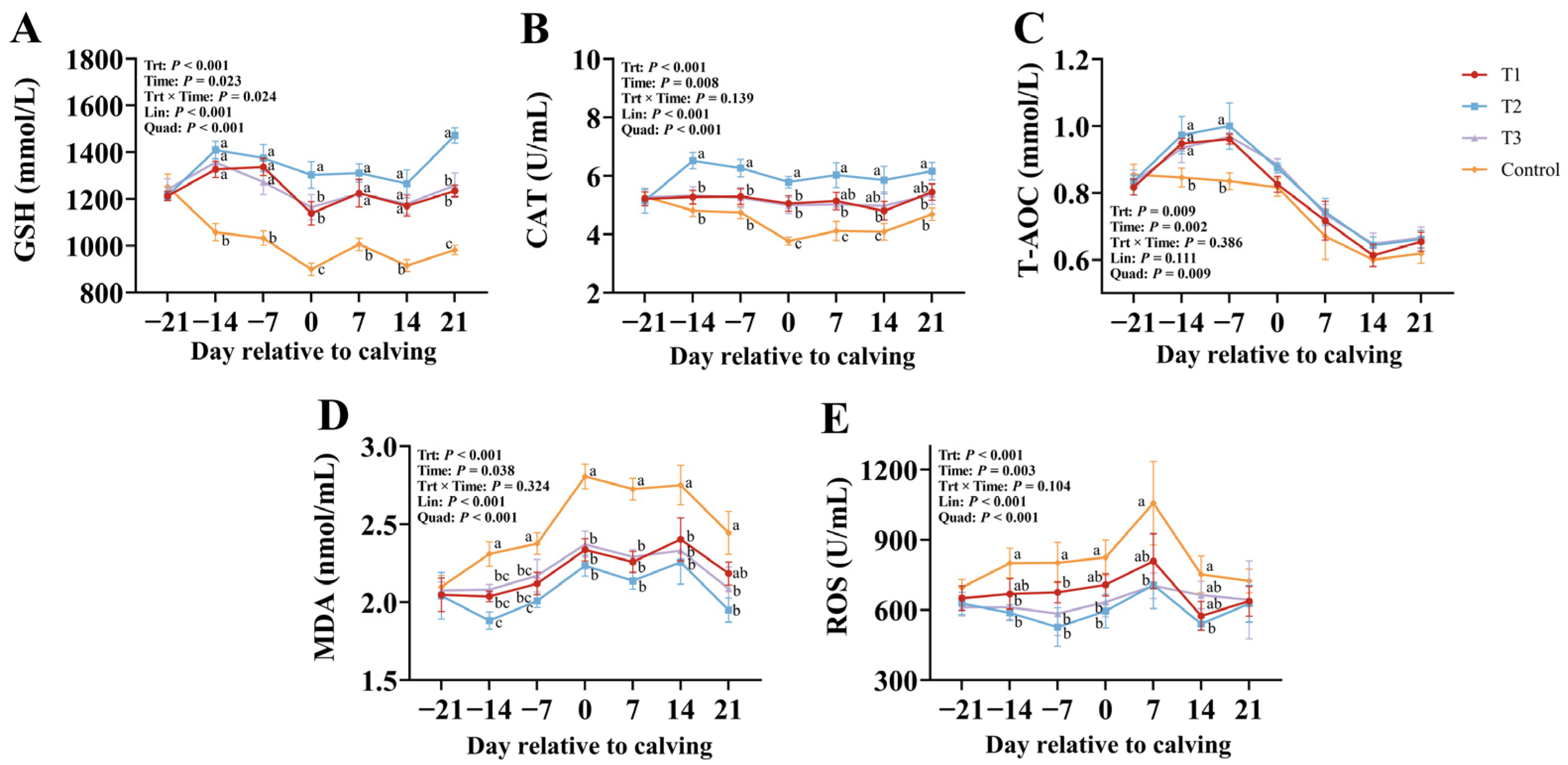
3.3. Effects of RPGSH Supplementation on Oxidative Stress Biomarkers in Dairy Cows
3.4. Effects of RPGSH Supplementation on Inflammation Biomarkers in Dairy Cows
3.5. Correlation Between Blood GSH Concentration and Postpartum Serum Indices
3.6. Incidence of Postpartum Diseases and Correlation with Blood GSH Levels in Dairy Cows
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meikle, A.; Cavestany, D.; Carriquiry, M.; Adnen, M.; Artegoitia, V.; Pereira, G.; Pessina, P.; Rama, G.; Femandez, A.; Breijo, M.; et al. Advances in knowledge of the dairy cow during the transition period in uruguay: A multidisciplinary approach. Agrocienc. Urug. 2013, 26, 141–152. [Google Scholar] [CrossRef]
- Nikkhah, A.; Alimirzaei, M. Management updates on prepartal stress effects on transition cow and calf health. World’s Vet. J. 2023, 13, 250–257. [Google Scholar] [CrossRef]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Hernández, J.; Valverde, I.; Pereira, V.; Sotillo, J.; Alonso, M.L.; Benedito, J.L. Plasma malonaldehyde (MDA) and total antioxidant status (TAS) during lactation in dairy cows. Res. Vet. Sci. 2006, 80, 133–139. [Google Scholar] [CrossRef]
- Olthof, L.A.; Domecq, J.J.; Bradford, B.J. Analysis of Jersey versus Holstein breed profitability on north central US dairies. JDS Commun. 2023, 4, 344–348. [Google Scholar] [CrossRef]
- Prodanović, R.; Nedić, S.; Vujanac, I.; Bojkovski, J.; Nedić, S.; Jovanović, L.; Kirovski, D.; Borozan, S. Dietary Supplementation of Chestnut Tannins in Prepartum Dairy Cows Improves Antioxidant Defense Mechanisms Interacting with Thyroid Status. Metabolites 2023, 13, 334. [Google Scholar] [CrossRef]
- Gu, F.F.; Jiang, L.; Wang, D.M.; Zhao, F.Q.; Liu, J.X. Supplementation with N-carbamoylglutamate during the transition period improves the function of neutrophils and reduces inflammation and oxidative stress in dairy cows. J. Dairy Sci. 2022, 105, 5786–5795. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Sciuto, A. Antioxidant Properties of Glutathione and Its Role in Tissue Protection, 1st ed.; Taylor & Francis: London, UK, 1997. [Google Scholar]
- Jain, S.K.; Parsanathan, R.; Achari, A.E.; Kanikarla-Marie, P.; Bocchini, J.A., Jr. Glutathione stimulates vitamin D regulatory and glucose-metabolism genes, lowers oxidative stress and inflammation, and increases 25-Hydroxy-vitamin D levels in blood: A novel approach to treat 25-Hydroxyvitamin D deficiency. Antioxid. Redox Signal. 2018, 29, 1792–1807. [Google Scholar] [CrossRef]
- Radu, C.; Feștilă, I.; Cocan, D.; Cosier, V. Biomarkers of oxidative stress during the transition period in romanian dairy cows breeds. Proenvironment 2015, 8, 84–89. [Google Scholar]
- Preynat, A.; Lapierre, H.; Thivierge, M.C.; Palin, M.F.; Matte, J.J.; Desrochers, A.; Girard, C.L. Effects of supplements of folic acid, vitamin B12, and rumen-protected methionine on whole body metabolism of methionine and glucose in lactating dairy cows. J. Dairy Sci. 2009, 92, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Abeyta, M.A.; Al-Qaisi, M.; Horst, E.A.; Mayorga, E.J.; Rodriguez-Jimenez, S.; Goetz, B.M.; Carta, S.; Tucker, H.; Baumgard, L.H. Effects of dietary antioxidant supplementation on metabolism and inflammatory biomarkers in heat-stressed dairy cows. J. Dairy Sci. 2023, 106, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.N.; Alharthi, A.S.; Liang, Y.; Lopes, M.G.; Lopreiato, V.; Vailati-Riboni, M.; Loor, J.J. Multifaceted role of one-carbon metabolism on immunometabolic control and growth during pregnancy, lactation and the neonatal period in dairy cattle. J. Anim. Sci. Biotechnol. 2021, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J. Physiological adaptations in transition dairy cows. Agric. Food Sci. 2004, 87, 74–84. [Google Scholar]
- Keshri, A.; Bashir, Z.; Kumari, V.; Prasad, K.; Joysowal, M.; Singh, M.; Singh, D.; Tarun, A.; Shukla, S. Role of micronutrients during peri-parturient period of dairy animals—A review. Biol. Rhythm Res. 2019, 52, 1018–1030. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef]
- Spears, J.W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC International: Gaithersburg, MD, USA, 1990. [Google Scholar]
- Soest, P.J.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- GB/T 6436-2018; Determination of Calcium in Feeds. Standards Press of China: Beijing, China, 2018.
- GB/T 6437-2018; Determination of Phosphorus in Feeds-Spectrophotometry. Standards Press of China: Beijing, China, 2018.
- GB/T 20194-2018; Determination of Starch in Feeds-Spectrophotometry. Standards Press of China: Beijing, China, 2018.
- NRC (National Research Council). Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- NY/T 34-2004; China National Feeding Standard of Dairy Cattle. China Agricultural Press: Beijing, China, 2004.
- Tyrrell, H.F.; Reid, J.T. Prediction of the energy value of cow’s milk. J. Dairy Sci. 1965, 48, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Vanbaale, M.J.; Ledwith, D.R.; Thompson, J.M.; Burgos, R.; Collier, R.J.; Baumgard, L.H. Effect of increased milking frequency in early lactation with or without recombinant bovine somatotropin. J. Dairy Sci. 2005, 88, 3905–3912. [Google Scholar] [CrossRef] [PubMed]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A body condition scoring chart for Holstein dairy cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Macmillan, K.; López Helguera, I.; Behrouzi, A.; Gobikrushanth, M.; Hoff, B.; Colazo, M.G. Accuracy of a cow-side test for the diagnosis of hyperketonemia and hypoglycemia in lactating dairy cows. Res. Vet. Sci. 2017, 115, 327–331. [Google Scholar] [CrossRef]
- Kasai, S.; Prasad, A.; Kumagai, R.; Takanohashi, K. Scanning electrochemical microscopy-somatic cell count as a method for diagnosis of bovine mastitis. Biology 2022, 11, 549. [Google Scholar] [CrossRef]
- Lomb, J.; Weary, D.M.; Mills, K.E.; von Keyserlingk, M.A.G. Effects of metritis on stall use and social behavior at the lying stall. J. Dairy Sci. 2018, 101, 7471–7479. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Liu, J.; Liu, J.X.; Ferguson, J.D. Effects of rumen-protected γ-aminobutyric acid on feed intake, lactation performance, and antioxidative status in early lactating dairy cows. J. Dairy Sci. 2013, 96, 3222–3267. [Google Scholar] [CrossRef]
- Santos, N.W.; Santos, G.T.; Silva-Kazama, D.C.; Grande, P.A.; Pintro, P.T.; Marchi, F.E.; Jobim, C.C.; Petit, H.V. Production, composition and antioxidants in milk of dairy cows fed diets containing soybean oil and grape residue silage. Livest. Sci. 2014, 159, 37–45. [Google Scholar] [CrossRef]
- Sun, F.; Cao, Y.; Cai, C.; Li, S.; Yu, C.; Yao, J. Regulation of nutritional metabolism in transition dairy cows: Energy homeostasis and health in response to post-ruminal choline and methionine. PLoS ONE 2016, 11, e0160659. [Google Scholar] [CrossRef]
- Deniz, A.; Aksoy, K.; Metin, M. Transition period and subclinical ketosis in dairy cattle: Association with milk production, metabolic and reproductive disorders and economic aspects. Med. Weter. 2020, 76, 495–502. [Google Scholar] [CrossRef]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Tankersley, N.S.; DePeters, E.J.; Graham, T.W. Case study: Effects of water, fresh cow YMCP plus, and rumen fluid transfaunate supplementation following calving on milk yield, reproductive efficiency, and incidence of common health disorders in holstein cows. Prof. Anim. Sci. 2007, 23, 513–520. [Google Scholar] [CrossRef]
- Musco, N.R.; Tudisco, M.; Grossi, V. Effect of a high forage: Concentrate ratio on milk yield, blood parameters and oxidative status in lactating cows. Anim. Prod. Sci. 2020, 60, 1531–1538. [Google Scholar] [CrossRef]
- Fabjanowska, J.; Klebaniuk, R.; Kowalczuk-Vasilev, E.; Samolińska, W.; Moczulska, M.; Kiczorowska, B.; Milewski, S. Effect of supplementation of dairy cows with herbal mixtures on their performance and selected blood indices. In Proceedings of the 2nd International PhD Student’s Conference at the University of Life Sciences in Lublin, Poland: Environment-Plant-Animal-Product, Lublin, Poland, 19–20 April 2023. [Google Scholar]
- Marques, L.T.; Fischer, V.; Zanela, M.B.; Junior, W.S. Suplementação de vacas holandesas em estádio avançado de lactação. Cienc. Rural 2010, 40, 1392–1398. [Google Scholar] [CrossRef]
- Plemyashov, K.V.; Korochkina, E.; Nikitin, V.V. The efficacy of vitamin-mineral supplementation using to dairy cows in the transition period. Vet. Med. J. 2022, 25, 38–41. [Google Scholar] [CrossRef]
- Wanapat, M.; Foiklang, S.; Sukjai, S.; Tamkhonburi, P.; Gunun, N.; Gunun, P.; Phesatcha, K.; Norrapoke, T.; Kang, S. Feeding tropical dairy cattle with local protein and energy sources for sustainable production. J. Appl. Anim. Res. 2018, 46, 232–236. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2014, 99, 1003–1016. [Google Scholar] [CrossRef]
- Rafa, H.; Nan, M.I.; Andrei, S. The impact of oxidative stress on reproductive disorders in cows—A review. Sci. Pap. J. Vet. Ser. 2023, 66, 5–11. [Google Scholar] [CrossRef]
- Youssef, M.; El-Ashker, M. Significance of insulin resistance and oxidative stress in dairy cattle with subclinical ketosis during the transition period. Trop. Anim. Health Prod. 2016, 49, 239–244. [Google Scholar] [CrossRef]
- Khatti, A.; Mehrotra, S.; Patel, P.K.; Singh, G.; Maurya, V.P.; Mahla, A.S.; Chaudhari, R.K.; Das, G.K.; Singh, M.; Sarkar, M.; et al. Supplementation of vitamin E, selenium and increased energy allowance mitigates the transition stress and improves postpartum reproductive performance in the crossbred cow. Theriogenology 2017, 104, 142–148. [Google Scholar] [CrossRef]
- Wisnieski, L.; Norby, B.; Pierce, S.J.; Becker, T.; Gandy, J.C.; Sordillo, L.M. Predictive models for early lactation diseases in transition dairy cattle at dry-off. Prev. Vet. Med. 2019, 163, 68–78. [Google Scholar] [CrossRef]

| Item | Prepartum | Postpartum |
|---|---|---|
| Ingredients (% of DM) | ||
| Soybean meal | 2.66 | 4.31 |
| DDGS | 3.72 | - |
| Alfalfa (first-cut hay) | 2.21 | 5.74 |
| Sugar beet pulp | 8.86 | 2.87 |
| Corn gluten meal | 4.43 | 1.72 |
| Oat grass | 11.52 | 2.30 |
| Corn | 8.86 | 17.51 |
| Silage | 39.86 | 34.45 |
| CaCO3 | 0.89 | - |
| H2O | 13.29 | 11.48 |
| Na2CO3 | - | 0.71 |
| CaHPO4 | - | 0.29 |
| Fat powder | - | 0.86 |
| Rumen-protected glucose | - | 1.44 |
| Cottonseed | - | 2.87 |
| Urea | - | 0.52 |
| Soybean husk | - | 7.18 |
| Molasses | - | 2.87 |
| Premix 1 | 3.70 | 2.88 |
| Nutrient level, % of DM | ||
| Crude protein | 15.7 | 16.7 |
| Starch | 16.8 | 21.5 |
| NEL 2, MCal/kg | 1.52 | 1.76 |
| NDF | 33.7 | 30.7 |
| Calcium | 1.00 | 1.00 |
| Phosphorus | 0.40 | 0.40 |
| Item 1 | Treatment 2 | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | Control | Trt | Time | Trt × Time | Lin | Quad | ||
| Milk yield, kg/d | 31.28 | 33.00 | 31.09 | 30.37 | 0.946 | 0.495 | <0.001 | 0.980 | 0.428 | 0.185 |
| DMI 3, kg/d | 16.17 | 16.45 | 16.34 | 16.15 | 0.170 | 0.798 | <0.001 | 0.711 | 0.833 | 0.315 |
| Fat, % | 3.83 | 4.06 | 3.83 | 3.74 | 0.053 | 0.699 | 0.164 | 0.840 | 0.600 | 0.412 |
| Protein, % | 3.43 | 3.42 | 3.38 | 3.45 | 0.051 | 0.943 | 0.028 | 0.704 | 0.995 | 0.651 |
| Lactose, % | 4.81 | 4.81 | 4.84 | 4.80 | 0.011 | 0.597 | <0.001 | 0.217 | 0.734 | 0.524 |
| SCC, cells/μL | 121.93 | 101.68 a | 116.70 | 138.25 b | 8.07 | 0.140 | 0.083 | 0.964 | 0.217 | 0.049 |
| UN, mg/dL | 13.41 | 13.80 | 13.49 | 14.21 | 0.389 | 0.845 | <0.001 | 0.851 | 0.482 | 0.849 |
| TS, % | 13.51 | 13.26 | 13.29 | 13.14 | 0.075 | 0.570 | <0.001 | 0.412 | 0.612 | 0.236 |
| ECM, kg/d | 32.58 | 35.62 | 32.30 | 30.76 | 1.14 | 0.307 | 0.005 | 0.975 | 0.234 | 0.051 |
| FCM, kg/d | 35.54 | 39.12 a | 35.43 | 33.06 b | 1.17 | 0.235 | 0.008 | 0.907 | 0.167 | 0.032 |
| Feed efficiency 4 | 1.92 | 2.06 a | 1.89 | 1.76 b | 0.075 | 0.306 | 0.003 | 0.984 | 0.169 | 0.044 |
| Item | Time (d) | Treatment 1 | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | Control | Trt | Time | Trt × Time | Lin | Quad | |||
| Calcium, mmol/L | −21 | 2.21 | 2.14 | 2.23 | 2.28 | 0.013 | 0.427 | 0.150 | 0.504 | 0.298 | 0.937 |
| −14 | 2.27 | 2.34 | 2.30 | 2.41 | |||||||
| −7 | 2.16 | 2.26 | 2.23 | 2.34 | |||||||
| 0 | 2.25 | 2.32 | 2.27 | 2.33 | |||||||
| 7 | 2.11 | 2.30 | 2.18 | 2.23 | |||||||
| 14 | 2.20 | 2.19 | 2.13 | 2.09 | |||||||
| 21 | 2.25 | 2.21 | 2.23 | 2.17 | |||||||
| Phosphorus, mmol/L | −21 | 2.00 | 1.94 | 1.95 | 1.97 | 0.021 | 0.114 | 0.21 | 0.940 | 0.821 | 0.103 |
| −14 | 2.09 | 1.99 | 2.07 | 2.03 | |||||||
| −7 | 2.04 | 1.98 | 1.96 | 1.89 | |||||||
| 0 | 1.97 | 1.98 | 2.00 | 1.96 | |||||||
| 7 | 2.18 | 2.12 | 2.08 | 2.11 | |||||||
| 14 | 1.99 | 1.97 | 2.00 | 2.02 | |||||||
| 21 | 2.12 | 1.99 | 2.03 | 2.01 | |||||||
| BHBA, mmol/L | −21 | 0.64 | 0.49 | 0.55 | 0.73 | 0.017 | 0.169 | <0.001 | 0.366 | 0.247 | 0.108 |
| −14 | 0.64 | 0.72 | 0.78 | 0.80 | |||||||
| −7 | 0.56 | 0.61 | 0.61 | 0.65 | |||||||
| 0 | 0.69 | 0.65 | 0.66 | 0.73 | |||||||
| 7 | 0.78 | 0.64 | 0.55 | 0.62 | |||||||
| 14 | 0.93 | 0.75 a | 0.93 | 0.93 b | |||||||
| 21 | 0.82 | 0.68 a | 0.86 | 0.91 b | |||||||
| Glucose, mmol/L | −21 | 3.62 | 3.67 | 3.62 | 3.60 | 0.032 | 0.183 | <0.001 | 0.878 | 0.072 | 0.035 |
| −14 | 3.33 | 3.43 | 3.42 | 3.37 | |||||||
| −7 | 3.97 | 3.83 | 3.85 | 3.85 | |||||||
| 0 | 4.40 | 4.52 | 4.50 | 4.38 | |||||||
| 7 | 3.35 | 3.67 | 3.48 | 3.31 | |||||||
| 14 | 3.18 | 3.40 a | 3.15 | 2.97 b | |||||||
| 21 | 3.28 | 3.47 a | 3.27 | 2.85 b | |||||||
| Item 1 | Prepartum 2 | Day of Calving 3 | Postpartum 4 | |||
|---|---|---|---|---|---|---|
| R-Value | p-Value | R-Value | p-Value | R-Value | p-Value | |
| CAT | 0.820 ** | <0.001 | 0.767 ** | <0.001 | 0.712 ** | <0.001 |
| T-AOC | 0.467 ** | 0.002 | 0.348 * | 0.028 | 0.341 * | 0.015 |
| MDA | −0.773 ** | <0.001 | −0.693 ** | <0.001 | −0.829 ** | <0.001 |
| ROS | −0.473 ** | 0.002 | −0.454 ** | 0.001 | −0.462 ** | <0.001 |
| HP | −0.821 ** | <0.001 | −0.802 ** | <0.001 | −0.849 ** | <0.001 |
| COR | −0.778 ** | <0.001 | −0.801 ** | <0.001 | −0.850 ** | <0.001 |
| CRP | −0.794 ** | <0.001 | −0.704 ** | <0.001 | −0.733 ** | <0.001 |
| IL−6 | −0.838 ** | <0.001 | −0.756 ** | <0.001 | −0.859 ** | <0.001 |
| Item | Treatment 1 | |||
|---|---|---|---|---|
| T1 | T2 | T3 | Control | |
| N | 10 | 10 | 10 | 10 |
| Ketosis | 10 (1/10) | - | 10 (1/10) | 1 (1/10) |
| Mastitis | 10 (1/10) | - | 10 (1/10) | 2 (2/10) |
| Metritis | 10 (1/10) | 10 (1/10) | 10 (1/10) | 3 (3/10) |
| Disease | Prepartum 1 | Day of Calving 2 | Postpartum 3 | |||
|---|---|---|---|---|---|---|
| R-Value | p-Value | R-Value | p-Value | R-Value | p-Value | |
| Ketosis | −0.308 * | 0.029 | −0.374 ** | 0.007 | −0.336 * | 0.017 |
| Mastitis | −0. 448 ** | 0.001 | −0.488 ** | <0.001 | −0.357 * | 0.011 |
| Metritis | −0.462 ** | 0.001 | −0.428 ** | 0.002 | −0.365 ** | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Jiang, X.; Sun, R.; Bai, Y.; Xu, C.; Song, Y.; Xia, C. Effects of Supplementing Rumen-Protected Glutathione on Lactation Performance, Nutrients, Oxidative Stress, Inflammation, and Health in Dairy Cows During the Transition Period. Vet. Sci. 2025, 12, 84. https://doi.org/10.3390/vetsci12020084
Hao Y, Jiang X, Sun R, Bai Y, Xu C, Song Y, Xia C. Effects of Supplementing Rumen-Protected Glutathione on Lactation Performance, Nutrients, Oxidative Stress, Inflammation, and Health in Dairy Cows During the Transition Period. Veterinary Sciences. 2025; 12(2):84. https://doi.org/10.3390/vetsci12020084
Chicago/Turabian StyleHao, Yu, Xuejie Jiang, Rui Sun, Yunlong Bai, Chuang Xu, Yuxi Song, and Cheng Xia. 2025. "Effects of Supplementing Rumen-Protected Glutathione on Lactation Performance, Nutrients, Oxidative Stress, Inflammation, and Health in Dairy Cows During the Transition Period" Veterinary Sciences 12, no. 2: 84. https://doi.org/10.3390/vetsci12020084
APA StyleHao, Y., Jiang, X., Sun, R., Bai, Y., Xu, C., Song, Y., & Xia, C. (2025). Effects of Supplementing Rumen-Protected Glutathione on Lactation Performance, Nutrients, Oxidative Stress, Inflammation, and Health in Dairy Cows During the Transition Period. Veterinary Sciences, 12(2), 84. https://doi.org/10.3390/vetsci12020084






